In this issue of Blood, Léon and colleagues describe the effects of romiplostim, a thrombopoietin (Tpo) mimetic peptide, in the mouse model of inherited platelet dysfunction because of mutation of the myosin 9 gene.1
The diagnosis of immune thrombocytopenia (ITP) may sometimes conceal more rare cases of inherited disorders of the platelet function, particularly in patients found to be resistant to steroids or splenectomy. On the whole, these last include a crowded list of dysfunctions, involving platelet surface constituents or intracellular components.2 Because two Tpo receptor agonists, eltrombopag and romiplostin, have been approved for chronic ITP adult patients unresponsive to glucocorticoids, intravenous immunoglobulin, or splenectomy,3 their potential use also in inherited thrombocytopenia is attractive.
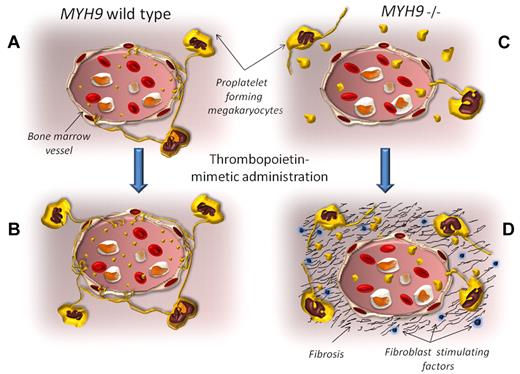
In normal conditions, proplatelet-forming megakaryocytes release nascent platelet directly in the bloodstream (A). Thrombopoietic stimulating agents expand the megakaryocyte pool and increase platelet production and release (B). The MYH9−/− mice replicate the megakaryocyte and platelet abnormalities observed in patients with MYH9-related diseases. The proplatelet formation occurs distant from vessels, with consequent platelet release inside the bone marrow environment. Only a few giant platelets are released in the bloodstream (C). When romiplostin is administered to these animals, the MYH9−/− megakaryocyte pool expands, and it can be hypothesized that the release of giant platelets inside the bone marrow is boosted. As a result, several growth factors for fibroblasts are secreted from platelets into the environment (including transforming growth factor-β, basic fibroblast growth factor, and platelet-derived growth factor), inducing the progressive bone marrow fibrosis (D).
In normal conditions, proplatelet-forming megakaryocytes release nascent platelet directly in the bloodstream (A). Thrombopoietic stimulating agents expand the megakaryocyte pool and increase platelet production and release (B). The MYH9−/− mice replicate the megakaryocyte and platelet abnormalities observed in patients with MYH9-related diseases. The proplatelet formation occurs distant from vessels, with consequent platelet release inside the bone marrow environment. Only a few giant platelets are released in the bloodstream (C). When romiplostin is administered to these animals, the MYH9−/− megakaryocyte pool expands, and it can be hypothesized that the release of giant platelets inside the bone marrow is boosted. As a result, several growth factors for fibroblasts are secreted from platelets into the environment (including transforming growth factor-β, basic fibroblast growth factor, and platelet-derived growth factor), inducing the progressive bone marrow fibrosis (D).
Léon et al report the effects of romiplostim in the mouse model of inherited platelet dysfunction because of mutation of the myosin 9 gene (MYH9).1 So far, at least 45 mutations of MYH9 have been described, accounting for an ensemble of autosomal-dominant inherited diseases, grouped as MYH9-related diseases (MYH9-RD), all characterized by the presence of thrombocytopenia with giant platelets and Döhle body–like inclusions within leukocytes.4,5 These three alterations, also known as May-Hegglin anomaly, can variably associate with other phenotypic peculiarities, including presenile cataract, proteinuric nephropathy, and progressive sensorineural hearing loss.4,5 Depending on the entity, the bleeding tendency is extremely variable, ranging from asymptomatic individuals to patients experiencing severe hemorrhages: in the latter, however, no therapy other than platelet transfusion is so far available.
The MYH9 gene encodes for the isoform A of nonmuscle myosin of class II, one of the myosin superfamily motor protein members. After the hydrolysis of ATP at the catalytic sites, the myosin-II interacts with actin filaments and regulates the cytoskeleton in all eukaryotic cells. Indeed, myosin-II drives several processes requiring energy and motion, such as cell migration, cell adhesion, and differentiation or tissue morphogenesis. In general, most cell types possess more than one single isoform, but cells from granulocytic and megakaryocytic lineages express uniquely the myosin-IIA isoform; myosin-IIA is also abundantly, though not exclusively, expressed in kidney, eye, and cochlea, all organs frequently involved in MYH9-RD.
The pathogenesis of thrombocytopenia in MYH9-RD is not fully understood. Many evidences suggest that, in these patients, megakaryocytic migration within the bone marrow and proplatelet ability formation are deeply disturbed. Seemingly, myosin-IIA is a key regulator of proplatelet formation, the physiologic mechanism by which megakaryocytes stretch out branching processes along marrow sinusoids and release nascent platelets into the bloodstream. In particular, myosin-IIA inhibits proplatelet formation triggered by type I-collagen, thus preventing the platelet release at a distance from sinusoid vessels. Actually, megakaryocytes isolated from patients with MYH9-RD formed proplatelets in vitro even in adhesion to collagen type I, suggesting an ectopic platelet release. Indeed, it is conceivable that MYH9 mutations result in abnormal platelet production inside the bone marrow, with few giant platelets released into the bloodstream.5
Because MYH9 knockout mice have an early embryonic mortality, Léon and colleagues have used the loxP/Cre recombinase strategy to specifically ablate the MYH9 expression into the megakaryocytic lineage.6 Like MYH9-mutated patients, MYH9−/− mice show thrombocytopenia, large and immature platelets, and impaired platelet contractile activity. Léon et al have found that romiplostin administration heightens the platelet count in MYH9−/− mice significantly less than in wild-type controls, despite the greater increase of bone marrow megakaryocytes in the former. Moreover, under romiplostin administration, MYH9−/− platelet express low levels of glycoprotein VI and glycoprotein Ib-IX-V complexes. Finally, romiplostin administration causes in MYH9−/− mice a progressive increase of reticulin fibers in the bone marrow, not observed in wild-type controls.1
All these findings suggest that romiplostin, by expanding the MYH9−/− megakaryocyte pool, emphasizes the platelet release inside bone marrow, likewise amplifying the secretion of growth factors inducing fibrosis such as platelet-derived growth factor, transforming growth factor-β, and basic fibroblast growth factor. In turn, the increased fiber deposition further stimulates the propletelet formation by megakaryocyes and, in the meanwhile, induces them to release metalloproteases, thus explaining the increased cleavage of adhesive glycoprotein complexes7 (see figure). The megakaryocytic lineage is crucial in the induction of bone marrow fibrosis and chronic stimulation of megakaryocytopoiesis is a well-known system to induce marrow fibrosis in animal models.8
We have recently reported that patients with thrombocytosis because of the inherited MPLSer505Asn activating mutation have not only a significant risk of thrombosis, but also evolve to bone marrow fibrosis. In these patients there is an unequivocal association between fibrosis and aging, with progressive increase of reticulin fibers, which in some middle-age and in most elderly patients appears diffuse and surrounded by focal bundles of collagen.9 The problem of marrow fibrosis in ITP patients undergoing Tpo mimetic therapy is truly felt, so that monitoring of cell counts and the peripheral blood smear is recommended. If new morphologic abnormalities or cytopenias are noted or if there is a loss of response to treatment, a bone marrow biopsy with staining for reticulin and collagen should be performed.10
A recent Italian trial has first reported that patients with MYH9 mutations significantly benefit from 6-week eltrombopag treatment, with increase of platelet count and disappearance of bleeding tendency in most of them.11 Whereas the experiences gathered so far in ITP patients indicate that Tpo-mimetic-induced fibrosis is reversible if drug therapy is discontinued, the irreversibility of marrow fibrosis associated with MPLSer505Asn mutation provides ground for reflection. In addition, data presented here by Léon and colleagues cast doubt that, under prolonged Tpo stimulation, patients with thrombocytopenia sustained by ineffective megakaryocytopoiesis would be predisposed to this complication.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal