Abstract
The LAM2001 phase 3 trial, involving 832 patients with acute myeloid leukemia (AML; median: 46 years) proposed HLA-identical sibling allograft HSCT for all patients with an identified donor. The trial compared reduced-intensity conditioning (RIC) for patients older than 50 years of age (N = 47) and myeloablative conditioning for younger patients (N = 117). BM HSCT was performed in the younger patients, while the older ones received a consolidation course, followed by peripheral blood allo-HSCT using RIC. The incidence of grade II-IV acute GVHD, was 51.9% (95% confidence interval [CI]: 42.1-61.8) and 11.3% (1.6-21.2) after myeloablative or RIC, respectively (P < .0001) and that of chronic GVHD 45.8% (95% CI: 34.8-56.7) and 41.7% (24.7-58.6; NS). Cumulative incidence of nonrelapse mortality at 108 months was 15.8% (95% CI: 9.8-23.2) for myeloablative, and 6.5% (0.2-16.2) for RIC (NS). CI of relapse at 108 months was 21.7% (95% CI: 13.9-28.6) and 28.6% (16.5-43.4; NS). Overall survival at 108 months was 63.4% (95% CI: 54.6-72.2) and 65.8% (52.2-72.2), respectively, after myeloablative or RIC (NS). RIC peripheral blood stem cell allo-HSCT is prospectively feasible for patients between the ages of 51 and 60 years without excess of relapse or nonrelapse mortality, and compares favorably with myeloablative marrow allo-HSCT proposed to younger patients. This study was registered at clinicaltrials.gov as no. NCT01015196.
Introduction
Great progress has been achieved since the introduction of aracytine and anthracyclins in the initial management of patients with acute myeloid leukemia (AML); complete remission (CR) rates have considerably improved, most failures being attributable to early death rather than induction toxicity.1 However, the rate of relapse remains unacceptable, and new strategies are required to improve the long-term outcome of AML patients. Part of the progress can be attributed to postremission chemotherapy,2 but HSCT is another consideration, be it autologous or allogeneic, the latter with related or unrelated donors.
In the recent years, the Groupe Ouest-Est d'étude des Leucémies Aiguës et autres Maladies du Sang (GOELAMS) group has indeed developed risk-adapted strategies to improve the outcome of patients with AML in first remission. The trial reported here, designed in 2001, tested different strategies in relation to HSCT. For patients lacking genoidentical donors, one aim was to compare intensive consolidation followed by 1 or 2 autologous peripheral stem cell transplantation(s) (SCT).3 For patients with a sibling donor, the second aim proposed an early allo-HST with an age-adapted conditioning regimen. A myeloablative regimen was applied for patients younger than 50 years of age. For patients between 50 and 60 years of age, a reduced–intensity conditioning (RIC) regimen was applied after an early intensification and before allo-HSCT. The results of the allogeneic HSCT arm of this trial are presented here, showing that this strategy yielded similar results in the 2 age groups.
Methods
Patient eligibility
From November 2001 to April 2005, 832 previously untreated patients (age ≤ 60 years) with AML, issued from 28 centers, were enrolled in the LAM-2001 GOELAMS study. Patients with AML3 or isolated extramedullary disease, as well as patients with a previous diagnosis of myelodysplastic or myeloproliferative disorder were excluded. Patients were also excluded from the study if they were considered ineligible to receive the planned treatment (World Health Organization Eastern Cooperative Oncology Group [WHO ECOG] status ≥ 3, severe arrhythmia, progressive coronary artery disease, acute heart failure, or left ventricular ejection fraction < 40%, renal or liver dysfunction, psychiatric disease, or inadequate familial support). All patients underwent diagnostic BM aspiration with evaluation of morphology (≥ 20% blasts), immunophenotyping, and cytogenetics. Whenever possible, sampling for molecular testing (FLT-ITD/D835, NPM, CEBPα) was performed at diagnosis. Cytogenetic analyses were performed according to the International System for Human Cytogenetic Nomenclature (ISCN).4 Three prognostic groups were defined: (1) low risk: t(8;21)(q22;q22), inv(16)(p13q22); (2) high risk: complex abnormalities (≥ 3), del(5q), −5, −7, 3q rearrangements, t(9;22), t(6;9), 11q23/MLL rearrangements except t(9;11); and (3) intermediate risk: all other cases. HLA typing was performed as early as possible after diagnosis.
In accordance with the Declaration of Helsinki, the study was reviewed and approved by the Comité de Protection des Personnes ethics committee (Nantes, France; ID 2000/5/00) and all patients provided written informed consent before their participation in the study.
Study design
The treatments applied have been extensively described elsewhere.3 Briefly, after inclusion, all patients were randomized to receive induction chemotherapy with either daunorubicin, 60 mg/m2 on days 1 to 3, or idarubicin, 8 mg/m2 on days 1 to 5, associated with continuous infusion of cytosine-arabinoside (ara-c), 200 mg/m2 on days 1 to 7. Response assessment was performed on day 15. If < 5% BM blasts were present, no additional chemotherapy was prescribed and evaluation for CR was performed between days 28 and 35. If there were > 5% blasts in the BM, a second induction course was given including daunorubicin 35 mg/m2 or idarubicin 8 mg/m2 on days 17 and 18, according to initial randomization and ara-c 1000 mg/m2 bid on days 17 to 20, followed by G-CSF (lenograstim). Patients in CR could proceed to further steps of the trial according to the identification (or not) of an HLA-identical sibling, at diagnosis or second randomization. Patients not in CR could receive one additional induction course similar to the first intensive consolidation course (ICC) and were excluded from the protocol if not in CR after this.
Patients without a donor proceeded to second randomization to receive one (arm A) or 2 (arm B) autologous HSCTs (auto-HSCTs), as late consolidation course(s). Results of this part of the study have been reported elsewhere.3
Patients with an HLA-identical sibling donor planned to receive an allo-HSCT. If younger than 51 years old, they received BM allogeneic SCT after a myeloablative conditioning regimen consisting of cyclophosphamide (120 mg/kg over 2 days) and total body irradiation (6 fractions of 2 Gy). GVHD prophylaxis included a combination of cyclosporin (CSA) and a short course of methotrexate (15 mg/m2 on day +1; 10 mg/m2 on day +3 and day +6). A mini-consolidation course (daunorubicin 60 mg/m2 or idarubicin 12 mg/m2 for 2 days, ara-c 100 mg/m2 SC [subcutaneous] for 7 days) was administered to allow planning of the transplant procedure. Patients older than 51 years of age received a mini-consolidation as previously described, followed by one ICC (daunorubicin 60 mg/m2 or idarubicin 12 mg/m2 for 2 days, ara-c 3 g/m2 IV × 2/day for 4 days) before peripheral blood stem cell (PBSC) transplantation after RIC, combining oral busulfan, 8 mg/kg over 2 days, fludarabine, 120 mg/m2 over 4 days, rabbit anti-thymocyte globulins (Genzyme), 2.5 mg/kg on days −4 and −3, and GVHD prophylaxis with CSA alone.
Statistical methods
Descriptive statistics are reported as frequencies, or medians and range. Normality of sample distribution was checked with the Kolmogorov-Smirnov test. Comparisons of median values were performed using the Mann-Whitney rank sum test. The χ2 test or Fisher exact test were used to test for differences between groups. Statistical analysis was performed on an intention-to-treat basis using Medcalc software. Outcome data were updated at the date of September 11, 2008. Overall survival (OS) was calculated from the date of the first randomization until the date of death from any cause, with censoring of other patients at the date of last follow-up. Leukemia-free survival (LFS) was calculated from the date of first CR until the date of relapse or death of any cause. For patients who did not relapse, observation was censored on the date of last follow-up. Survival data except cumulative incidences were estimated by the Kaplan-Meier method, then compared by the log-rank test, with hazard ratio (HR) estimated by the Cox model. Factors associated with a significant impact in univariate analysis were retained for multivariate logistic regression. Type 1 error was fixed at the 5% level. All tests were 2-tailed. Failure events as relapse and deaths from causes related to the transplantation were calculated performing competing risk analysis using the R software (R Foundation for Statistical Computing).5
Results
Patient and transplantation characteristics
Between November 2001 and April 2005, a total of 832 patients were included in the study.3 Nine patients were considered ineligible, 8 with a wrong diagnosis or age (2 older than 60, 1 AML3, and 5 Ph+ CML [chronic myeloid leukemia] in acute phase). One patient was excluded because he died before receiving chemotherapy. There was no significant difference between the 2 groups (idarubicin, n = 412 or daunorubicin, n = 411) of the first randomization regarding initial characteristics.4 Of the 823 patients evaluable for induction treatment, 676 (82%) achieved a CR, including 579 patients treated with one course of chemotherapy (86% of CR patients). There were 24 (2.9%) early deaths, 12 (1.4%) deaths during aplasia, 7 (0.8%) deaths after hematologic recovery, and 104 (12.6%) patients with induction failure. The CR rate was not significantly different between the 2 induction treatment arms (idarubicin 83% vs daunorubicin 81%, not significant [NS]).
Of 676 patients in CR, 640 were assigned to the intensive treatment as scheduled by the trial. The 36 remaining patients were not considered for intensive postremission therapy for the following reasons: extrahematologic toxicity (n = 5), protocol violation (n = 17), refusal (n = 4), early relapse (n = 10). Among these 640 patients, 410 had no sibling donor identified and were eligible for the second randomization: they were assigned to receive one (arm A, n = 206) or 2 (arm B, n = 204) courses of auto-SCT. Two hundred thirty patients had an HLA-identical sibling donor. Of them, 29 with good prognosis and in CR1 after one induction were assigned to receive 2 courses ICC, while 201 were eligible for an allograft. Of them, 10 left the study early before scheduling transplantation (relapse 1, major early toxicities 4, major protocol violations 5).
No significant differences were observed between patients who had or did not have a sibling donor; the only difference between patients assigned to receive an allograft being age as per protocol. The median age and follow-up for patients assigned to receive auto-SCT (n = 410) were 46 years old (range: 17-60) and 53 months (range: 9-83), respectively. For patients with a sibling donor they were 44 years old (range: 17-60) and 53 months (range: 9-83), respectively.
Finally, of the 191 patients with an identified HLA-identical sibling donor, 164 effectively received transplantations according to the planned strategy in first remission. The remaining 27 patients could not receive the planned allo-HSCT for the following reasons: patient's refusal (n = 2), donor's refusal (n = 2), relapse (n = 11), nonleukemic deaths (n = 2), protocol violation, including 2 cord blood transplantations (n = 6), poor performance status (n = 3), and one donor's exclusion. Of the 164 who received transplantations, 117 received a myeloablative regimen and 47 a reduced-intensity regimen (Figure 2). Patients' characteristics between the 2 groups (Table 1) were comparable, except that there were more male patients in the myeloablative group (P = .02), more older patients requiring 2 courses of chemotherapy to achieve CR (P = .03) and, as expected by the protocol, older age for RIC. Nine patients eligible for the myeloablative regimen were conditioned with RIC and 2 patients aged 56 and 52 eligible for RIC were treated with a myeloablative regimen.
Characteristics of the overall cohort of patients who effectively received allogenic SCTs
| Patients . | Allogeneic HSCT, N = 164 . | Myeloablative, n = 117 . | RIC, n = 47 . | P* . |
|---|---|---|---|---|
| Median follow-up for alive patients (range), mo | 88 (16-119) | 88 (30-119) | 88 (16-119) | NS |
| Median age (range), y | 44 (17-60) | 39 (17-50) | 54 (34-60) | < .001 |
| Male sex (%) | 84 (51) | 67 (57) | 17 (36) | .02 |
| Previous solid tumor | 4 | 3 | 1 | NS |
| Performance status (%) | ||||
| 0-1 | 151 (92) | 108 (92.3) | 43 (91.5) | NS |
| 2 | 13 (8) | 9 (7.7) | 4 (8.5) | |
| Median initial WBC count (range), G/L | 11.8 (0.7-308) | 12.6 (0.7-308) | 9.3 (1-159) | NS |
| FAB classification | ||||
| (0/1/2/4/5/6/7) | 8/37/36/29/13/5/0 | 5/29/25/21/12/4/0 | 3/8/11/8/1/1/0 | NS |
| Not classified | 36 | 21 | 15 | |
| Karyotype | ||||
| Not evaluable (%) | 2 (1.2) | 1 (0.8) | 1 (2.1) | |
| Failure | 12 (7.3) | 10 (8.5) | 2 (4.3) | |
| Partial failure | 7 (4.2) | 6 (5.2) | 1 (2.1) | |
| Evaluable (%)† | ||||
| Favorable (1) | 18 (10.9) | 14 (12) | 4 (8.5) | |
| Intermediate (2) | 101 (61.5) | 72 (61.5) | 29 (61.7) | |
| High-risk (3) | 24 (14.6) | 14 (12) | 10 (21.3) | NS |
| First randomization (%) | ||||
| Idarubicin | 88 (53.6) | 59 (50.4) | 29 (61.7) | |
| Daunorubicin | 76 (46.3) | 58 (49.6) | 18 (38.3) | NS‡ |
| Complete remission (%) | ||||
| CR in one course | 120 (73.2) | 87 (74.3) | 33 (70.2) | |
| CR in two courses | 44 (26.8) | 30 (25.7) | 14 (29.8) | .03 |
| Donor-recipient CMV serostatus (%) | ||||
| +/+ | 44 (26.8) | 26 (22.2) | 18 (38.3) | |
| +/− | 22 (13.4) | 17 (14.5) | 5 (10.6) | |
| −/+ | 26 (15.9) | 15 (12.8) | 11 (23.4) | |
| −/− | 65 (39.6) | 52 (44.4) | 13 (27.7) | .04 |
| Missing | 7 (4.3) | 7 (6) | 0 | |
| Donor-recipient sex match (%) | ||||
| Male-male | 48 (29.3) | 38 (32.5) | 10 (21.3) | |
| Male-female | 43 (26.2) | 29 (24.8) | 14 (29.8) | |
| Female-male | 34 (20.7) | 27 (23) | 7 (14.9) | |
| Female-female | 33 (20.1) | 17 (14.5) | 16 (34) | .03 |
| Missing | 6 (3.7) | 6 (5.1) | 0 | |
| Type of donor (%) | ||||
| HLA-identical sibling | 162 (98.8) | 115 (98.3) | 47 (100) | |
| Mismatched relative | 2 (1.2) | 2 (1.7) |
| Patients . | Allogeneic HSCT, N = 164 . | Myeloablative, n = 117 . | RIC, n = 47 . | P* . |
|---|---|---|---|---|
| Median follow-up for alive patients (range), mo | 88 (16-119) | 88 (30-119) | 88 (16-119) | NS |
| Median age (range), y | 44 (17-60) | 39 (17-50) | 54 (34-60) | < .001 |
| Male sex (%) | 84 (51) | 67 (57) | 17 (36) | .02 |
| Previous solid tumor | 4 | 3 | 1 | NS |
| Performance status (%) | ||||
| 0-1 | 151 (92) | 108 (92.3) | 43 (91.5) | NS |
| 2 | 13 (8) | 9 (7.7) | 4 (8.5) | |
| Median initial WBC count (range), G/L | 11.8 (0.7-308) | 12.6 (0.7-308) | 9.3 (1-159) | NS |
| FAB classification | ||||
| (0/1/2/4/5/6/7) | 8/37/36/29/13/5/0 | 5/29/25/21/12/4/0 | 3/8/11/8/1/1/0 | NS |
| Not classified | 36 | 21 | 15 | |
| Karyotype | ||||
| Not evaluable (%) | 2 (1.2) | 1 (0.8) | 1 (2.1) | |
| Failure | 12 (7.3) | 10 (8.5) | 2 (4.3) | |
| Partial failure | 7 (4.2) | 6 (5.2) | 1 (2.1) | |
| Evaluable (%)† | ||||
| Favorable (1) | 18 (10.9) | 14 (12) | 4 (8.5) | |
| Intermediate (2) | 101 (61.5) | 72 (61.5) | 29 (61.7) | |
| High-risk (3) | 24 (14.6) | 14 (12) | 10 (21.3) | NS |
| First randomization (%) | ||||
| Idarubicin | 88 (53.6) | 59 (50.4) | 29 (61.7) | |
| Daunorubicin | 76 (46.3) | 58 (49.6) | 18 (38.3) | NS‡ |
| Complete remission (%) | ||||
| CR in one course | 120 (73.2) | 87 (74.3) | 33 (70.2) | |
| CR in two courses | 44 (26.8) | 30 (25.7) | 14 (29.8) | .03 |
| Donor-recipient CMV serostatus (%) | ||||
| +/+ | 44 (26.8) | 26 (22.2) | 18 (38.3) | |
| +/− | 22 (13.4) | 17 (14.5) | 5 (10.6) | |
| −/+ | 26 (15.9) | 15 (12.8) | 11 (23.4) | |
| −/− | 65 (39.6) | 52 (44.4) | 13 (27.7) | .04 |
| Missing | 7 (4.3) | 7 (6) | 0 | |
| Donor-recipient sex match (%) | ||||
| Male-male | 48 (29.3) | 38 (32.5) | 10 (21.3) | |
| Male-female | 43 (26.2) | 29 (24.8) | 14 (29.8) | |
| Female-male | 34 (20.7) | 27 (23) | 7 (14.9) | |
| Female-female | 33 (20.1) | 17 (14.5) | 16 (34) | .03 |
| Missing | 6 (3.7) | 6 (5.1) | 0 | |
| Type of donor (%) | ||||
| HLA-identical sibling | 162 (98.8) | 115 (98.3) | 47 (100) | |
| Mismatched relative | 2 (1.2) | 2 (1.7) |
SCT indicates stem cell transplantation; WBC, white blood cell; FAB, French-American-British; NS, non significant; and CR, complete remission.
Explain symbol.
Karyotype: (1) t(8;21) or inv(16); (2) all other chromosomal abnormalities; (3) high risk: −5, 5q−, −7, 3q abnormalities, t(6;9), multiple abnormalities (more than five abnormalities).
Explain symbol.
Sex mismatches were 17 female/male, 27 male/female in the myeloablative arm and 16 female/male, 7 male/female in the RIC arm (P = .03).
Transplantation outcomes
Transplantation outcomes as shown in Table 2 can be further explained as follows.
Clinical evolution of allo-SCT recipients in the myeloablative and RIC arms of the trial
| . | Myeloablative . | RIC . | P . |
|---|---|---|---|
| Acute GVHD grade II-IV, % (95% CI) | 51.9 (42.1-61.8) | 11.3 (1.6-21.2) | < .0001 |
| Chronic GVHD, % (95% CI) | 45.8 (34.8-56.7) | 41.7 (24.7-58.6) | NS (.83) |
| Cumulative incidence of NRM, % (95% CI), mo | |||
| 36 | 12.9 (7.5-19.7) | 4.2 (0.7-12.9) | NS (.33) |
| 72 | 15.8 (9.8-23.2) | 6.5 (0.2-16.2) | |
| 108 | 15.8 (9.8-23.2) | 6.5 (0.2-16.2) | |
| Cumulative incidence of relapse, % (95% CI), mo | |||
| 36 | 18.9 (12.4-26.6) | 20.7 (15.7-41) | NS (.33) |
| 72 | 21.7 (13.9-28.6) | 28.6 (16.5-43.4) | |
| 108 | 21.7 (13.9-28.6) | 28.6 (16.5-43.4) | |
| EFS, % (95% CI), mo | |||
| 36 | 68.1 (59.5-76.4) | 68.1 (54.6-80.9) | |
| 72 | 63.4 (54.6-72.2) | 65.8 (52.2-72.2) | NS (.83) |
| 108 | 63.4 (54.6-72.2) | 65.8 (52.2-72.2) | |
| OS, % (95% CI), mo | |||
| 36 | 73.4 (65.2-81.1) | 76.2 (63.1-87.3) | |
| 72 | 68 (59.3-76.3) | 69.3 (55.6-82.1) | NS (.82) |
| 108 | 68 (59.3-76.3) | 69.3 (55.6-82.1) |
| . | Myeloablative . | RIC . | P . |
|---|---|---|---|
| Acute GVHD grade II-IV, % (95% CI) | 51.9 (42.1-61.8) | 11.3 (1.6-21.2) | < .0001 |
| Chronic GVHD, % (95% CI) | 45.8 (34.8-56.7) | 41.7 (24.7-58.6) | NS (.83) |
| Cumulative incidence of NRM, % (95% CI), mo | |||
| 36 | 12.9 (7.5-19.7) | 4.2 (0.7-12.9) | NS (.33) |
| 72 | 15.8 (9.8-23.2) | 6.5 (0.2-16.2) | |
| 108 | 15.8 (9.8-23.2) | 6.5 (0.2-16.2) | |
| Cumulative incidence of relapse, % (95% CI), mo | |||
| 36 | 18.9 (12.4-26.6) | 20.7 (15.7-41) | NS (.33) |
| 72 | 21.7 (13.9-28.6) | 28.6 (16.5-43.4) | |
| 108 | 21.7 (13.9-28.6) | 28.6 (16.5-43.4) | |
| EFS, % (95% CI), mo | |||
| 36 | 68.1 (59.5-76.4) | 68.1 (54.6-80.9) | |
| 72 | 63.4 (54.6-72.2) | 65.8 (52.2-72.2) | NS (.83) |
| 108 | 63.4 (54.6-72.2) | 65.8 (52.2-72.2) | |
| OS, % (95% CI), mo | |||
| 36 | 73.4 (65.2-81.1) | 76.2 (63.1-87.3) | |
| 72 | 68 (59.3-76.3) | 69.3 (55.6-82.1) | NS (.82) |
| 108 | 68 (59.3-76.3) | 69.3 (55.6-82.1) |
Clinical evolution of allo-SCT recipients in the myeloablative and RIC arms of the trial shows similar evolution except for the higher incidence of acute GVHD in patients receiving a myealoablative conditioning regimen.
SCT indicates stem cell transplantation; RIC, reduced-intensity conditioning; CI, confidence interval; NS, non significant; NRM, nonrelapse mortality; EFS, event-free survival; and OS, overall survival.
GVHD.
Among patients receiving a myeloablative conditioning, 56 developed acute GVHD (grade 1, n = 10; grade 2, n = 32; grade 3, n = 10; grade 4, n = 4) and 34 chronic GHVD, extensive in 20 cases. Within the group of patients conditioned with RIC, 12 developed acute GVHD (grade 1, n = 6; grade 2, n = 2; grade 3, n = 2; grade 4, n = 1; unknown, n = 1) and 16 chronic GVHD, extensive in 5. The overall incidence of grade II-IV acute GVHD was 51.9% (95% confidence interval [CI]: 42.1-61.8) and 11.3% (95% CI: 1.6-21.2) for myeloablative and RIC, respectively (P < .0001), and for chronic GVHD 45.8% (95% CI: 34.8-56.7) and 41.7% (95% CI: 24.7-58.6; P = .83).
Survival.
The OS at 108 months, after allo-HSCT of patients in first CR, was 68% (95% CI: 59.3-76.3) and 69.3% (95% CI: 55.6-82.1), respectively, after myeloablative conditioning or RIC (Figure 1; NS). This analysis only considered patients who received an allo-SCT. On an intent-to-treat basis, results were not statistically different (P = .35).
Overall survival after allogenic SCT shows similar outcome in the 2 age groups and conditioning regimen.
Overall survival after allogenic SCT shows similar outcome in the 2 age groups and conditioning regimen.
Event-free survival (EFS) at 108 months was 63.4% (95% CI: 54.6-72.2) and 65.8% (95% CI: 52.2-79.1), respectively (NS). On an intent-to-treat basis, results were still not different between the 2 arms (P = .23).
Nonrelapse mortality.
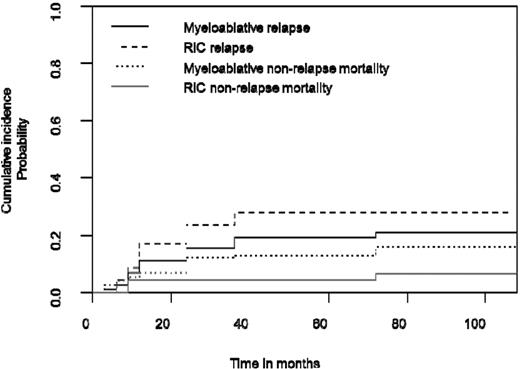
The cumulative incidence of nonrelapse mortality (NRM) at 36 months was 12.9% (95% CI: 7.6-19.7) for myeloablative conditioning and 4.2% (95% CI: 0.7-12.9) for RIC (Figure 2). With a longer follow-up at 108 months, these figures were 15.8% (95% CI: 9.8-23.2) and 6.5% (95% CI: 0.2-16.3), respectively (NS). All but 2 nonrelapse deaths were due to treatment-related toxicities.
Myeloablative and RIC estimated cumulative incidence curves with NRM and relapse as competing events show similar outcome in the 2 age groups with adapted conditioning regimen.
Myeloablative and RIC estimated cumulative incidence curves with NRM and relapse as competing events show similar outcome in the 2 age groups with adapted conditioning regimen.
Relapse.
The cumulative incidence of relapse at 36 months was 18.9% (95% CI: 12.4-26.6) for myeloablative conditioning and 20.7% (95% CI:15.7-41) for RIC, and at 108 months, 21.7% (95% CI: 13.9-28.6) and 28.6% (95% CI:16.5-43.4), respectively (NS; Figure 2).
Comparison of auto- and allo-HSCT arms outcome.
As reported previously,3 similar outcomes were observed in this trial between allo-SCT patients and those, without related donor, who received one or 2 auto-HSCT. In an intent-to-treat analysis, and considering only those with intermediate- or high-risk cytogenetics, allo-SCT (RIC or myeloablative) did not reach significantly better EFS and OS than auto-SCT (P = .23 and 0.29, respectively).
Discussion
The results reported for this LAM-2001 trial of the GOELAMS group indicate that, in a prospective multicentric study, allo-HSCT performed in early first remission may yield comparable results for unselected older patients, conditioned with RIC, and for younger patients conditioned with a myeloablative regimen.
It must be noted that the study, initiated before 2001, did not include NPM1/FLT3 molecular studies, and retrospective analysis has only been possible for 37 allografted patients with a normal karyotype (16 patients with NPM1+/FLT3-ITD−; R. Guièze, P. Cornillet-Lefebvre, B. Lioure, et al, manuscript in preparation). The possible impact of this prognosis factor therefore cannot be appreciated here. Moreover, good-prognosis patients characterized by AML without hyperleukocytosis and core binding factor (CBF) cytogenetics [inv16, t(8;21)] in remission after the first induction course were excluded from the study. Numerous reports have described retrospective results of RIC HSCT for older AML patients, often with more advanced diseases or heterogeneous initial chemotherapies.6-12 This cohort of unselected patients, treated prospectively, has, however, several points of interest including the fact that 2 different sources of HSCs were used yet yielded similar results. Within the smaller group of older patients, between 50 and 60 years of age, the probability of relapse was similar to that of the younger patients conditioned with the myeloablative regimen, even including ATG. Significantly less acute GVHD were observed after RIC but, in this trial, no statistical difference was observed for the incidence of chronic GVHD. As this trial was designed in an era where the natural history of GVHD after RIC was not fully appreciated, late acute GVHD was probably partly included in the chronic group. However, the low incidence of chronic GVHD among the older patients suggests that, overall, the incidence of GVHD was still lower in this subgroup of patients.
Even without statistical difference in terms of NRM (P = .12), there was a trend for increased toxicity after myeloablative regimen, as observed in other settings.13,14 However, direct comparison of the 2 transplantation approaches would be biased in this trial because older patients received more intense chemotherapy before the procedure than patients of the younger group who were conditioned without intensive course after the mini-consolidation. In this older group, a potent GVL effect is suggested after RIC, probably associated with an optimal leukemic burden reduction before transplantation. The upper age limit of 60 years is certainly insufficient today to confirm a definite improvement for “older” patients and complementary strategies are needed to evaluate this approach. This question has remained unsolved since the 1990s and is discussed regularly.15,16 Strategies in allogeneic SCT are evolving with time and numerous approaches of conditioning regimen are still under study, as well as new immunosuppressive combinations for the prevention of GVHD, thus complicating comparisons. In this study, the use of ATG was probably beneficial to lower toxicities and did not result in a significant increase in the probability of relapse. The availability of IV busulfan, which is largely used in current studies, will probably help to further decrease morbidity.
If this trial is confirmed and a prospective feasibility is proven to include allo-HSCT in the strategy for older high-risk patients, the question remains of what will be the upper age limit for the next decade. Another pending question is that of the best donor's source to be chosen without increasing NRM. Here, it is interesting to note that similar results were obtained using either BM or PBMCs, but the best source of cells remains to be determined. Another possible source to better explore is that of cord blood. Of note, both the patients excluded from this report because they received such HSCT died, one 7 months and the other one 29 months after HSCT, the second one in relapse.
Other promising immunotherapeutic approaches have also been recently developed for the intensification regimen in older patients, such as infusion of mismatched stem cells after chemotherapy,17 but results need to be confirmed and balanced with allo-HSCT. In conclusion, in this trial, long-term disease control of adult AML using RIC allo-HSCT for older patients compared favorably with a younger group of patients conditioned more intensively in a prospective manner.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Nicole Raus from the Societé Francaise de Greffes de Moelle et Thérapie Cellulaurie and the numerous Attachés de Recherche Clinique and Technicien d'Etude Clinique who helped with data collection and monitoring, especially Roselyne Delepine and Cindy Grandjenette.
Authorship
Contribution: B.L., A.P., J.-L.H., N.I., and J.-Y.C. wrote the protocol; B.L. and J.-Y.C. analyzed data, recruited patients, provided clinical care, performed bibliographic searches, and wrote the manuscript; B.L., A.P., A.H., P.C., N.F., D. Blaise, B.W., M.D., J.C., M.C., M.H., F.L., T.L., E.R., M.O.-U., C.B., J.-L.H., D. Bouscary, N.I., and J.Y.C. recruited patients and provided clinical care; M.C.B. performed data collection management, validation, and statistical analyses and wrote the manuscript; I.L., O.B., and P.C.-L. produced and validated cytogenetic and molecular data; and L.F. helped perform data collection.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Yves Cahn, MD, UMR 5525 CNRS-UJF, Clinique Universitaire d'Hématologie, CHU Michallon, B.P. 217, 38043 Grenoble Cedex 09, France; e-mail: jycahn@chu-grenoble.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal