Abstract
Asthma is a chronic airway disease characterized by paroxysmal airflow obstruction evoked by irritative stimuli on a background of allergic lung inflammation. Currently, there is no cure for asthma, only symptomatic treatment. In recent years, our understanding of the involvement of coagulation and anticoagulant pathways, the fibrinolytic system, and platelets in the pathophysiology of asthma has increased considerably. Asthma is associated with a procoagulant state in the bronchoalveolar space, further aggravated by impaired local activities of the anticoagulant protein C system and fibrinolysis. Protease-activated receptors have been implicated as the molecular link between coagulation and allergic inflammation in asthma. This review summarizes current knowledge of the impact of the disturbed hemostatic balance in the lungs on asthma severity and manifestations and identifies new possible targets for asthma treatment.
Introduction
Asthma is a disease of chronic airway inflammation causing symptoms of paroxysmal airflow obstruction, airway hyper-responsiveness to irritative stimuli, wheezing, chest tightness, and coughing.1 These symptoms occur against a background of allergic inflammation, characterized by infiltration of mast cells, eosinophils, and T-helper 2 (Th2) lymphocytes into the airway wall and mucus hypersecretion. Many patients with chronic asthma show progressive decline of lung function that is thought to be the result of structural remodeling of the airway wall2 and have frequent exacerbations and steroid resistance,3 posing a major clinical challenge and health problem.4 New therapeutic approaches need to be developed targeting the inflammatory background that triggers asthma symptoms.5
Historically, coagulation and fibrinolysis have been considered as processes that take place in the vascular compartment. It is now appreciated that the airways represent a body compartment in which coagulation and anticoagulant mechanisms can be initiated and regulated locally.6 In addition to the activation of coagulation in lung inflammatory disorders that is probably induced by leakage of plasma proteins into the bronchoalveolar space, essential mediators of coagulation can be found locally in the lung, including tissue factor (TF) that initiates coagulation and thrombin, which transforms fibrinogen to fibrin.7 Several diseases associated with abundant lung inflammation, including acute respiratory distress syndrome, pneumonia, and lung fibrosis,6,8 have been shown to result in similar changes in bronchoalveolar levels of proteins implicated in coagulation and fibrinolysis, tipping the physiologic equilibrium of preventing fibrin clot formation toward a net procoagulant state. In particular, for asthma this disturbed hemostatic balance in the airways is of importance for the perpetuation of allergic inflammation (Figure 1) in which cytokines and protease-activated receptors (PARs) play an important role. In addition, platelets have been found to actively participate in many manifestations of asthma. This review summarizes current knowledge of the role of coagulation and anticoagulant pathways in the pathophysiology of asthma.
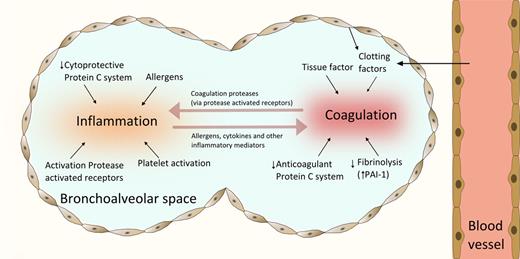
Interaction between coagulation and allergic inflammation in asthma. Coagulation is activated in the airways of patients with asthma by leak of clotting factors and TF expressed on various cell types, including alveolar epithelium, macrophages, and eosinophils. Fibrin deposition is further facilitated by decreased activity of the anticoagulant protein C system and inhibition of fibrinolysis by enhanced production of PAI-1. Allergens are responsible for an inflammatory response in the lungs, which is aggravated by proinflammatory effects of platelets and decreased cytoprotective effects of the PC system. PARs play an important role as the molecular link between coagulation and inflammation; these receptors are activated by proteases expressed by either allergens or factors involved the regulation of coagulation.
Interaction between coagulation and allergic inflammation in asthma. Coagulation is activated in the airways of patients with asthma by leak of clotting factors and TF expressed on various cell types, including alveolar epithelium, macrophages, and eosinophils. Fibrin deposition is further facilitated by decreased activity of the anticoagulant protein C system and inhibition of fibrinolysis by enhanced production of PAI-1. Allergens are responsible for an inflammatory response in the lungs, which is aggravated by proinflammatory effects of platelets and decreased cytoprotective effects of the PC system. PARs play an important role as the molecular link between coagulation and inflammation; these receptors are activated by proteases expressed by either allergens or factors involved the regulation of coagulation.
Activation of coagulation in patients with asthma
Fibrin is the end product of coagulation and is generated after cleavage of fibrinogen by thrombin.7 Although fibrin is typically formed at sites of vascular injury, it can also be generated in the pulmonary compartment, wherein fibrin production is necessary for normal airway epithelial repair after epithelial damage.9 Severe asthma can be associated with exaggerated intra-alveolar fibrin production, as demonstrated by massive fibrin depositions in the alveoli and distal airways of an asthma patient who died from a severe asthma attack that did not respond to treatment.10 Elevated concentrations of thrombin and thrombin–anti-thrombin complexes have been detected in sputum of patients with asthma11,12 as well as in bronchoalveolar lavage (BAL) fluid after allergen challenge,13,14 further supporting the existence of local coagulation activation in asthma. Thrombin activity in BAL fluid induced by segmental allergen provocation correlated with the degree of airway inflammation.14 Mast cells have been implicated as regulators of fibrin metabolism in asthma: on allergen challenge, mast cells release tryptase, which can cleave the α- and β-chain of fibrinogen, thereby removing the thrombin cleavage site and inhibiting fibrin generation by thrombin.15 Mast cells are also essential for the release of cytokines, heparin, and histamine, which may induce plasma leakage. The relevance of mast cells for lung coagulation in vivo warrants further research.
TF is considered the main initiator of coagulation. TF is a 47-kDa transmembrane glycoprotein that binds and activates clotting factor VII (FVII), generating FVIIa. Blood is not exposed to active TF under physiologic conditions. TF becomes exposed on the surface of mononuclear, epithelial, and endothelial cells on stimulation by bacterial and/or environmental products, such as lipopolysaccharide or proinflammatory cytokines or is activated from the circulating microvesicular form during inflammatory conditions. Alternatively, TF located at extravascular sites can become exposed to blood at sites of endothelial disruption. In the lung, alveolar epithelium exposes high TF levels, preventing bleeding of the fragile lung tissue.16 Patients with severe asthma demonstrated increased soluble TF levels in induced sputum compared with those with moderate asthma and healthy controls that was significantly and positively correlated with the amount of eosinophils.17 In addition, intrabronchial allergen challenge leads to increased soluble TF levels in BAL fluids of patients with mild asthma.14 The cellular source of this soluble TF and whether it is captured in microvesicles have not been studied thus far. Not only alveolar epithelium and macrophages may contribute to the pool of pulmonary TF, as eosinophils, the main inflammatory cells in asthma, may provide an additional source of intrapulmonary TF.18 Targeting the extracellular domain of TF with specific antibodies suppressed the initial phase of the eosinophil passage across activated endothelium in vitro. This indicates a function for TF, either directly or indirectly via thrombin generation, in transendothelial migration of eosinophils to the site of allergic inflammation.18 Of note, the expression of TF by eosinophils is not undisputed: other investigators could not confirm the presence of TF in preparations of isolated eosinophils.19 Perhaps slight differences in eosinophil activation states could have caused these different results. Allergens derived from house dust mite, especially the major house dust mite allergen Der p1, are capable of degrading tight junctions resulting in interruption of the airway epithelial cell lining and underlying endothelial cell lining, thereby facilitating contact between TF and plasma.20 The house dust mite allergen Der p2 functionally mimics MD-2, the direct lipopolysaccharide receptor in the MD-2 TLR-4 lipopolysaccharide signaling complex. This interaction may further contribute to inflammatory signals that might lead to increased TF expression and access to FVII and other plasma components after exposure to house dust mite antigens.21 In summary, patients with asthma show evidence of up-regulation of pulmonary coagulation via an increase in TF activity. Although limited activation of coagulation can assist in epithelial repair, pulmonary coagulation resulting from a crosstalk between epithelial, endothelial, and inflammatory cells can have a major impact on asthma pathophysiology, as indicated by experimental models of allergic inflammation discussed below.
Experimental evidence that coagulation contributes to asthma pathophysiology
The significance of increased coagulation activation in the lungs for the pathophysiology of asthma has been demonstrated in mouse studies. Exposure of mice to aerosolized fibrinogen followed by thrombin (which is expected to result in fibrin generation) caused increased airway hyper-responsiveness; thrombin or aerosolized fibrinogen alone was not sufficient to increase airway hyper-responsiveness to methacholine.10 Together, these data clearly indicate that elevated fibrin concentrations in the airways can produce a lung function disorder characteristic for asthma.
Interventions targeting specific components of the coagulation system have been studied in the classic mouse model of allergic lung inflammation induced by ovalbumin challenge via the airways after prior sensitization (Figure 2). This model induces a clinical syndrome that at least in part resembles allergic asthma, characterized by eosinophilic lung inflammation, airway hyper-responsivenss, increased immune globulin E (IgE) levels, mucus hypersecretion, and eventually airway remodeling.22 Moreover, the similarity between the coagulation systems of mice and humans is considerable,23 making the mouse an attractive animal for studies on asthma and coagulation. Genetically modified FVIItTA/tTA mice with a very low expression of FVII demonstrated reduced coagulation activation in their lungs on ovalbumin challenge.24 Normal wild-type mice exposed to allergen showed increased FVII mRNA levels in whole lung homogenates and increased expression of FVII protein in bronchial epithelial cells, which was virtually absent in FVIItTA/tTA mice. Importantly, FVIItTA/tTA mice displayed a diminished influx of eosinophils into BAL fluid, together with lower levels of the chemoattractant eotaxin and the Th2 cytokines IL-4, IL-5, and IL-13. In addition, airway hyper-responsiveness and mucus layer thickness were reduced in allergen-challenged FVIItTA/tTA mice.24 FVIIa itself did not induce mucin production by human respiratory epithelial cells in vitro; however, addition of exogenous FX resulted in FXa production in these cell cultures, indicating the presence of functional TF/FVIIa, as well as enhanced mucin production.24 Addition of FXa to respiratory epithelial cells also induced mucin production.25 Together, these data suggest that TF/FVIIa mediated its effect on allergic lung inflammation at least in part indirectly, via its role in the formation of FXa. In accordance with this hypothesis, FXa activity was found increased in BAL fluid of mice challenged with ovalbumin during 16 weeks (a model of chronic allergic lung inflammation associated with airway remodeling), concurrent with elevated FX mRNA levels in whole lung homogenates and alveolar macrophages.25 Treatment of mice with the FXa inhibitor fondaparinux during the last 3 weeks of allergen challenges resulted in attenuation of airway hyper-responsiveness without altering infiltration of inflammatory cells into the lung and decreased the thickness of the mucosal layer and lung collagen deposition.25 The results of these studies introduce a novel participant in the asthmatic response, indicating that coagulation plays an important role in experimentally induced allergic lung inflammation and that FXa/thrombin functions in airway remodeling by stimulating mucin and collagen deposition.
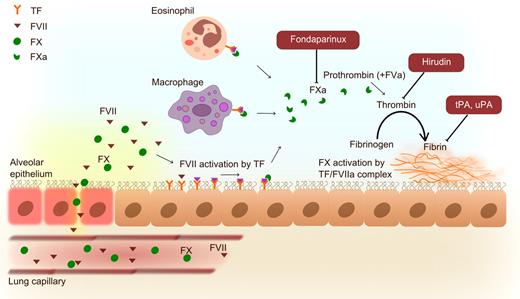
Activation of bronchoalveolar coagulation in asthma with interventions examined in the murine ovalbumin allergic lung inflammation model. Plasma (containing clotting factors, such as FVII and FX) leaks from lung capillaries as a consequence of the inflammatory response. TF expression on epithelial cells, eosinophils, and macrophages initiates intra-alveolar coagulation by activation of FVII (which can also be produced by epithelial cells). Interventions with the anticoagulants fondaparinux (FXa inhibitor) and hirudin (thrombin inhibitor) and the plasminogen activators tPA and uPA improve the disturbed pulmonary hemostatic balance and concurrently diminish allergic inflammation and asthma parameters in experimental settings.
Activation of bronchoalveolar coagulation in asthma with interventions examined in the murine ovalbumin allergic lung inflammation model. Plasma (containing clotting factors, such as FVII and FX) leaks from lung capillaries as a consequence of the inflammatory response. TF expression on epithelial cells, eosinophils, and macrophages initiates intra-alveolar coagulation by activation of FVII (which can also be produced by epithelial cells). Interventions with the anticoagulants fondaparinux (FXa inhibitor) and hirudin (thrombin inhibitor) and the plasminogen activators tPA and uPA improve the disturbed pulmonary hemostatic balance and concurrently diminish allergic inflammation and asthma parameters in experimental settings.
Thrombin has been implicated in asthma pathophysiology by both in vivo and in vitro studies. Administration of the thrombin inhibitor PEG-hirudin decreased airway hyper-responsiveness to methacholine of mice with acute allergic lung inflammation.10 Thrombin can mediate proinflammatory effects on a cellular level via cleavage of protease-activated receptor 1 (PAR1).26 Thrombin stimulated mucin secretion by primary human bronchial epithelial cells; this effect could be mimicked by specific stimulation of PAR1.27 In accordance with these in vitro observations, intranasally instilled thrombin-induced secretion of mucosubstance in nasal epithelium of rats, mediated by PAR1.27 Relevant for asthma, thrombin-PAR1 stimulation causes smooth muscle cell proliferation in vitro by stimulating platelet-derived-growth factor production28 and can induce connective tissue growth factor in fibroblasts,28 which is considered to contribute to development of fibrosis. In accordance, incubation of BAL fluid harvested from patients with atopic asthma challenged with allergen in a lung segment induced proliferation of fibroblasts in vitro, which could be inhibited by hirudin.29 In addition, thrombin can increase the bronchial tone in human bronchial rings.30
In summary, coagulation proteins, predominantly FXa and thrombin, can contribute to allergic inflammation by downstream production of fibrin or by effects outside their role in hemostasis. Good examples of the latter are the increase of mucin production caused by FXa and the activation of PAR1 on endothelial and epithelial cells by thrombin. Administration of coagulation factors can reproduce pathophysiologic alterations characteristic of asthma in animals in vivo and in relevant in vitro systems; conversely, inhibition of coagulation attenuates functional, immunologic, and morphologic features of allergic lung inflammation in mice elicited by ovalbumin sensitization and challenge.
Anticoagulant pathways: the PC system in asthma
The protein C (PC) anticoagulant system has been implicated in asthma pathophysiology.31 This pathway is initiated when thrombin binds to thrombomodulin on the cell surface, forming the thrombomodulin/thrombin complex that converts PC to activated PC (APC). Thrombomodulin/thrombin-dependent activation of PC is augmented by the endothelial PC receptor (EPCR). The biologic effects of APC can be divided in anticoagulant and cytoprotective effects,31 which include alteration in gene expression, anti-inflammatory and antiapoptotic effects, and protection of endothelial and epithelial barrier functions; these latter effects are dependent on EPCR and mediated by PAR1. The administration of recombinant APC has been found to exert beneficial effects in various preclinical models of inflammatory diseases, including sepsis, acute lung injury, stroke, ischemia/reperfusion injury, and wound healing; both the anticoagulant and the cytoprotective pathways have been implicated herein.31 Patients with asthma show evidence of an impaired function of the PC system within their lungs. In patients with mild allergic asthma bronchoalveolar levels of APC decreased 4 hours after a bronchial allergen challenge and were significantly lower than healthy controls.14 In addition, APC/thrombin and APC/PC ratios were decreased in induced sputum of patients with asthma, pointing to an imbalance between coagulation and the PC system.32 For the understanding of the involvement of the PC pathway in asthma pathophysiology, it is important to note that essential components of the PC pathway, in particular PC, thrombomodulin, EPCR, and PAR1 are all expressed by the respiratory epithelium32-34 and that as a consequence thereof respiratory epithelial cells can produce APC in the presence of thrombin.32 Conceivably, the reduced function of the PC pathway in asthma in part is caused by down-regulation of PC and EPCR: bronchial epithelial cells exposed to eotaxin or RANTES (Regulated on Activation, Normal T-cell Expressed, and Secreted; CCL5) in vitro displayed a reduced expression of PC and EPCR mRNA.32 Similar to patients with asthma, sensitized mice challenged with ovalbumin displayed reduced APC/thrombin ratios in BAL fluid.35 Importantly, inhalation of recombinant APC before exposure to aerosolized ovalbumin strongly attenuated allergic inflammation as reflected by a reduced influx of eosinophils and lower levels of the Th2 cytokines IL-4, IL-5, and IL-13 in BAL fluid; moreover, APC-treated mice had lower bronchoalveolar levels of IgE and diminished airway hyper-responsiveness.35 The inhibitory effect of APC on eosinophil influx could be reversed by an anti-EPCR antibody, but not by a PAR1 antagonist, indicating that APC exerts this effect via an EPCR-dependent but PAR1-independent mechanism.35 These in vivo findings are corroborated by in vitro studies showing inhibitory effects of APC and PC on eosinophil36 and lymphocyte37 migration toward chemoattractants; the effects on both cell types could be prevented by an EPCR antibody; these data suggest that cell migration may in part depend on the APC/thrombin balance.
Overall, these data indicate that a reduced function of the PC system may contribute to the perpetuation of inflammation in allergic asthma and that restoration of this function, for example by the administration of recombinant APC, may be of benefit to patients with asthma. Knowledge on how APC acts on inflammation has dramatically increased over the past few years, in particular in the field of systemic inflammatory syndromes, such as sepsis and endotoxemia. In systemic inflammation models, recombinant APC protects against mortality by effects that can be mediated by either EPCR-PAR1–dependent (endothelial cells, dendritic cells) or CD11b/CD18-PAR1–dependent (macrophages) mechanisms.38,39 Importantly, these protective APC effects do not rely on the anticoagulant properties of this protein: APC mutants lacking anticoagulant properties but with retained capacity to activate PAR1 are still able to protect mice from endotoxin or sepsis-induced death.40 As such, these non-anticoagulant APC mutants are promising new anti-inflammatory drugs, especially because they do not carry the risk of bleeding complications. At present, however, no data are available on the activity of these APC mutants in asthma models. In addition, for asthma it remains to be established via which cell type APC may exert anti-inflammatory effects. Of note, a very recent study has indicated that indeed a cytoprotective-selective APC mutant that lacks most of APC's anticoagulant activity has a similar capacity as wild-type APC to attenuate the development of pulmonary edema and to decrease mortality in mice with Pseudomonas pneumonia, suggesting that non-anticoagulant APC mutants are active in the lungs.41 Of note, recombinant human APC (Xigris), which was registered for the use in patients with severe sepsis, was recently withdrawn from the market after the “PROWESS-SHOCK” trial in patients with septic shock showed no clinical benefit (or harm) in subjects who had received APC (European Medicines Agency, press release October 25, 2011).
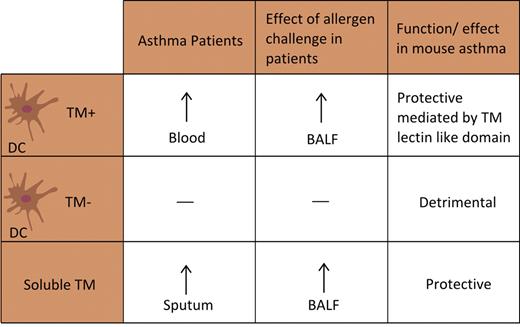
Recent investigations have implicated thrombomodulin in the pathogenesis of asthma (Figure 3). Although extensively characterized as the receptor expressed by the vascular endothelium that is essential for APC generation after binding thrombin, it has now become clear that thrombomodulin expressed by dendritic cells is involved in allergic asthma. Thrombomodulin-positive dendritic cells were more prevalent in peripheral blood of patients with allergy and asthma than in subjects without asthma42,43 ; the percentage of thrombomodulin-positive dendritic cells correlated with the extent of airflow limitation as determined by the percent forced expiratory volume in 1 second.44 Exposure of peripheral blood dendritic cells to house dust mite resulted in higher thrombomodulin expression in atopic than in nonatopic subjects.43 In addition, segmental allergen challenge in patients with mild allergic asthma increased soluble thombomodulin levels and thrombomodulin expression on dendritic cells in BAL fluid.14,45 Moreover, patients with asthma showed increased soluble thrombomodulin levels in induced sputum.32 The presence of soluble thrombomodulin in the airways, of which the cellular source remains to be established, may serve to inhibit local inflammation during asthma: although these observational data suggest a detrimental role for thrombomodulin in asthma, mouse studies indicate that thrombomodulin probably plays a protective role in the allergic inflammation accompanying ovalbumin sensitization and challenge.44 Indeed, inhalation of recombinant soluble thrombomodulin reduced the levels of IgE and Th2 cytokines as well as eosinophil numbers in BAL fluid of challenged mice. Thrombomodulin appeared to impact on dendritic cell function; adoptive transfer of thrombomodulin-treated dendritic cells reduced the severity of experimental asthma as measured by lung function and the extent of allergic inflammation. In addition, mice that were adoptively transferred with thrombomodulin-negative dendritic cells had more disease than those transferred with unsorted dendritic cells; conversely, animals adoptively transferred with thrombomodulin-positive dendritic cells were less responsive to ovalbumin. The lectin domain of thrombomodulin was responsible for the interaction with dendritic cells: wild-type mice treated with ovalbumin-pulsed dendritic cells deficient for the lectin domain of thrombomodulin showed more disease than wild-type mice treated with ovalbumin-pulsed dendritic cells from wild-type mice.44 Together, these mouse investigations identify thrombomodulin as a potential protective receptor in asthma by an effect that is unrelated to its anticoagulant properties; if these data can be confirmed in humans, the reported increases in the number of thrombomodulin-positive dendritic cells in patients with asthma would imply a compensatory rather than an asthma triggering response.42-44
Association between thrombomodulin (TM) expression on dendritic cells (DC) and soluble TM, and human and experimental asthma.
Association between thrombomodulin (TM) expression on dendritic cells (DC) and soluble TM, and human and experimental asthma.
PARs: the role of PAR2 in asthma
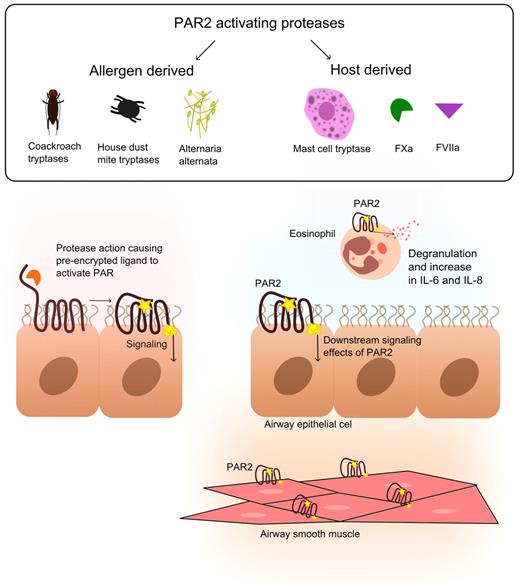
PARs belong to a family of G protein–coupled receptors that can be activated by serine proteases via proteolytic cleavage.46 PARs carry their own ligand, which remains hidden until unmasked by proteolytic cleavage. The PAR family consists of 4 members (PAR1-PAR4); each subtype displays a unique activation site that is recognized by specific proteases. All PARs have been detected in lungs, in particular in epithelium and airway smooth muscle.46 PAR1 and PAR2 have also been found on endothelium, macrophages, and migratory cells, such as mast cells and neutrophils. PAR1 can be activated by thrombin and APC and thereby play a role in asthma (see above). PAR2 has been implicated more directly in the pathophysiology of asthma (Figure 4). PAR2 can be activated by coagulation factors FVIIa and FXa, thereby providing a direct link between coagulation and inflammation. Notably, also other proteases can cleave and activate PAR2, both derived from the host (mast cell tryptase, trypsin, proteinase 3, elastase, and granzyme A) and from allergens (Der p1, p3, and p9 from house dust mite,47 Alternaria alternata,48 and cockroach extract49 ).
The role of PAR2 in allergic lung inflammation. Different sources of PAR2-activating proteases (both host-derived and allergen-derived) in allergic lung inflammation cause PAR2 activation on eosinophils, airway epithelial cells, and airway smooth muscle. PAR2 has been implicated as the molecular link between these proteases (including FXa and FVIIa) and allergic lung inflammation.
The role of PAR2 in allergic lung inflammation. Different sources of PAR2-activating proteases (both host-derived and allergen-derived) in allergic lung inflammation cause PAR2 activation on eosinophils, airway epithelial cells, and airway smooth muscle. PAR2 has been implicated as the molecular link between these proteases (including FXa and FVIIa) and allergic lung inflammation.
Increased expression of PAR2 is reported in bronchial epithelium of patients with asthma.50 Sensitized PAR2-deficient mice demonstrated a markedly diminished influx of eosinophils in BAL fluid after ovalbumin challenge; conversely, in transgenic mice overexpressing PAR2, eosinophil recruitment was higher.51 The effects in both PAR2-deficient and PAR2-overexpressing mice were apparent at 24 hours after ovalbumin challenge but not at later time points or after multiple ovalbumin challenges, indicating an involvement of PAR2, especially in the acute response to allergen exposure. In addition, deletion of PAR2 tended to diminish ovalbumin-induced airway hyper-responsiveness, whereas overexpression of PAR2 exacerbated this response.51 In accordance, PAR2 activation in the airways led to allergic sensitization to concurrently inhaled antigens52 and to exaggerated allergen-induced airway inflammation and airway hyper-responsiveness in sensitized mice.53 The concept that proteases expressed by allergens can activate PAR2 and thereby facilitate inflammation was recently supported by studies in which cockroach extract (which does express PAR2-activating protease activity) was used as challenging antigen; these investigations indeed revealed that allergic sensitization to cockroach extract, and the resultant allergic airway inflammation depended on the ability of the extract to activate PAR2.54 Notably, most reports on PAR2 function focused on epithelial cells; relevant for allergic asthma, however, mast cells and eosinophils also express PAR2 and activation thereof results in histamine release, and degranulation and cytokine release, respectively.46 The contribution of different cell types in PAR2-mediated allergic inflammation in vivo warrants further research.
Together, these data indicate that activation of PAR2 may facilitate airway inflammation and airway hyper-responsiveness in allergic asthma. Different proteases implicated in asthma are able to cleave PAR2, including mast cell tryptase and proteases expressed by allergens (house dust mite, cockroach, and A alternata). Asthma is associated with generation of the coagulation proteases FVIIa and FXa (see above), but it is currently unknown what their contribution is to PAR2-mediated responses during allergic inflammation.
Platelets and asthma
Platelets are anucleated fragments of their megakaryocyte-precursors and form the cellular components of a blood clot. Clinical evidence for a role of platelets in asthma is derived from studies demonstrating increased activation of platelets in patients with atopic asthma.55,56 In addition, increased circulating platelet-leukocyte aggregates have been detected in patients with asthma attacks and after allergen challenge.57,58 Similarly, allergen-challenged mice showed platelet-leukocyte complexes in their circulation.59 Importantly, platelets expressing P-selectin on their surface are required for the influx of eosinophils into the lungs of mice with allergic inflammation, as indicated by experiments with platelet-depleted mice transfused with either wild-type platelets (which restored eosinophil recruitment) or P-selectin–deficient platelets (which failed to do so).60 In accordance, P-selectin–deficient mice exhibited diminished leukocyte infiltration on challenge with cockroach allergen.61 Furthermore, in an in vitro flow model, more eosinophils from patients with asthma adhered to activated endothelium than eosinophils from healthy controls, a process that was dependent on platelets and P-selectin.62 Platelets have also been shown to contribute to chronic consequences of asthma: airway wall remodeling failed to occur in allergen-sensitized mice depleted of platelets.63 Platelets have been detected in BAL fluid of allergen-challenged mice63 and extravascularly in bronchial tissue and in the intra-alveolar space in patients with asthma,64 indicating diapedesis of platelets in areas of allergic inflammation and suggesting that platelets can contribute to lung inflammation via mechanisms that are independent of leukocytes. This latter hypothesis is supported by recent studies revealing that platelets of allergen-sensitized mice can undergo chemotaxis in response to the sensitizing allergen in vivo and in vitro.65 Notably, in ovalbumin-sensitized and challenged mice, platelets migrated out of blood vessels into lung tissue directly underneath the airways; platelet influx preceded the influx of leukocytes, and many platelets were not adjacent to leukocytes.65 Direct activation of platelets via IgE receptors was required for allergen-induced platelet migration, considering that platelets deficient in the FcRγ chain (and thus deficient for IgE receptors) transfused into wild-type mice were unable to migrate into lung parenchyma. Murine platelets indeed possess the high affinity receptor for IgE (FcϵRI), which was up-regulated on allergen sensitization and challenge.66 These findings are further corroborated by the expression of both high- and low-affinity receptors for IgE on human platelets66,67 and the observation that a larger proportion of platelets from patients with allergic asthma express IgE binding sites on their surface compared with healthy controls.66 In vitro, platelets isolated from allergen-sensitized mice migrated toward either sensitizing antigen or an anti-IgE antibody, a chemotactic response that required FcϵRI expression.65 Similarly, platelets harvested from patients with asthma (but not from nonallergic control subjects) migrated toward the specific sensitizing antigen and toward an anti-IgE antibody in vitro.65
Platelets possess preformed granules filled with proinflammatory and procoagulant mediators, some of which have been linked to bronchoconstriction, including thromboxanes, histamine, serotonin, and platelet activating factor (PAF). PAF is a lipid derivative of phosphorylcholine released from platelets, mast cells, and IgE-sensitized basophils that bridges inflammatory and coagulation processes. Several reports point to a role for PAF in bronchoconstriction: injection of PAF into guinea pigs produced bronchoconstriction68 ; inhalation of PAF caused a dose-related bronchoconstriction and a prolonged increase in airway hyper-responsiveness in healthy subjects69 ; and antigen-induced airway hyper-responsiveness could be inhibited by an antagonist of PAF in guinea pigs.70 Approximately 5% of the Japanese population has a loss of function mutation in PAF acetylhydrolase that results in excess PAF and prolonged generation of PAF. The homozygous genotype was found more frequently in children with atopic asthma than in their parents or controls.71 The prevalence of PAF acetylhydrolase deficiency is higher in asthmatics compared with healthy subjects.72 These reports suggest a functional relationship of PAF in the pathophysiology of asthma. The potential role of platelets in lung function disorders accompanying asthma is further supported by findings that bronchoconstriction induced by either intratracheal instillation of lipopolysaccharide73 or intravenous injection of thrombin74 required the presence of functionally intact platelets in the circulation. In accordance, platelet depletion resulted in a significant inhibition of allergen-induced airway hyper-responsiveness to inhaled histamine in rabbits,75 and P-selectin-deficient mice demonstrated lower levels of airway hyper-responsiveness than wild-type mice61 with cockroach allergen-induced lung inflammation. Moreover, intravenous administration of platelet agonists induced bronchospasms and an accumulation of platelets in the lungs of experimental animals.76,77
Together, these data identify platelets as important players in the development of allergic inflammation and in the recruitment of eosinophils. Modulation of platelet function seems an attractive new approach in asthma that warrants further investigation.
Fibrinolysis in asthma
Fibrinolysis is the process of fibrin cleavage by plasmin into fibrin degradation products, which is essential for the resolution of blood clots. Plasmin is formed from plasminogen by tissue-type plasminogen activator (tPA) or urokinase-type plasminogen activator (uPA). Plasminogen activator inhibitor type I (PAI-1) is the main inhibitor of both tPA and uPA. Many different cell types present in the lung can produce tPA, uPA, and PAI-1, including endothelial cells, macrophages, fibroblasts, mast cells, and bronchial epithelial cells.6,8 Asthma is associated with inhibition of fibrinolysis in the airways, primarily because of enhanced production of PAI-1.78 PAI-1 levels were increased in sputum of patients with asthma17,79 and in BAL fluid of rodents sensitized and challenged with ovalbumin,10,80,81 which was accompanied by decreased plasminogen activator activity.10 Administration of uPA improved various features characteristic for asthma, including airway hyper-responsiveness and subepithelial fibrosis in the lungs of mice exposed to ovalbumin82 ; similarly, inhalation of aerosolized tPA reduced airway hyper-responisveness in this model.10 Mast cells are probably an important source for PAI-1 in the asthmatic lung: lung tissue of patients with asthma and rats challenged with ovalbumin showed high numbers of PAI-1–positive mast cells.81,83 In addition, transcription of PAI-1 was highly up-regulated in human mast cells stimulated with IgE in vitro84 and mast cell-deficient mice had approximately 50% less PAI-1 in their airways compared with normal mice after ovalbumin challenge in vivo.78
PAI-1 has been implicated as a mediator of the allergic immune response: in a murine allergic rhinitis model, PAI-1–deficient mice showed a suppressed Th2 response and fewer symptoms.85 In addition, PAI-1–deficient mice displayed a reduced airway hyper-responsiveness in the ovalbumin-induced allergic lung inflammation model.82 In a model of chronic asthma produced by exposure to aerosolized ovalbumin during 4 weeks, PAI-1 deficiency did not influence peribronchial eosinophilic infiltration, goblet cell hyperplasia, or ovalbumin-specific IgE levels.80 PAI-1-deficient mice did show reduced collagen and fibrin deposition and enhanced matrix metalloproteinase-9 activity, indicating that the plasmin system regulates extracellular matrix deposition in the airways independently of the effect of PAI-1 on inflammatory cell recruitment.80,82 Epidemiologic studies support the importance of PAI-1 for the pathophysiology of asthma: a 4G/5G polymorphism in the promoter region of PAI-1 has been linked to the risk and severity of asthma.86 The 4G/5G polymorphism regulates the extent of PAI-1 release on exposure to allergen: a challenge with house dust mite caused an increase in plasma PAI-1 levels in all patients with asthma with the 4G allele and in only one-third of 5G homozygotes,87 a clinical finding further corroborated by in vitro studies showing that in stimulated human mast cells the transcriptional activity of the 4G-PAI-1 promoter is higher than that of the 5G-PAI-1 promoter.88
Remarkably, plasminogen-deficient mice demonstrated attenuated leukocyte recruitment into the lungs and reduced early histologic changes, including collagen deposition in the peribronchial areas and mucus metaplasia in allergic pulmonary inflammation induced by ovalbumin sensitization and challenge.89 In accordance, administration of the plasminogen inhibitor tranexamic acid reduced eosinophil and lymphocyte numbers, mucus production, and collagen deposition in the lungs of ovalbumin-treated wild-type mice.89 These results are unexpected in light of the impact of PAI-1 deficiency described above because plasminogen deficiency or inhibition, opposite to PAI-1 deficiency, will result in reduced fibrinolysis. It is therefore conceivable that PAI-1 contributes to asthma pathophysiology by a mechanism that is not directly related to its role in fibrinolysis; indeed, PAI-1 has been implicated in processes and diseases that are not or only partially related to its capacity to inhibit plasminogen generation, including wound healing, atherosclerosis, metabolic diseases, and tumor angiogenesis, processes that may depend on its interference in cellular migration and cellular matrix binding.90
Thrombin activatable fibrinolysis inhibitor (TAFI) is known to be an important regulator of fibrinolysis after thrombin-induced activation by inhibiting binding sites on fibrin for plasminogen and tPA. TAFI can also inactivate complement proteins C3a and C5a. TAFI-deficient mice showed enhanced airway hyper-responsiveness and lung injury in the ovalbumin asthma model, which was partly the result of diminished inhibition of complement.91 The role of TAFI in human asthma warrants further investigation.
Clinical experience with anticoagulant treatment of asthma: inhaled heparin
Heparin is a glycosaminoglycan widely used as anticoagulant in clinical practice. Heparin binds to anti-thrombin causing a conformational change that results in its activation, and inactivation of thrombin and other proteases involved in blood clotting, most notably FXa. Besides its anticoagulant properties, heparin possesses a wide range of anti-inflammatory activities, including inhibition of inflammatory mediators, such as eosinophilic cation protein, peroxidase, neutrophil elastase, and cathepsin G, inhibition of lymphocyte activation, neutrophil chemotaxis, smooth muscle growth, vascular tone, and complement activation.92 Early studies described subjective improvement of asthma symptoms using intravenous heparin.93,94 Patients with mild to severe asthma exposed to inhaled heparin experienced subjective, but no objective, improvement.95 Subsequent studies with inhaled heparin demonstrated reduced bronchoconstrictive responses in patients with exercise-induced asthma96,97 ; inhaled enoxaparin (a low molecular weight heparin) demonstrated similar protective effects.98 In accordance, in subjects with asthma and house dust mite allergy, nebulized heparin administered 10 minutes before challenge inhibited bronchospasms99 and 5 treatments with nebulized heparin between 90 minutes before until 6 hours after allergen exposure attenuated the early and reduced the late allergic response.100 Of note, however, trials of inhaled heparin on the bronchoconstrictive response to methacholine have yielded mixed results.101,102 Bleeding complications have not been reported after inhalation of heparin. At present, inhaled heparin is not used in clinical practice as adjunctive therapy for asthma attacks.
In conclusion, mediators and cells classically involved in procoagulant and anticoagulant pathways play a role in asthma pathophysiology. Patients with asthma display signs of enhanced activation of coagulation in their airways, impaired function of the anticoagulant PC system, and attenuated fibrinolysis. Inhibition of coagulation in mouse models of allergic asthma reduces eosinophil recruitment, lung inflammation, and airway remodeling and improves lung function. Platelets can contribute to many features of asthma, at least in part by their capacity to directly respond to IgE, release inflammatory mediators, and form aggregates with leukocytes. Administration of inhaled APC exerts strong anti- inflammatory effects in ovalbumin-challenged mice. Considering the wide range of cytoprotective properties of this protein, these effects may be unrelated to APC induced inhibition of coagulation. PARs have been identified as the link between coagulation and inflammation; in experimental asthma, PAR2 activation, either by clotting proteases or proteases expressed by common allergens, contributes to disease severity. Clearly, this abundance of preclinical data provides ample opportunities for identification of new therapeutic targets for patients with asthma, in particular for those with severe, refractory disease. As such, this relatively new area of asthma research holds promise for the future and the development of novel drugs with new mechanisms of action for asthma treatment.
Acknowledgments
J.D.d.B. and C.J.M. were supported by The Netherlands Asthma Foundation (projects 3.2.08.009 and 3.2.11.021, respectively).
Authorship
Contribution: J.D.d.B. and T.v.d.P. drafted the first version of the manuscript; C.J.M., C.v.V., and E.H.D.B. added essential information; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Daan de Boer, Academic Medical Center, University of Amsterdam, Center for Experimental and Molecular Medicine, Meibergdreef 9, Room G2-130, 1105 AZ Amsterdam, The Netherlands; e-mail: j.d.deboer@amc.uva.nl.