In this issue of Blood, Neufeld and colleagues present promising results on the safety and efficacy of a novel iron chelator for the treatment of transfusional iron overload.1
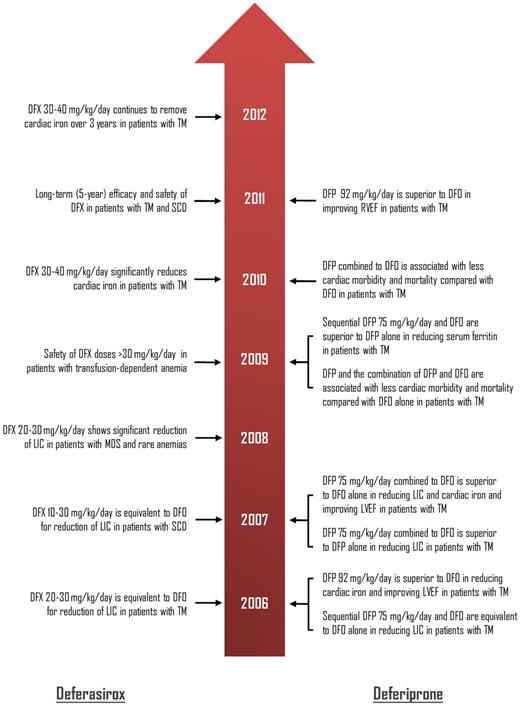
Timeline showing the most recent (2006 onwards) key data from prospective clinical trials regarding the efficacy of deferasirox and deferiprone in patients with transfusion-dependent anemias. The original studies are reviewed and listed in Musallam and Taher.4 DFO indicates deferoxmaine; DFX, deferasirox; DFP, deferiprone; LIC, liver iron concentration; LVEF, left-ventricular ejection fraction; RVEF, right-ventricular ejection fraction; TM, β-thalassemia major; SCD, sickle cell disease; and MDS, myelodysplastic syndromes.
Timeline showing the most recent (2006 onwards) key data from prospective clinical trials regarding the efficacy of deferasirox and deferiprone in patients with transfusion-dependent anemias. The original studies are reviewed and listed in Musallam and Taher.4 DFO indicates deferoxmaine; DFX, deferasirox; DFP, deferiprone; LIC, liver iron concentration; LVEF, left-ventricular ejection fraction; RVEF, right-ventricular ejection fraction; TM, β-thalassemia major; SCD, sickle cell disease; and MDS, myelodysplastic syndromes.
Transfusional iron overload remains a primary concern in patients with congenital and acquired anemias. If left untreated, chronic iron overload causes significant damage to the heart, liver, and endocrine glands and can lead to premature death. The subcutaneous agent deferoxamine (DFO) was the first chelator introduced for the treatment of transfusional iron overload in the mid-1960s. As demonstrated consistently in studies of large cohorts of patients with β-thalassemia major (TM), the introduction of DFO had a profound effect on long-term survival. In fact, Kaplan-Meier survival plots for patients who began treatment with DFO at a young age look remarkably similar to those for children receiving curative bone marrow transplants from matched siblings.2 However, poor compliance to the drug, attributed to the burden of prolonged and regular subcutaneous infusions, impinged on the observed survival benefit and imposed a new challenge.3 In the past two decades, the introduction of two oral iron chelators, deferiprone and deferasirox, has managed to reshape patient management. Data regarding the efficacy and safety of these two oral chelators are vast (see figure).4 Nevertheless, experience with both drugs has demonstrated some drawbacks, and the search for the ideal iron chelator continues.
In the present study by Neufeld and colleagues, FBS0701, an orally available tridentate chelator, was evaluated in a phase 2 trial on 51 patients with various transfusion- dependent hemoglobinopathies.1 Two once-daily doses of 14.5 or 29 mg/kg per day were randomly assigned to the study participants. After 24 weeks of treatment, patients receiving 29 mg/kg per day showed a statistically significant 0.3 mg/kg (dry weight) net decrease in liver iron concentration (LIC) measured by R2 magnetic resonance imaging, while patients receiving 14.5 mg/kg per day experienced a net increase in LIC. The observed decrease in LIC at the higher dose remains modest compared with that observed with deferasirox therapy5 ; however, this could be partly attributed to treatment duration and baseline patient characteristics. Thus, higher drug doses may be required if more clinically meaningful reductions are to be achieved. This brings us to the main advantage of this drug, a favorable safety profile that may permit trials of higher doses. First, the drug was not associated with any reductions in absolute neutrophil counts or rashes, which are noted with deferiprone and deferasirox therapy, respectively.4 Second, treatment was associated with a lower incidence of most gastrointestinal side effects compared with that reported with deferasirox therapy in core trials.6 Although gastrointestinal side effects attributed to deferasirox therapy are often mild and transient, the effect on patient compliance cannot be dismissed. Third, FBS0701 treatment was not associated with dose-dependent changes in serum creatinine. Fluctuations in serum creatinine level with deferasirox therapy have been a subject of concern. Although reports of overt renal impairment fail to provide convincing causal evidence and were mainly observed in elderly patients with several comorbidities,7 mild, dose-dependent increases in serum creatinine were observed in 38% of patients receiving deferasirox at doses of 20 to 30 mg/kg per day.5 These increases were sometimes transient, mostly within the normal range, and did not exceed twice the upper limit of normal. Safety data with deferasirox in TM children and adults have now been reported for up to 5 years of treatment and confirm absence of progressive increases in serum creatinine over longer-term treatment,8 even in heavily iron-loaded patients who require dose escalation to > 30 mg/kg per day.9 Even so, one cannot disregard the promising opportunity of FBS0701 for absolute renal safety. The other controversial concern with existing oral chelators is the effect on hepatic function and histology. With all fairness, data presented on FBS0701 cannot yet be interpreted, because 3 of 8 patients showing elevations in liver enzymes acquired hepatitis C virus infection through an unknown cause while on the study drug.
What should we expect next from FBS0701? The extensive clinical trial program devised for deferasirox should be a lead example for any iron chelator hoping to establish its efficacy and safety in the management of transfusional iron overload, especially in an era of evidence-based health care. The efficacy and safety of the drug at higher doses should be established and compared with available chelators in different disease and age subgroups. More importantly, the ability to chelate iron from the heart should be promptly investigated and compared with the remarkable success of deferiprone and its combination with DFO,10 as well as emerging evidence from deferasirox.11 Lastly, a favorable cost-effectiveness profile needs to be demonstrated before the drug becomes widely used, especially in developing countries where hemoglobinopathies are common yet resources are poor.
Conflict-of-interest disclosure: A.T.T. is a member of the Novartis speakers' bureau. K.M.M. declares no competing financial interests. ■