Abstract
Generation of human monocyte–derived dendritic cells (DCs) for cancer vaccination involves ex vivo maturation in the presence of proinflammatory cytokines and prostaglandin E(2) (PGE2). Although the inclusion of PGE2 during maturation is imperative for the induction of DC migration, PGE2 has unfavorable effects on the immunostimulatory capacity of these cells. Like PGE2, leukotrienes (LTs) are potent mediators of DC migration. We therefore sought to characterize the migratory and immunologic properties of DCs that matured in the presence of LTB4, LTC4, LTD4, and PGE2. Here, we demonstrate that DCs matured in the presence of LTC4, but not LTB4 or LTD4, are superior to PGE2-matured DCs in stimulating CD4+ T-cell responses and in inducing antigen-specific cytotoxic T lymphocytes (CTLs) in vitro without concomitant induction or recruitment of regulatory T cells (Tregs). LTC4-matured DCs migrate efficiently through layers of extracellular matrix and secrete higher levels of immunostimulatory IL-12p70 while producing reduced levels of immune-inhibitory IL-10, IL12p40, indoleamine-2,3-dioxidase, and TIMP-1 (tissue inhibitor of matrix metalloproteinases). Intracellular calcium mobilization and receptor antagonist studies reveal that, in contrast to LTD4, LTC4 did not signal through CysLTR1 in DCs. Collectively, our data suggest that LTC4 represents a promising candidate to replace PGE2 in DC maturation protocols for cancer vaccination.
Introduction
The most commonly used protocol for generating mature human dendritic cells (DCs) for cancer vaccination involves the ex vivo differentiation of DCs from CD14+ monocytes/macrophages, followed by culture in media supplemented with the proinflammatory cytokines TNF-α, IL-6, IL-1β, and PGE2 to induce maturation.1 Several studies have shown that the presence of the inflammatory mediator PGE2 during maturation of DCs is a prerequisite for the acquisition of chemotaxis toward lymph node–derived chemokines, such as CCL-19 and CCL-21.2,3
Unfortunately, PGE2 has unfavorable effects on the immunostimulatory capacity of DCs, hence limiting the efficacy of DC-based immunotherapy. PGE2 impairs the ability of DCs to produce bioactive IL-12p70,4,5 a cytokine essential to facilitate the generation of Th1 CD4+ T-cell responses, while stimulating the expression of immune inhibitory IL-12p40,6 IL-10,7 and indoleamine-2,3-dioxidase (IDO).8 IL-10 production by DCs limits proinflammatory cytokine production and may induce tolerance.9 The inhibitory effects of IDO on T cells include the depletion of the essential amino acid tryptophan resulting in the inhibition of T-cell proliferation.10,11 In addition, tryptophan catabolites have been reported to induce T-cell apoptosis and to exert cytotoxic effects on B cells and natural killer (NK) cells.12 Furthermore, PGE2 has been shown to enhance the expression of tissue inhibitor of matrix metalloproteinase (TIMP-1), thereby limiting DC migration through extracellular matrix in vitro, and potentially in vivo.13 Last, a recent study found that DCs matured in the presence of PGE2 are capable of expanding vaccine-induced immunosuppressive Tregs.14,15
To overcome PGE2-mediated immune-inhibitory effects, alternative maturation protocols for DCs have been investigated. Although DCs generated in the presence of GM-CSF/IL-15,16 and matured with Toll receptor 7/8 agonist R-848, TNF-α, IFN-γ, and PGE2 exhibited enhanced IL-12 production compared with TNF-α. IL-1β, IL-6, and PGE2-matured DCs,17 it remains unclear to what degree other immune-inhibitory properties of PGE2 were affected. Recently, another protocol for producing so-called α-Type-1 polarized DCs, using a maturation cocktail consisting of TNF-α. IL-1β, polyIC, IFN-α, and IFN-γ, without PGE2, has been described.18 Despite enhanced secretion of IL-12p70 and IL-23, the T-cell stimulatory properties of such α-Type-1 polarized DCs were no better than those of TNF-α, IL-1β, IL-6, and PGE2-matured DCs.19
Collectively, these data argue that there is an urgent need to develop improved DC maturation protocols omitting PGE2 to enhance the immunogenicity of DC-based cancer vaccines while preserving the migratory properties of DCs.
Leukotrienes (LTs) represent potent mediators of DC migration. The most biologically relevant LTs are LTB4, LTC4, and LTD4, with LTE4 being 8 to 12 times less active in its biologic activities than LTC4 and LTD4, respectively. LTB4 is well recognized as a potent stimulator of neutrophil aggregation and inducer of numerous inflammatory functions in leukocytes.20-22 There are two 7-pass transmembrane G-protein coupled receptors for LTB4, namely BLT1 (Yokomizo et al23 ) and BLT2 (Yokomizo et al24 ). Murine bone marrow–derived DCs migrate on exposure to LTB4, via up-regulation of the expression of CCR-7 and its ligand CCL-19,25 an effect that is abrogated in cells lacking the BLT1 receptor. Accordingly, BLT1−/− mice demonstrate diminished DC migration in a model of contact hypersensitivity. Human monocyte–derived DCs also migrate on exposure to LTB4,26 and chemotaxis is eliminated by blockade of the BLT2 receptor. These initial studies confirm an active role for LTB4 in DC activation, although it appears that there may be differences in receptor usage between species.
LTC4 and LTD4 act through 2 G-protein–coupled receptors, designated CysLTR1 (Lynch et al27 ) and CysLTR2 (Sarau et al28 and Heise et al29 ). These LTs are mediators of immediate hypersensitivity reactions and asthma exacerbations.30-32 Murine Langerhans cell migration in response to CCL-19 is impaired in mice lacking the multidrug transporter ATP-binding cassette c1 (also known as multidrug resistant protein 1, MRP-1) and migration toward CCL-19 could be restored by exogenous LTC433,34 Lastly, a previous study demonstrated that the expression of CysLT1 and CysLT2 receptors on human monocyte–derived DC may be dependent on the maturation stimulus,35 and in addition, provided some initial evidence for LTD4-induced DC chemotaxis.
In this study, we investigated the immunologic and migratory properties of human monocyte–derived DC that were matured in the presence of LTB4, LTC4, LTD4, and PGE2 and demonstrate that LTC4 may represent a promising candidate to replace PGE2 in DC maturation protocols. The use of LTC4 in this fashion may ultimately lead to DC-based cancer vaccines with enhanced immunogenicity and greater clinical impact.
Methods
Materials
Human recombinant CCL-19, TNF-α, and IL-β were obtained from Peprotech. LTB4, LTC4, LTD4, PGE2, MK-476 (Montelukast), anti-CysLTR1, and anti-CysLTR2 antibodies were purchased from Cayman Chemicals. Pertussis toxin was purchased from Tocris Bioscience. Ehrlich's reagent (4-[dimethyl-amino] benzaldehyde), brefeldin A, ionomycin, trichloroacetic acid, phorbol 12-myristate 13-acetate (PMA), (3,3′,5,5′)–tetramethylbenzidine, kynurenine sulfate, tryptophan, poly inosinic cytidylic acid (pIC), and lipopolysaccharide (LPS; from Salmonella abortus equi) were obtained from Sigma-Aldrich. Pluronic acid F127 was purchased from Invitrogen and FluoForte from Enzo Life Sciences. CD40L and Enhancer were obtained from Alexis Biochemicals. All antibodies were from BD Biosciences except for phycoerythrin-conjugated anti–human FoxP3 which was purchased from Ebiosciences. All reagents for magnetic-based cell separations were from Miltenyi Biotec.
Generation of human monocyte–derived DCs
CD14+ cells isolated via magnetic bead separation or elutriated monocytes/macrophages were used for DC culture by incubation in serum-free X-VIVO 15 medium (Cambrex Bio Science) supplemented with rhIL-4 (1000 U/mL) and rhGM-CSF (800 U/mL). After 5 days of culture, cells were harvested and characterized to ensure that they met the phenotype of immature DCs. DC purity was consistently > 90% and DCs were matured with cytokine cocktail (CC, 10 ng/mL TNF-α, 1000 U/mL IL-6, 10 ng/mL IL-1β), 1 μg/mL LPS, 12.5 μg/mL pIC, or 1.0 μg/mL recombinant CD40L plus 1.0 μg/mL enhancer. PGE2 was used at 1 μg/mL and LTs at 100nM.
Migration assays
DCs at 1 × 106 were plated into the upper chambers of 6-well transwell plates (Costar, 8 μm pore size). CCL19 (MIP-3β; Peprotech) at100 ng/mL was added to the lower chambers and migration of DCs was assessed after 2 hours at 37°C/5% CO2. DC migration in the absence of chemokine was subtracted as background. To determine DC migration through extracellular matrix components, inserts were coated with 2 mm of PBS-diluted Matrigel and DC migration was assessed after 6 hours. Lastly, chemotaxis assays for CD4+CD25+ T cells were performed in transwell plates with a 3-μm-pore-size polycarbonate filter (Corning) for 3 hours.
Polymerase chain reaction
Total RNA was extracted from DCs using the RNeasy Maxi kit (QIAGEN) and reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the Stratagene Mx3005P cycler and 2xBrilliant SYBR Green QPCR Mastermix (Stratagene) with the following primers: CCR-7, 5′-GGTGGCTCTCCTTGTCATTTTC-3′ and 5′-GGGAGGAACCAGGCTTTAAAGT-3′, TIMP-1, 5′-ACAGACGGCCTTCTGCAATTC-3′ and 5′-GGTGTAGAC-GAACCGGATGTCA-3′, MT-1, 5′-CCGATGTGGTGTTCCAGACA-3′ and 5′-TGGCCTCGTATGTGGCATACT-3′, MMP-9, 5′-CCTTTTGAGGGCGACCTCCAAG-3′ and 5′-CTGGATGACGATGTCTGCGT-3′, CysLTR1, 5′-CCTCAGCACCTATGCTTTGT-3′ and 5′-ATTGTCTTGTGGGGGCTCAA-3′, CysLTR2, 5′-AGACTGCATAAAGCTTTGGTTATC-3′ and 5′-ATACTCTTGTTTCCTTTCTCAA-CC-3′, BLT-1, 5′-GAGTTCATCTCTCTGCTGGC-3′ and 5′-CCAGGTTCA-GCACCATCAGG-3′, BLT-2, 5′-GAGACTCTGACCGCTTTCGT-3′ and 5′-AAGGTTGACTGCGTGGTAGG-3′, IDO, 5′-TGTCCGTAAGGTCTTGCCAAGA-3′ and 5′-CACCAATAGAGAGACCAGGAAGAATC-3′, β-actin, 5′-ATGTTTGAGACCTTCAACAC-3′ and 5′-CACGTCACACTTCATGATGG-3′. Differences in gene expression were calculated using the ΔΔCt method.
Enzyme-linked immunospot assays
IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed as described.36 HLA-A0201+ CD8+ T cells were isolated from CTL cultures by negative depletion using magnetic beads (Miltenyi Biotec). T cells at 2.5 × 104 were stimulated with 2.5 × 103 peptide-loaded (10μM) T2 cells for 16 hours. Spots were counted using the CTL ImmunoSpot S4 Analyzer (CTL Laboratories).
Intracellular calcium release
DCs were loaded with the fluorescent dye FluoForte in the presence of Pluronic acid F127 according to the manuals provided by the manufacturers. DCs (105/well) of a 96-well plate (Corning flat black, clear bottom) were analyzed under calcium-free conditions using the Infinite F200 Pro (Tecan) with excitation at 485 nm and emission at 535 nm.
ELISAs
Enzyme-linked immunosorbent assays (ELISAs) were performed according to the manuals provided by the manufacturers. The IL12p70 kit, sensitivity 5 pg/mL, and CCL-22/macrophage-derived chemokine (MDC) kit, sensitivity 62.5 pg/mL, were obtained from R&D Systems; the IL12p40 kit, sensitivity 31.3 pg/mL, was purchased from BD Biosciences; and the IL-10 assay, sensitivity 5 pg/mL, was from eBiosciences.
Cytolytic assays
Peptide-loaded DCs were cocultured with autologous T cells (isolated via magnetic bead-based separation) at a DC:T-cell ratio of 1:20. Cells were restimulated once after 7 days (DC:T cell ratio of 1:10) and analyzed for cytolytic activity after another 7 days in Europium release assays37 using peptide-loaded T2 cells as targets. Triplicate wells were averaged, and the percentage of specific lysis was determined. The effector-to-target ratio which caused 20% lysis was determined by regression analysis and lytic units (LU) were calculated as described.38
Cytokine production by stimulated CD4+ T cells
Production of cytokines by CD4+ T cells was assessed by intracellular cytokine staining and flow cytometry, as described.39 For detection of IFN-γ and IL-2, cells were activated for 6 hours in the presence of 50 ng/mL PMA and 1 μg/mL ionomycin and 10 μg/mL of brefeldin A was added during the last 2 hours of incubation. IL-4 and IL-5 production by CD4+ T cells was determined after 16 hours of stimulation
T-cell proliferation assays
Negative selection of T lymphocytes was performed by magnetic bead separation (Miltenyi Biotec). Purified cells were seeded into 96-well round-bottom microplates at 105 cells/well and the indicated numbers of allogeneic DCs in complete medium. Triplicate wells of T cells alone were used as the background control. After 5 days of culture, T-cell proliferation was determined using the WST-1 Cell Proliferation Reagent according to the manual provided by the manufacturer (Clontech).
Determination of IDO enzyme activity
Kynurenine levels were measured to determine IDO enzyme activity. DCs were washed and resuspended at 106 cells/mL in Hanks buffered saline solution supplemented with 100μM tryptophan. After 4 hours, supernatants were assayed for the presence of kynurenine. Supernatants were mixed with 30% trichloroacetic acid (2:1) and centrifuged at 10 000g for 15 minutes. The supernatant was incubated at 50°C for 20 minutes to hydrolyze N-formylkynurenine and was then added to an equal volume of Ehrlich reagent (100 mg p-dimethylbenzaldehyde, 5 mL glacial acetic acid) in a 96-well microtiter plate. Triplicate samples were run against a standard curve of kynurenine (0-100μM). Optical density was measured at 492 nm using a microplate reader. IDO activity was reported as the concentration of kynurenine produced.
Results
Expression of LT receptors by DCs
In the first series of experiments, we analyzed the expression of LT receptors CysLTR1, CysLTR2, BLT1, and BLT2 by monocyte-derived DCs in the presence or absence of well-established maturation stimuli, including a cytokine cocktail (CC) consisting of TNF-α, IL-6, and IL-1β, TLR-4 agonist lipopolysaccharide (LPS), TLR-3 agonist pIC, and CD40L. Figure 1 (left panel) illustrates the expression of LT receptor mRNA, as determined by quantitative RT PCR, in comparison with that of untreated, immature DCs (DC Imm). DCs expressed all leukotriene receptors analyzed. BLT1 mRNA was expressed at similar levels by immature, CC and pIC-matured DCs, whereas modest down-regulation (2- to 3-fold) was observed in CD40L and LPS-matured DCs. In contrast, BLT2 mRNA was consistently expressed by DCs and did not seem to change significantly on DC maturation. Expression of CysLTR1 and CysLTR2-mRNA was significantly up-regulated in CC and LPS-matured DCs, compared with immature DCs or pIC and CD40L-matured DCs.
Expression of leukotriene receptor mRNA and protein by human monocyte-derived DCs. (Left panel) DCs were matured for 1 hour with CC (10 ng/mL TNF-α, 1000 U/mL IL-6, 10 ng/mL IL-1β, 1 μg/mL LPS, 12.5 μg/mL pIC, or 1.0 μg/mL recombinant CD40L plus 1.0 μg/mL enhancer). RNA was isolated, reverse transcribed and amplified for 40 cycles using quantitative RT PCR. For comparison, RNA isolated from immature DCs (DC Imm) was also amplified. The data presented are from 1 representative experiments of 3 performed. (Right panel) DCs were matured for 12 hours as indicated and expression of CysLTR1 and CysLTR2 was analyzed by FACS. A representative result for 1 of 3 donors is presented.
Expression of leukotriene receptor mRNA and protein by human monocyte-derived DCs. (Left panel) DCs were matured for 1 hour with CC (10 ng/mL TNF-α, 1000 U/mL IL-6, 10 ng/mL IL-1β, 1 μg/mL LPS, 12.5 μg/mL pIC, or 1.0 μg/mL recombinant CD40L plus 1.0 μg/mL enhancer). RNA was isolated, reverse transcribed and amplified for 40 cycles using quantitative RT PCR. For comparison, RNA isolated from immature DCs (DC Imm) was also amplified. The data presented are from 1 representative experiments of 3 performed. (Right panel) DCs were matured for 12 hours as indicated and expression of CysLTR1 and CysLTR2 was analyzed by FACS. A representative result for 1 of 3 donors is presented.
The results obtained by RT PCR were corroborated at the protein level in fluorescence-activated cell sorter (FACS) analyses for CysLTR1 and CysLTR2 expression (Figure 1B). Immature DCs expressed both receptors on their cell surfaces (Figure 1B top panel) and receptor expression was further increased by maturation with CC or LPS. Maturation with pIC did not affect receptor expression, whereas stimulation with CD40L led to a very modest up-regulation of both receptors.
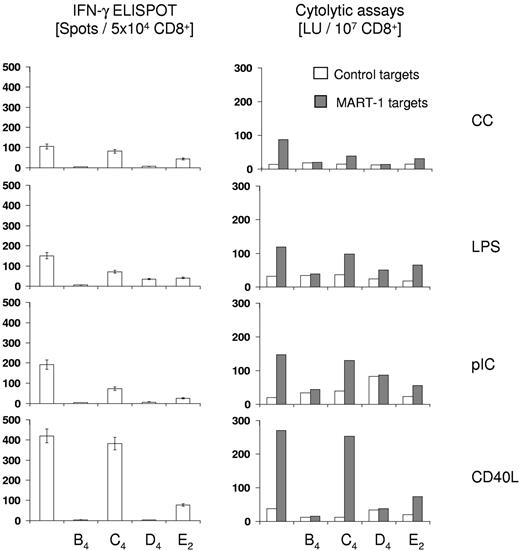
Induction of tumor-antigen specific CTL
DCs have the unique capability of inducing a primary specific immune response by presenting antigenic peptides to naive T cells and play a central role in cancer vaccination with the ultimate goal of generating tumor-specific and cytolytic T-cell responses. Therefore, the impact of the inclusion of LTB4, LTC4, LTD4, and PGE2 during maturation on the ability of DCs to stimulate a CTL response against a HLA-A0201–restricted peptide epitope derived from the melanoma antigen MART-1/Melan A was tested. DCs from healthy HLA-A2+ volunteers were matured with CC, LPS, pIC, or CD40L in the presence or absence of LTB4, LTC4, LTD4, and PGE2, loaded with MART peptide and cocultured with autologous T cells. T cells were restimulated once and isolated CD8+ T cells were analyzed for antigen-specific IFN-γ secretion in ELISPOT assays (Figure 1 left panel) or for antigen-specific lytic activity using peptide-pulsed T2 cells as targets in CTL assays (Figure 1 right panel). As shown in Figure 2, DCs exposed to any of the maturation stimuli were capable of stimulating a MART-1–specific CTL response. Maturation of DCs with CD40L was clearly superior to all the other maturation stimuli with respect to the ability of DCs to induce a MART-1–specific CTL response. Data from cytolytic assays correlated well with the enumeration of IFN-γ secreting CD8+ T cells in ELISPOT assays (Figure 1 left panel). Maturation of DCs in the presence of LTB4 or LTD4 almost completely impaired the induction of MART-specific CTL, independent of the maturation stimulus, as assessed in both CTL and ELISPOT assays. LTC4 and PGE2 similarly reduced the generation of MART-specific CTL responses by CC, LPS, and pIC-maturated DCs. However, although PGE2 greatly reduced the T-cell stimulatory function of CD40L-matured DCs, DCs matured with CD40L plus LTC4 proved to be potent inducers of primary MART-1 CTL responses, albeit with a slight reduction in frequency of MART-1–specific CD8 T cells compared with CD40L-matured DCs.
Cytolytic assays and IFN-γELISPOT analyses. For induction of antigen-specific CTL, T cells were isolated via magnetic bead separation from HLA-A0201–positive healthy volunteers. DCs were matured for 24 hours as indicated, loaded with the HLA-A0201-restriced MART-1/MelanA epitope ELAGIGILTV (10μM), and then used as stimulators at a stimulator to responder ratio of 1:20. After 7 days, cells were restimulated with autologous, peptide-loaded DCs at a stimulator to responder ratio of 1:10. Seven days later, CD8+ T cells were isolated and analyzed for cytolytic activity in standard europium release assays using MART-peptide pulsed or control peptide pulsed T2 cells as targets. Simultaneously, IFN-γ secreting CD8+ T cells were enumerated after addition of MART-1 peptide (10μM) in ELISPOT assays and counts obtained with control peptide were subtracted. The data are shown as the mean ± SEM of triplicate cultures and are from 1 representative experiment of 2 performed. Cytotoxicity is expressed as LU/107 cells at a reference lysis level of 20%.
Cytolytic assays and IFN-γELISPOT analyses. For induction of antigen-specific CTL, T cells were isolated via magnetic bead separation from HLA-A0201–positive healthy volunteers. DCs were matured for 24 hours as indicated, loaded with the HLA-A0201-restriced MART-1/MelanA epitope ELAGIGILTV (10μM), and then used as stimulators at a stimulator to responder ratio of 1:20. After 7 days, cells were restimulated with autologous, peptide-loaded DCs at a stimulator to responder ratio of 1:10. Seven days later, CD8+ T cells were isolated and analyzed for cytolytic activity in standard europium release assays using MART-peptide pulsed or control peptide pulsed T2 cells as targets. Simultaneously, IFN-γ secreting CD8+ T cells were enumerated after addition of MART-1 peptide (10μM) in ELISPOT assays and counts obtained with control peptide were subtracted. The data are shown as the mean ± SEM of triplicate cultures and are from 1 representative experiment of 2 performed. Cytotoxicity is expressed as LU/107 cells at a reference lysis level of 20%.
In conclusion, our cytolytic assays clearly highlight that LTB4 and LTD4 are not suitable agents to replace PGE2 in maturation protocols that aim to generate DCs that induce antigen-specific cellular immune responses. In the subsequent experiments, we therefore focused on a comparison of immunologic and migratory properties of CD40L + LTC4-matured DCs with those of DCs matured with the current “gold-standard” consisting of CCs in combination with PGE2.
Signal transduction of LTC4 and LTD4 in DCs
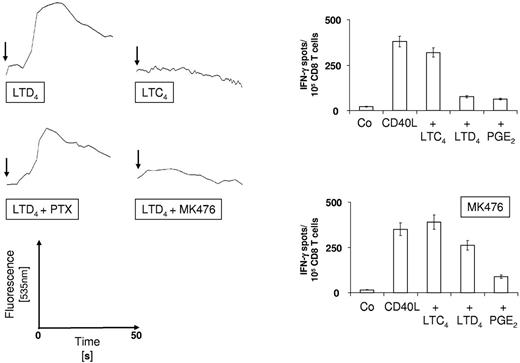
We observed that LTB4 almost completely abolished the induction of antigen-specific CTL, irrespective of the maturation stimulus used. This result is conceivable given that BLT1 is expressed on human monocyte–derived DCs. BLT1 is the high-affinity receptor for LTB4 (Kd BLT1 = 1nM vs Kd BLT2 = 20nM)40 and signaling through BLT1 results in the induction of strong Th2 responses.41 In contrast, LTC4 and LTD4 mediate their biologic effects through both CysLTR1 and CysLTR2, but only maturation in the presence of LTD4 resulted in a greatly diminished induction of CTL responses by DCs. We, therefore, investigated the mobilization of intracellular calcium by DCs in response to LTC4 and LTD4, an effect that is known to be mediated by signaling through CysLTR1 which couples primarily to the pertussis toxin-insensitive heterotrimeric Gq/11-protein.42 As shown in Figure 3A (top panel), mobilization of intracellular calcium was observed for DCs that were stimulated with CD40L and LTD4, but not for DCs treated with CD40L and LTC4. Even at a 10-fold higher concentration (1μM), no LTC4-induced Ca flux could be observed and there was no inhibition of intracellular Ca release when LTC4 and LTD4 were used in combination (data not shown).
Intracellular calcium mobilization and CysLTR1 antagonism. (Left panel) DCs were loaded with FluoForte and intracellular calcium release was determined using a Tecan Infinite F200 Pro fluorometer. CD40L was added at t-d and LTC4 or LTD4 were injected after 20 seconds at 37°C (arrow) and data were acquired for another 50 seconds. DCs were preincubated with 1 μg/mL PTX or 10μM CysLTR1 antagonist MK476) for 2 hours before stimulation with CD40L and LTD4. (Right panel) Peptide-specific CD8+ T-cell responses were induced as described using DC that were matured for 12 hours as indicated in the presence or absence of 10μM CysLTR1 antagonist MK476. Frequencies of antigen-specific cells were determined in IFN-γ ELISPOT assays. The data are shown as the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results.
Intracellular calcium mobilization and CysLTR1 antagonism. (Left panel) DCs were loaded with FluoForte and intracellular calcium release was determined using a Tecan Infinite F200 Pro fluorometer. CD40L was added at t-d and LTC4 or LTD4 were injected after 20 seconds at 37°C (arrow) and data were acquired for another 50 seconds. DCs were preincubated with 1 μg/mL PTX or 10μM CysLTR1 antagonist MK476) for 2 hours before stimulation with CD40L and LTD4. (Right panel) Peptide-specific CD8+ T-cell responses were induced as described using DC that were matured for 12 hours as indicated in the presence or absence of 10μM CysLTR1 antagonist MK476. Frequencies of antigen-specific cells were determined in IFN-γ ELISPOT assays. The data are shown as the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results.
Calcium flux was only slightly reduced by pretreatment of DCs with pertussis toxin (PTX; Figure 3A center panel, left), but was almost completely inhibited in the presence of the CysLTR1-specific antagonist MK-476 (Figure 3A center panel, right). We therefore conclude that LTC4, in contrast to LTD4, does not appear to signal through CysLTR1 in human monocyte-derived DCs, at least not under our experimental conditions.
We next asked whether blocking of CysLTR1 could restore the loss of immunostimulatory capacity of LTD4-matured DCs and stimulated MART-1–specific CD8+ T cells in the presence or absence of the CysLTR1-specific antagonist MK-476. As presented in Figure 3B, CD40L and CD40L/LTC4-matured DCs induced strong MART-1–specific T-cell responses, irrespective of the presence or absence of MK-476. In contrast, MK-476 greatly enhanced the T-cell stimulatory capacity of CD40L/LTD4-matured DCs. Last, the inhibitory effect of PGE2 on the induction of a T-cell response was, expectedly, not affected. These results further demonstrate that stimulation of DCs through CysLTR1 negatively impacts on their immunostimulatory function.
Generation of CD4+ T-cell responses
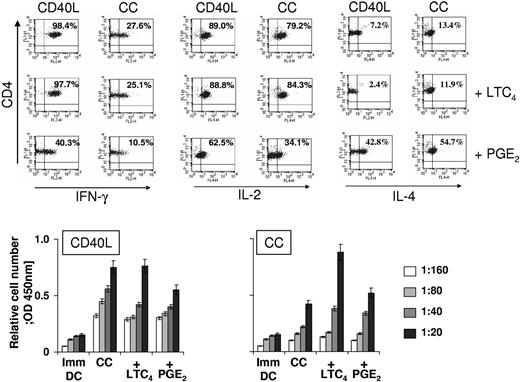
The impact of DC maturation on the induction of T-helper (Th) cell responses was next analyzed. Naive CD4+CD45RA+ T cells were stimulated with allogeneic DCs that had been matured with CC or CD40L in the presence or absence of LTC4 or PGE2. After 1 restimulation, the expression of Th1 cytokines (IL-2, IFN-γ) or Th2 cytokine (IL-4) by stimulated CD4+ T cells was determined. As shown in Figure 4 (top panel), CD40L and CD40L/LTC4-matured DCs strongly biased the immune response toward Th1. In contrast, stimulators that were matured with CD40L and PGE2 induced significantly lower numbers of Th cells that produced the Th1 cytokines IFN-γ and IL-2 and concomitantly induced Th cells that expressed the Th2 cytokine IL-4.
Induction of CD4+ T-cell responses. (Top panel) Polarization of naive CD4+CD45RA+ T cells. CD4+CD45RA+ naive T cells were isolated via magnetic bead-based techniques and restimulated once after 5 days with allogeneic DC (1:10) that were matured as indicated. After 10 days, cells were harvested and stimulated with PMA and ionomycin. For detection of IFN-γ and IL-2, cells were stimulated for 6 hours. For detection of IL-4 cells were stimulated for 16 hours. Brefeldin A was added during the last 2 hours of incubation. For detection of intracellular cytokines, cells were permeabilized with 0.1% saponin, stained with the indicated antibodies and analyzed by FACS. (Bottom panel) Allogeneic mixed lymphocyte reactions. T cells (105/well) that had been isolated using magnetic bead-mediated negative selection were cocultured at the indicated stimulator to responder ratios with allogeneic DCs that had been matured with CC or CD40L in the presence or absence of LTC4 or PGE2 for 24 hours. After 5 days, cell proliferation was determined using the WST-1 reagent according to the manual provided by the manufacturer. The data are shown as the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results.
Induction of CD4+ T-cell responses. (Top panel) Polarization of naive CD4+CD45RA+ T cells. CD4+CD45RA+ naive T cells were isolated via magnetic bead-based techniques and restimulated once after 5 days with allogeneic DC (1:10) that were matured as indicated. After 10 days, cells were harvested and stimulated with PMA and ionomycin. For detection of IFN-γ and IL-2, cells were stimulated for 6 hours. For detection of IL-4 cells were stimulated for 16 hours. Brefeldin A was added during the last 2 hours of incubation. For detection of intracellular cytokines, cells were permeabilized with 0.1% saponin, stained with the indicated antibodies and analyzed by FACS. (Bottom panel) Allogeneic mixed lymphocyte reactions. T cells (105/well) that had been isolated using magnetic bead-mediated negative selection were cocultured at the indicated stimulator to responder ratios with allogeneic DCs that had been matured with CC or CD40L in the presence or absence of LTC4 or PGE2 for 24 hours. After 5 days, cell proliferation was determined using the WST-1 reagent according to the manual provided by the manufacturer. The data are shown as the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results.
CC and CC/LTC4-matured DCs induced a mixed Th1/Th2 response (27.6% and 26.1% IFN-γ positive cells versus 13.4% and 11.9% IL-4 expressing Th cells), whereas CC/PGE2-matured DCs clearly skewed the immune response toward Th2 with 10.8% of CD4+ T cells expressing IFN-γ compared with 54.7% of cells expressing IL-4. In addition, these cells produced dramatically less IL-2 than all the other polarized Th cell cultures (34.1% vs 80%-90%).
The capacity of DCs to stimulate CD4+ T-cell responses was further assessed in mixed lymphocyte reactions. DCs were matured with CC or CD40L in the presence or absence of LTC4 or PGE2, and were subsequently cocultured with allogeneic T cells at different DC:T-cell ratios, as indicated. As shown in Figure 4 (bottom panel), DCs exposed to either maturation stimuli enhanced the proliferation of T cells compared with immature DCs (Imm DC). PGE2 further increased the T-cell stimulatory capacity of DCs when used in combination with CC, but decreased the allostimulatory function of CD40L-matured DCs. LTC4 significantly augmented T-cell proliferation induced by CC-matured DCs and did not negatively affect the stimulatory function of CD40L-matured DCs.
The allo-MLR results are quite divergent from the data obtained by polarization of naive CD4+ T cells or induction of CD8+ T-cell responses and argue that one must be cautious when solely relying on T-cell proliferation assays as an indicator for the immunostimulatory properties of DCs.
Induction of autologous regulatory T cells
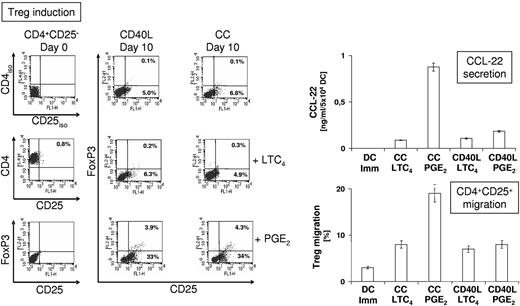
The efficacy of induction of Treg by DCs depends on the nature of the DC maturation stimulus, with CC/PGE2 being a strong inducer of Treg.14 To examine how maturation of DCs with LTC4 influenced the induction of Treg, CD4+CD25negFoxP3neg T cells were cocultured (Figure 5 left panel) for 10 days (without the addition of IL-2) with autologous DCs that had been matured for 24 hours with CC or CD40L in the presence or absence of LTC4 or PGE2. DCs matured with either agent induced CD4+CD25+ T cells (5.0% with CD40L-matured DCs and 6.8% with CC-matured DCs), whereas CD4+CD25+FoxP3+ Tregs were not detectable. Similar frequencies were obtained for DCs that had been matured in the presence of LTC4 (4.9% and 6.3% CD4+CD25+ T cells). In stark contrast, CD40L/PGE2 or CC/PGE2-matured DCs appeared to be potent simulators of Treg induction with 36.9% and 38.3% of cells, respectively, expressing CD25, with 10.2% and 11.2% of CD25+ cells representing CD4+CD25+FoxP3+ Tregs.
Induction and recruitment of autologous regulatory T cells by DC. (Left panel) CD4+CD25neg T cells were isolated via magnetic bead-based techniques and stimulated once with autologous DCs (at a ratio of 1:10), which were matured for 24 hours as indicated. After 10 days, cells were harvested and CD4, CD25, and FoxP3 expression was analyzed by flow cytometry. For detection of intracellular FoxP3, cells were permeabilized with 0.1% saponin. (Right panel) Secretion of CCL-22 by immature DCs and DCs that were matured as indicated was determined after 24 hours by ELISA. Supernatant equivalent to CCL-22 produced by 105 DCs was used to determine chemotaxis of isolated CD4+CD25+ T cells over 3 hours in transwell assays (3 μm pore size).
Induction and recruitment of autologous regulatory T cells by DC. (Left panel) CD4+CD25neg T cells were isolated via magnetic bead-based techniques and stimulated once with autologous DCs (at a ratio of 1:10), which were matured for 24 hours as indicated. After 10 days, cells were harvested and CD4, CD25, and FoxP3 expression was analyzed by flow cytometry. For detection of intracellular FoxP3, cells were permeabilized with 0.1% saponin. (Right panel) Secretion of CCL-22 by immature DCs and DCs that were matured as indicated was determined after 24 hours by ELISA. Supernatant equivalent to CCL-22 produced by 105 DCs was used to determine chemotaxis of isolated CD4+CD25+ T cells over 3 hours in transwell assays (3 μm pore size).
CCL-22 (C-C motif chemokine ligand 22) has been described as the key DC-produced chemokine that recruits regulatory T cells through its Treg-expressed receptor CCR-4 (C-C chemokine receptor type 4) and the production of CCL-22 is highly elevated in PGE2-matured DCs.43 We investigated CCL-22 production by immature DCs, CC/LTC4, CC/PGE2, CD40L/LTC4, and CD40L/PGE2-matured in ELISA assays (Figure 5 right panel, top). Indeed, PGE2 greatly enhanced CCL-22 secretion in CC-matured DCs; however, this effect was not observed in CD40L/PGE2–matured DCs, which produced similar levels of CCL-22 to CC/LTC4 and CD40L/LTC4–matured DCs. Consistently, supernatant from CC/PGE2–matured DCs induced more efficient chemotaxis of isolated CD4+CD25+ T cells in transwell assays than supernatant obtained from immature DCs, CC/LTC4, CD40L/LTC4, and CD40L/PGE2-matured DCs (Figure 5 right panel, bottom). From these results, we conclude that enhancement of CCL-22 production by DC is not an effect intrinsic to PGE2, but a synergistic effect of CC and PGE2 stimulation.
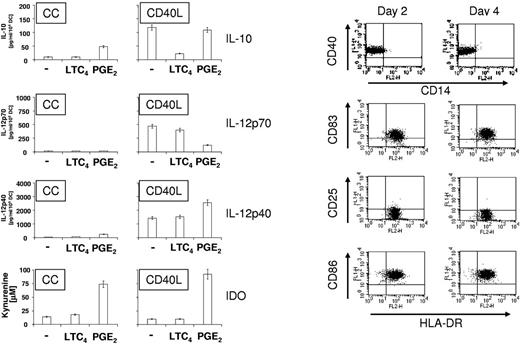
Cytokine secretion and IDO activity of mature DCs
DCs matured in the presence of PGE2 have been shown to secrete immunosuppressive IL-10 and IL-12p40 while inhibiting the production of the Th1 cytokine IL-12p70. In addition, PGE2 has been shown to up-regulate the expression of IDO.44,45 To compare the cytokine production and IDO activity of PGE2 and LTC4-matured DCs, DCs were matured with CC and CD40L in the presence or absence of PGE2 or LTC4. As shown in Figure 6 (left panel), PGE2 stimulated the expression of IL-10 by CC-matured DCs (48.4 ± 5.4 pg/mL), whereas IL-10 was not detectable in the presence of LTC4 plus CC or with CC alone. In contrast, CD40L-matured DCs produced IL-10 (118.5 ± 14.8 pg/mL) and the amount of IL-10 did not significantly change in the presence of PGE2 (109.2 ± 11.5 pg/mL). However, LTC4 caused a significant down-regulation of IL-10 production of CD40L-matured DCs (22.2 ± 4.4 pg/mL). IL-12p70 production could not be detected for CC-matured DCs under any condition, whereas CD40L-induced IL-12p70 changed insignificantly in the presence of LTC4 (468.0 ± 52.8 and 398.6 ± 38.8 pg/mL) but was down-regulated in the presence of PGE2 (124 ± 14.6 pg/mL). IL12p40 was up-regulated approximately 4-fold in CC-matured DCs in the presence of PGE2 (242.0 ± 26.4 pg/mL). CD40L-matured DCs expressed high levels of IL-12p40 (1430 ± 184 pg/mL) and IL-12p40 secretion was further increased in the presence of PGE2 (2560 ± 240 pg/mL), but did not change significantly in the presence of LTC4. In agreement with previous reports,37,38 PGE2 up-regulated IDO and IDO activity in CC and CD40L-matured DCs (74% and 92% conversion of tryptophan to kynurenin). In contrast, only 10%-20% of tryptophan was metabolized by CC, CC/LTC4, CD40L, and CD40L/LTC4 matured DCs.
Cytokine secretion and IDO activity of mature DCs. (Left panel) DCs were matured for 24 hours with CC or CD40L in the presence or absence of LTC4 or PGE2. Supernatants were harvested and secretion of IL-10, IL-12p70, and IL-12p40 were analyzed by ELISA. To determine IDO enzyme activity in mature DCs, conversion of tryptophan into kynurenine was determined. The data shown are the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results. (Right panel) Stability of mature DC phenotype in the absence of IL-4 and GM-CSF. DCs were matured for 2 days with CD40L in the presence of LTC4. Cells were then frozen, thawed and cultured for another 2 days in the absence of any cytokines and the expression of HLA-DR and maturation markers CD25, CD83, and CD86 was analyzed by FACS. The data presented are from 1 of 3 experiments that all yielded similar results.
Cytokine secretion and IDO activity of mature DCs. (Left panel) DCs were matured for 24 hours with CC or CD40L in the presence or absence of LTC4 or PGE2. Supernatants were harvested and secretion of IL-10, IL-12p70, and IL-12p40 were analyzed by ELISA. To determine IDO enzyme activity in mature DCs, conversion of tryptophan into kynurenine was determined. The data shown are the mean ± SEM of triplicate cultures and represent 1 of 2 experiments that yielded similar results. (Right panel) Stability of mature DC phenotype in the absence of IL-4 and GM-CSF. DCs were matured for 2 days with CD40L in the presence of LTC4. Cells were then frozen, thawed and cultured for another 2 days in the absence of any cytokines and the expression of HLA-DR and maturation markers CD25, CD83, and CD86 was analyzed by FACS. The data presented are from 1 of 3 experiments that all yielded similar results.
Phenotype of mature DCs in the absence of cytokines
A prerequisite for the generation of DC vaccines is that the DCs can be cryopreserved, that they remain viable, and maintain a stable DC phenotype after recovery. This is particularly important because DC migration through the afferent lymphatics to the T-cell area of lymph nodes has been shown to require 24 to 48 hours.46,47 As shown in Figure 6 (right panel), DCs were matured with CD40L and LTC4 for 2 days, cryopreserved, thawed, and then cultured for another 2 days in the absence of any cytokines or serum. After 2 days of maturation, DCs were CD14neg and expressed high levels of the maturation markers CD40, CD83, and CD86 (> 90%). However, in contrast to PGE2-matured DCs (data not shown), they lacked the expression of CD25. After thawing and another 2 days of culture in the absence of cytokines, the DC phenotype was unchanged and we conclude that DCs can be stably matured with CD40L and LTC4.
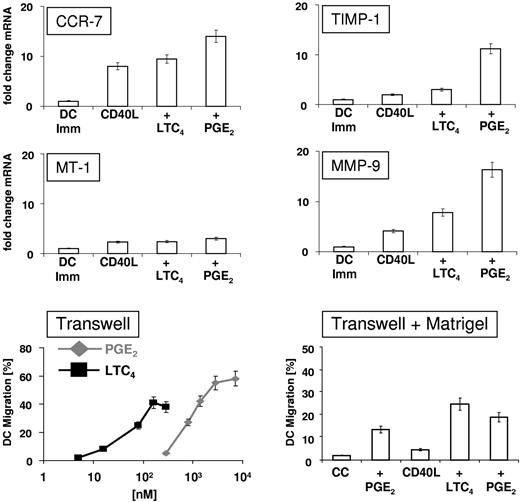
Expression of molecules involved in DC migration
CCR-7, the matrix metalloproteinases MT-1 and MMP-9, and the tissue inhibitor of MMPs TIMP-1 have been implicated in DC migration, and furthermore, have been shown to be up-regulated by PGE2. Therefore, mRNA levels were analyzed in immature, CD40L-matured, CD40L/LTC4–matured, and CD40L/PGE2–matured DCs using quantitative RT-PCR (Figure 7 top panel). CD40L up-regulated the expression of CCR-7 16.8-fold compared with immature DCs. LTC-4 induced a 19.2-fold increase in CCR-7 mRNA and PGE2 increased mRNA expression 26.5-fold. MT-1 mRNA was only up-regulated approximately 2-fold in mature DCs, compared with immature DCs, independent of the maturation stimulus. TIMP-1 mRNA was up-regulated 2.5-fold by CD40L-mediated DCs maturation and 3.4-fold by CD40L/LTC4-induced maturation, whereas an up-regulation of mRNA expression of 11.3-fold was observed in the presence of PGE2 during CD40L-induced maturation. Lastly, MMP-9 mRNA expression was up-regulated 4.3-fold in CD40L-matured DCs. This up-regulation was further increased by the addition of LTC4 (7.8-fold), although the most pronounced increase in MMP-9 mRNA expression was seen in the presence of PGE2 (16.3-fold).
Expression of molecules involved in DC migration. (Top panel) DCs were matured for 2 hours as indicated and the expression levels of mRNA encoding CCR-7, MMP)-9, MT-1 MMP, and TIMP-1 were determined by SYBR green-based quantitative RT PCR. The data shown are the mean ± SEM of triplicates and represent 1 of 2 experiments that yielded similar results. (Bottom panel, left) Transwell migration assays. DCs were matured for 48 hours with CD40L and increasing amounts of LTC4 or PGE2. Migration was assessed in transwell plates with polycarbonate inserts (8 μm pore size) for 2 hours at 37°C. CCL-19 (100 ng/mL) was added to the lower chambers of transwell plates. The data shown represent the mean ± SEM of duplicate cultures and represent 1 of 2 experiments that yielded similar results. (Bottom panel, right) Membranes of transwell inserts were coated with Matrigel (laminin, collagen, proteoglycans, entactin, nidogen) and migration assays were performed using DCs that had been matured as indicated. CCL-19 (100 ng/mL) was added to the lower chambers and migration of DCs was assessed after 6 hours at 37°C/5% CO2. DC migration in the absence of chemokine was subtracted as background. The data are shown as the mean of duplicate cultures ± SEM and represent 1 of 2 experiments that yielded similar results.
Expression of molecules involved in DC migration. (Top panel) DCs were matured for 2 hours as indicated and the expression levels of mRNA encoding CCR-7, MMP)-9, MT-1 MMP, and TIMP-1 were determined by SYBR green-based quantitative RT PCR. The data shown are the mean ± SEM of triplicates and represent 1 of 2 experiments that yielded similar results. (Bottom panel, left) Transwell migration assays. DCs were matured for 48 hours with CD40L and increasing amounts of LTC4 or PGE2. Migration was assessed in transwell plates with polycarbonate inserts (8 μm pore size) for 2 hours at 37°C. CCL-19 (100 ng/mL) was added to the lower chambers of transwell plates. The data shown represent the mean ± SEM of duplicate cultures and represent 1 of 2 experiments that yielded similar results. (Bottom panel, right) Membranes of transwell inserts were coated with Matrigel (laminin, collagen, proteoglycans, entactin, nidogen) and migration assays were performed using DCs that had been matured as indicated. CCL-19 (100 ng/mL) was added to the lower chambers and migration of DCs was assessed after 6 hours at 37°C/5% CO2. DC migration in the absence of chemokine was subtracted as background. The data are shown as the mean of duplicate cultures ± SEM and represent 1 of 2 experiments that yielded similar results.
DC Migration
The migratory function of CD40L-matured DCs toward CCL-19 as a function of LTC4 and PGE2 concentrations was analyzed in transwell assays. As shown in Figure 7 (bottom panel, left), DCs migrated in a concentration-dependent fashion toward CCL-19 and optimal migration was obtained at ∼ 100nM for LTC4 and ∼ 2.5μM for PGE2, with PGE2 being a slightly more potent inducer of chemotaxis. These concentrations are in agreement with previous studies and correlate well with the concentrations used in our assays (100 nM LTC4 and 2.8μM PGE2). We had to omit CC/LTC4-matured DCs from this assay because of the fact that these cells did not readily detach from cell culture dishes and, hence retained their tendency to adhere to surfaces (data not shown). Next, we evaluated the migration of mature DCs toward CCL-19 through Matrigel, an assay that resembles migration of intradermally injected DCs, to some extent. A comparison between DCs matured with CD40L and LTC4 or PGE2 was made. As shown in Figure 7 (bottom panel, right), only 2% of DCs migrated if matured with CC, whereas the addition of PGE2 to the CC increased the number of migrating cells to 13.5%. However, 4.6% of CD40L-matured DCs migrated through Matrigel. Migration of CD40L-matured DCs was enhanced by PGE2 (19.6% migration), although the most efficient migration was observed when DCs were matured using of CD40L plus LTC4 (28.8% migration). These results clearly indicate that, in this assay, migration of CD40L/LTC4-matured DCs appeared to be superior to migration of DCs matured using the current “gold standard” of CC with PGE2.
Discussion
In this study, we demonstrate that LTC4 represents a potent stimulator of migration for human monocyte–derived DCs and, in contrast to LTB4, LTD4, and PGE2, does not negatively interfere with the immunostimulatory properties of mature DCs.
In agreement with previous studies26,42 we found that DCs expressed both BLT1 and BLT2-mRNAs with a modest down-regulation of BLT1-mRNA in CD40L and LPS-matured DCs. The expression of BLT1, the high-affinity receptor for LTB4, on DCs may explain the immunoinhibitory effect of LTB4 on the induction of T-cell responses, because signaling through BLT1 is known to skew immune responses toward Th2.41
CysLTR1 and CysLTR2-mRNAs were detected in both immature and mature DCs, with a pronounced up-regulation of both mRNAs in CC and LPS-matured DCs and this effect was confirmed at the protein level. These data are consistent with results obtained in recent studies,35,48 except that Thivierge et al found an LPS-dependent down-regulation of CysLTR1.35 This discrepancy might be explained by differences in LPS concentration, the source of LPS, or the time point of RNA isolation.
We further demonstrate that LTC4, but not LTD4-matured DCs, are potent inducers of antigen-specific CTL and Th1 CD4+ T-cell responses, at least in part, because of increased IL12-p70 and decreased IL-10 and IDO production compared with PGE2-matured DCs. Our calcium flux results and CysLTR1 blocking experiments provide solid evidence for an immunoinhibitory function of this receptor on DCs and argue that signaling of LTC4 occurred exclusively through CysLTR2. This is conceivable, given that CysLTR1 is the primary receptor for LTD4 with a 50-times lower binding affinity for LTC4 (dissociation constant 0.4nM versus 21nM), whereas LTC4 and LTD4 bind with similar affinity to CysLTR2 (∼ 10nM), as determined in recombinant systems.40 In addition, in a recent study, LTC4 was shown to exert antagonistic effects on immature and mature murine bone marrow–derived DCs. Although LTC4 activated immature DCs, it demonstrated a CysLTR1-dependent inhibition of induction of Th-1 responses when added to LPS-matured DCs, which had up regulated CysLTR1, further highlighting the immunoinhibitory function of CysLTR1 on DCs. Last, the question whether LTD4 signaled exclusively through CysLTR1 or through CysLTR1 and CysLTR2 in DCs remains a subject for further investigation. In particular, because there is a considerable “overlap” between both signaling pathways in terms of ERK 1, 2, p38 MAPK, and NF-κB/AP-1 activation49-51
Admittedly, it appears counterintuitive to assume that LTC4, a molecule typically involved in Th2-mediated diseases such as asthma or allergies, would improve the Th1 skewing capacity of DCs. However, our observations are not without precedence. In a previous study, in vitro stimulation of DCs with both LTC4, LTD4, and antigen simultaneously resulted in both IL-10 and IL-12 production; however, although IL-10 production was suppressed by CysLTR1 blockade, IL-12 production was increased.52 These findings suggest that CysLTR1 engagement on murine DCs primes a Th2 response by promoting the production of Th2-type cytokines. It is reasonable to assume that the observed increase in IL-12, a Th1 cytokine, was because of unopposed activation of CysLTR2, the primary receptor for LTC4. The finding that CysLTs are involved in stimulating Th1-type immune responses is further supported by data reported by Schultz et al,53 who found that using LTC4 transporter multidrug resistance protein-deficient mrp1−/− mice, blockade of the cysLT pathway decreased IL-12 and IFN-γ production against Streptococcus pneumoniae by a mechanism that involved increased release of LTB4 because of product inhibition of LTC4-synthase.
Another noteworthy observation in this study is the fact that LTC4-matured DCs, like PGE2-matured DCs, expressed high levels of CD83 and CD86 although lacking the expression of CD25. CD25 is known to be released by proteolytic shedding from the cell surface and to down-regulate T-cell activation by acting as a soluble antagonist of IL-2.54-56 The lack of CD25 expression by LTC4-matured DCs may, therefore, represent a further contributing factor to the enhanced immunogenicity of these cells.
Lastly, it has been demonstrated that the expression of matrix metalloproteinases MT-1 (MMP-14) and MMP-9 is a major contributing factor to the migratory capacity of DCs through the degradation of extracellular matrix components.13,57 In this context, MMP-9 is of paramount importance because it cleaves collagen IV, a major component of basement membranes. Even though we only observed a modest up-regulation of MMP-9 mRNA in LTC4-matured DCs compared with PGE2-matured DCs, and we detected no differences in MT-1 expression, we speculate the superior migration through Matrigel observed with LTC4 vs. PGE2 is due to the differential effect of these 2 agents on TIMP-1 induction. As such, the increased induction of TIMP-1 in response to PGE2 probably inhibited the migration-promoting effects of MMP-9, a hypothesis supported by a previous report demonstrating that the balance of MMP-9 and TIMP expression is of crucial for DC migration in vivo.58
Taken together, we have shown that LTC4 may represent a promising candidate to replace PGE2 in DC maturation protocols that may lead to enhanced immunogenicity of DC-based cancer vaccines. The results presented in this study warrant further investigation of this novel maturation protocol in animal tumor models, and ultimately, in clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by VA Merit Review Grants (D.S.T. and S.K.P.) and a National Institutes of Health T32 grant (N.D.R.).
National Institutes of Health
Authorship
Contribution: J.D. wrote the paper, analyzed data and designed, and performed the research; T.S. and W.T.L. assisted with intracellular calcium release measurements: N.d.R. and W.T.L. helped with editing the paper; and D.S.T. and S.K.P. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Scott K. Pruitt, Duke University Medical Center, Dept of General Surgery, Durham, NC, 27710; e-mail: scott.pruitt@duke.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal