Abstract
Mounting evidence suggests that agonist-initiated signaling in platelets is closely regulated to avoid excessive responses to injury. A variety of physiologic agonists induce a cascade of signaling events termed as inside-out signaling that culminate in exposure of high-affinity binding sites on integrin αIIbβ3. Once platelet activation has occurred, integrin αIIbβ3 stabilizes thrombus formation by providing agonist-independent “outside-in” signals mediated in part by contractile signaling. Junctional adhesion molecule A (JAM-A), a member of the cortical thymocyte marker of the Xenopus (CTX) family, was initially identified as a receptor for a platelet stimulatory mAb. Here we show that JAM-A in resting platelets functions as an endogenous inhibitor of platelet function. Genetic ablation of Jam-A in mice enhances thrombotic function of platelets in vivo. The absence of Jam-A results in increase in platelet aggregation ex vivo. This gain of function is not because of enhanced inside-out signaling because granular secretion, Thromboxane A2 (TxA2) generation, as well as fibrinogen receptor activation, are normal in the absence of Jam-A. Interestingly, integrin outside-in signaling such as platelet spreading and clot retraction is augmented in Jam-A–deficient platelets. We conclude that JAM-A normally limits platelet accumulation by inhibiting integrin outside-in signaling thus preventing premature platelet activation.
Introduction
Platelet activation is carefully regulated through both positive and negative regulators.1 The ultimate result of platelet activation by agonist is conversion of integrin αIIbβ3 from its low-affinity state to a high-affinity state capable of binding soluble ligands such as fibrinogen through a process known as inside-out signaling.2 Ligand binding to the activated integrin induces a cascade of signaling events known as outside-in signaling that stabilizes platelet aggregates and supports the process of clot retraction.3 Timely and rapid activation of integrin is important for the process of hemostasis, but unwanted activation results in thrombosis.4 Significant progress has been made toward understanding the process of agonist-induced platelet activation.5 However, little is known about the process through which unwanted or accidental activation of integrin is discouraged. Here we show that junctional adhesion molecule A is a negative regulator of integrin function and provides protection from thrombosis.
Junctional adhesion molecule A (JAM-A) was initially identified as a receptor for a platelet stimulatory mAb F11 (mAbF11) and it was shown that activation of platelets by this Ab occurs through cross-linking of JAM-A with FcγRIIA receptor on platelet surface.6,7 Subsequently, it was been proposed that JAM-A may be regulating platelet function during thrombosis and atherosclerosis.8-11 Prior work has shown that JAM-A is expressed on human and mouse platelets, where it can be found on the surface of both resting and activated platelets.12 Later, JAM-A has been shown to be a member of the cortical thymocyte marker of the Xenopus (CTX) family of cell adhesion molecules (CAMs) that contains 2 extracellular Ig domains.13 In addition to platelets, JAM-A is shown to be expressed on epithelial and endothelial cells as well as on leukocytes.14 On epithelial and endothelial cells, JAM-A is exclusively localized to the tight junctions.15,16 Although significant progress has been made in the elucidation of JAM-A function as a tight-junction protein, not much is known about its function on platelets that lack tight junctions.
We generated a Jam-A knockout (Jam-Agt/gt) mouse by disrupting F11r (Jam-A gene) using the gene-trap technique.17,18 Using these mice, we show that Jam-A negatively regulates platelet function in vivo, as Jam-A–deficient mice show a prothrombotic phenotype. This gain of function is not because of the lack of endothelial Jam-A because transplantation of Jam-A–deficient platelets in a wild-type (Jam-Awt/wt) background also exhibits a prothrombotic phenotype. In addition, Jam-A–deficient platelets hyperaggregate in response to a low dose of physiologic agonists. The hyperreactivity of platelets is not due to augmentation of granular secretion, Thromboxane A2 (TxA2) generation, or integrin activation because there was no difference in these processes between Jam-Awt/wt and Jam-Agt/gt platelets. We attribute the cause of hyperreactivity of platelets to enhanced outside-in signaling because β3 integrin tyrosine phosphorylation (β3Y773), platelet spreading, and clot retraction process are augmented in Jam-Agt/gt platelets.
Methods
Materials
ADP was purchased from Chronolog. AYPGKF and AYPGQV were purchased from AnaSpec. Human fibrinogen (Fg) was obtained from Enzyme Research. All other chemicals unless indicated, were of analytical grade purchased from Sigma-Aldrich).
Abs
Anti-integrin β3Y773 phospho-specific Ab was obtained from Abcam; phospho-specific p38, or ERK1/2, or myosin light chain were purchased from Cell Signaling Technology; anti–PECAM-1, FITC-Fg, P-selectin, and anti–JAM-A were obtained from BD Pharmingen. Isotype-specific control IgGs were obtained from Santa Cruz Biotechnology. PE-conjugated JON/A and the corresponding control IgG were obtained from Emfret Analytics.
Animals
The Jam-A knockout (Jam-Agt/gt) mouse strain was generated by disrupting F11r (Jam-A gene) using the gene-trap technique.17,18 Jam-Agt/gt mice were backcrossed for 10 generations to obtain congenic C57BL/6 background. The genotype of Jam-A knockout mice (Jam-Agt/gt) used in this study was confirmed using Southern blotting, PCR, and Western blotting.17 Age-matched wild-type C57BL/6 mice were used as controls. Approval for animal experimental studies was received from the University of Delaware Institutional Animal Care and Use Committee.
Murine and human platelet preparation
Whole human blood was drawn into acid/citrate/dextrose (ACD) 6:1 (v/v) or in 3.8% sodium citrate 9:1 (v/v) by venipuncture from healthy, drug-free volunteers older than 18 years of age under informed consent. Approval was obtained from the University of Delaware Institutional Review Board for these studies according to the Declaration of Helsinki. Washed human platelets were obtained as described previously.19 Isolation of the murine platelet heparinized syringe was used to draw blood from the posterior vena cava from 10- to 14-week-old mice and transferred into a tube containing anticoagulant. Blood was diluted using Tyrode buffer (1:1) without calcium, and platelet-rich plasma (PRP) was obtained by centrifugation at 200g for 10 minutes at room temperature (RT).20 In some experiments, PRP was pooled together from 3 to 4 mice in the presence or absence of aspirin (1mM) or apyrase (1 U) and centrifuged at 400g for 10 minutes at RT. Platelet pellet was resuspended in Tyrode buffer containing 1mM calcium, to a concentration of 2 × 108/mL. Platelets were kept at RT for 45 minutes before experimentation and used within 3 hours of isolation. Western blotting of platelet proteins was performed as described previously.21
Tail-bleeding assay
Tail bleeding of Jam-Awt/wt and Jam-Agt/gt was performed on an anesthetized mouse before genotyping as described previously.20 Time in seconds required for cessation of blood flow was recorded.
Platelet aggregation and secretion
Platelet aggregation of Jam-Awt/wt and Jam-Agt/gt was performed using PRP containing 2 × 108/mL platelets using a Chrono-Log lumi-aggregometer (Chrono-Log) as described previously.20 Aggregation traces were recorded using Aggrolink software (Chrono-Log). Secretion of granular ATP was performed by addition of the luciferin-luciferase reagent. Each experiment was repeated at least 3 times independently.
TxA2 assay
Agonist-induced TxA2 generation was assayed using the TxB2 EIA kit (Enzo Life Sciences). Washed mouse platelets (4 × 108/mL) from Jam-Awt/wt and Jam-Agt/gt were stimulated with 0.1 U of thrombin for 5 minutes and centrifuged. The supernatant was collected and the TxB2 assay was performed as per the manufacturer's instruction.
DIC and platelet spreading
Differential interference contrast microscopy (DIC) images of the platelets were obtained as described previously.20 Fully spread platelets were differentiated from nonspread platelets using the criteria of Goodman.22 Using these criteria, fully spread platelets were defined as the hyaloplasm being extensively spread with no distinct pseudopodia. All others were considered nonspread. To compute the surface area of individual platelets, images of at least 50 platelets per view in triplicate were manually outlined using ImageJ software. The percentage of platelets that showed a fully spread morphology was determined from 3 randomly chosen fields per experiment. Each experiment was repeated at least 3 times. Images were processed in Adobe Photoshop.
Flow cytometry
Flow cytometric analysis was performed as described previously.20 FITC-labeled fibrinogen binding to washed platelets (0.6 × 108/mL) isolated from Jam-Awt/wt and Jam-Agt/gt mice on activation with agonist was performed as described previously.20 In addition, PE-labeled JON/A Ab binding was performed as described by the manufacturer's instructions (Emfret Analytics).
In vivo thrombosis model
A FeCl3-induced carotid artery thrombosis assay was performed as described.20 An injury to the carotid artery was inflicted by placing a piece of Whatman 1 filter paper (1 × 1 mm) saturated with freshly prepared 7.5% FeCl3 (anhydrous; Sigma-Aldrich) on the adventitial surface of the carotid artery, proximal to the flow probe, for 2 minutes. The artery was then rinsed with PBS and the flow was monitored for 45 minutes. The time for complete occlusion (defined as lack of detectable blood flow) to take place after the initiation of arterial injury with 7.5% FeCl3 was recorded.
Laser-induced thrombus formation in mouse cremaster muscle arterioles
Thrombus formation was visualized in the cremaster muscle microcirculation of male Jam-Awt/wt and Jam-Agt/gt mice according to the procedures developed by Falati et al.23 Briefly, following anesthesia, the cremaster muscle was exteriorized, cleaned of connective tissue, and spread flat on the pedestal of a custom-built platform for visualization of the cremaster microcirculation by intravital microscopy. After a 10-minute stabilization period, Alexa 488–labeled anti-CD41 Ab F(ab)2 fragments (MWReg30, 240 μg/kg; BD Biosciences) were administered via a catheter in the jugular vein. After an additional 5 minutes, an arteriole (30-45 μm diameter) was selected for injury and a pulsed nitrogen dye laser was fired through the microscope objective to induce thrombus formation. Brightfield and fluorescent (to visualize fluorescently labeled platelets) images were captured using a digital CCD camera (SensiCam; Cooke) coupled to Slidebook 5.0 image acquisition software (Intelligent Imaging Innovations). Up to 10 injuries were made in each mouse and 4 mice were studied in each group. Data are reported as the background-subtracted median-integrated fluorescence intensity.
BM transplantation experiment
To determine the contribution of platelet Jam-A versus endothelial cell Jam-A to thrombus formation using the cremaster injury model, we generated radiation chimera mice as described previously.24 Fetal liver cells were isolated from 14-16 days after coitus and Jam-Agt/gt mice fetuses and injected retro-orbitally into lethally irradiated male Jam-Awt/wt recipients (Cesium-137, 10 Gy, Gammacell 40 Exactor; MDS Nordion). Intravital microscopy experiments were performed as described in the previous section 4 weeks after transplantation. Hematopoietic reconstitution was confirmed by performing complete blood cell counts before intravital experiments.
Pulmonary thromboembolism
The acute vascular thromboembolism experiments were performed as described previously using anesthetized Jam-Awt/wt and Jam-Agt/gt mice.25 A mixture of collagen (0.4 mg/kg; Chronolog) and epinephrine (60 mg/kg; Sigma-Aldrich) in 100 μL of PBS was administered through the tail vein injection. Time of cessation of respiration (time needed to the onset of respiratory arrest that lasted at least 2-3 minutes) was recorded. Two minutes after the onset of respiratory arrest, but while the heart was still beating or at the completion of the 30-minute observation period, 0.5 mL of Evans blue solution (1% in saline; Sigma-Aldrich) was injected into the heart. Lungs were excised, photographed using Nikon Cool pix camera, formalin-fixed, and embedded into paraffin. Histologic analysis was performed on H&E-stained sections of lung from each mouse.
Clot retraction
The clot retraction assay was performed using washed platelets isolated from Jam-Awt/wt and Jam-Agt/gt mice following a procedure described previously.26 Platelets (3 × 108/mL) were resuspended in modified Tyrode-HEPES buffer (20mM HEPES, 137mM NaCl, 2.7mM KCl, 1mM MgCl2, and 3.3mM NaH2PO4, 1 mg/mL glucose, 35 mg/mL BSA, 2 mg/mL purified Fg, and 1mM CaCl2) and mixed with thrombin 1 U/mL in a glass borosilicate tube. Samples of platelet-poor plasma were used as controls. Clot retraction was monitored every 15 minutes for 2 hours at 37°C and documented photographically by using Nikon camera. Images were analyzed using the NIH Image J software.
Statistical analysis
Statistical analysis of the data were performed using the Student t test (mean + SEM value). P < .05 was regarded as statistically significant. Each experiment was repeated independently at least 3 times.
Results
Genetic ablation of Jam-A results in shorter tail-bleeding time and faster vessel occlusion
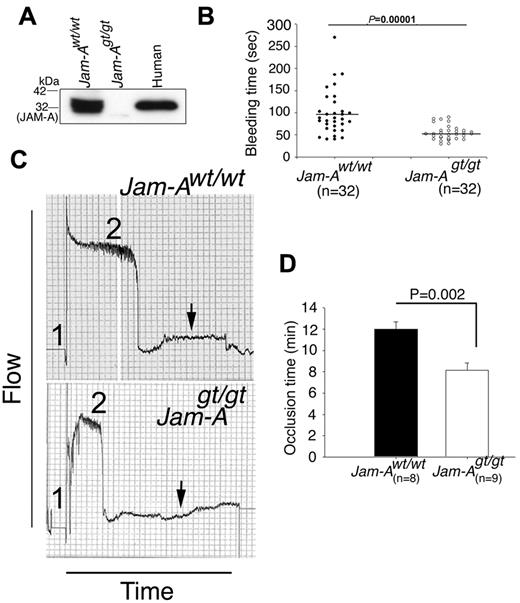
To determine the role of JAM-A in platelet function, we made use of a previously generated Jam-A knockout mouse (Jam-Agt/gt), which had been established by disrupting the mouse F11R (Jam-A gene) using the gene-trap technique.17 These mice were backcrossed for 10 generations to obtain a congenic C57BL/6 background. Jam-Agt/gt mice are healthy, fertile, and show no visible defects, although sperm motility, corneal epithelial morphology, and FGF2-induced angiogenesis are all flawed.17,27,28 The platelet count and size in Jam-Agt/gt mice were comparable with the Jam-Awt/wt mice. The absence of Jam-A in the platelets of Jam-Agt/gt mice was confirmed by Western blotting (Figure 1A). We determined whether Jam-A deficiency results in defective thrombotic functions using 4 well-established in vivo thrombosis assays. First, we examined the tail-bleeding time of Jam-Agt/gt mice compared with Jam-Awt/wt of the same genetic background (Figure 1B). We found that the average tail-bleeding time for the Jam-Awt/wt mice was about 100 seconds, consistent with values reported in the literature.20 Interestingly, the Jam-Agt/gt mice had a significantly shorter average tail-bleeding time (56 seconds, P = .00001), suggesting that Jam-A deficiency results in a prothrombotic phenotype (Figure 1B).
Ablation of Jam-A results in prothrombotic phenotype. (A) Western blot analysis of proteins from Jam-Awt/wt and Jam-Agt/gt platelets. Human platelet lysate was used as a positive control. (B) Tail-bleeding time of Jam-Awt/wt and Jam-Agt/gt mice before genotyping (n = 32). Mean bleeding time is denoted by a horizontal line. (C) Carotid artery blood flow in Jam-Awt/wt and Jam-Agt/gt mice following exposure to 7.5% FeCl3. Shown is the representative flow trace of Jam-Awt/wt and Jam-Agt/gt mice (n = 8). Duration of injury is denoted by no. 1 and the time to occlude is denoted by no. 2. (D) Quantitation of occlusion time from panel C.
Ablation of Jam-A results in prothrombotic phenotype. (A) Western blot analysis of proteins from Jam-Awt/wt and Jam-Agt/gt platelets. Human platelet lysate was used as a positive control. (B) Tail-bleeding time of Jam-Awt/wt and Jam-Agt/gt mice before genotyping (n = 32). Mean bleeding time is denoted by a horizontal line. (C) Carotid artery blood flow in Jam-Awt/wt and Jam-Agt/gt mice following exposure to 7.5% FeCl3. Shown is the representative flow trace of Jam-Awt/wt and Jam-Agt/gt mice (n = 8). Duration of injury is denoted by no. 1 and the time to occlude is denoted by no. 2. (D) Quantitation of occlusion time from panel C.
To further evaluate this thrombotic phenotype, we performed the FeCl3-induced carotid artery thrombosis assay, a well-established in vivo thrombosis model.29,30 The time for complete occlusion of the carotid artery after injury with 7.5% FeCl3 was compared in age- and sex-matched Jam-Awt/wt and Jam-Agt/gt mice. We found that ablation of Jam-A significantly shortens the time required for complete and stable vessel occlusion on vessel injury (Figure 1C-D, P = .002), again suggesting a prothrombotic phenotype in Jam-A–deficient mice.
Lack of Jam-A results in enhanced thrombus formation in laser-induced injury model
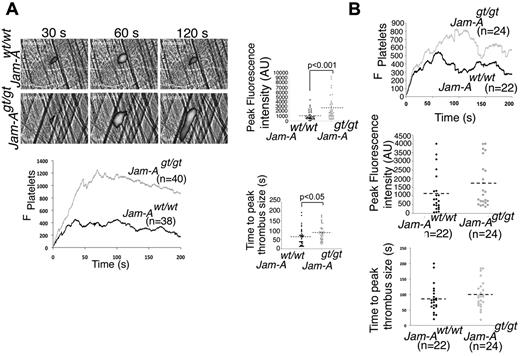
Next, thrombus formation was directly visualized in the cremaster muscle microcirculation of male Jam-Awt/wt and Jam-Agt/gt mice following laser injury.23 We found that platelet-rich thrombus formation was substantially augmented in Jam-Agt/gt mice compared with Jam-Awt/wt mice (Figure 2A, supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Interestingly, the rate of thrombus formation and time to peak were significantly enhanced in Jam-Agt/gt mice compared with the Jam-Awt/wt. Furthermore, the extent of thrombus formation (peak fluorescence intensity) was also significantly augmented in these mice (Figure 2A, P = .001). It is possible that the observed prothrombotic effect could be due to the absence of Jam-A on platelet or endothelial cells, or both. To differentiate the contributions of platelet and endothelial cell Jam-A in impeding thrombus formation in vivo, we generated radiation chimera mice by injecting fetal liver cells isolated from Jam-Agt/gt or Jam-Awt/wt fetuses into lethally irradiated male Jam-Awt/wt recipients.24 Mice receiving Jam-Agt/gt cells will have intact endothelial Jam-A, but lack platelet Jam-A. Successful reconstitution of hematopoiesis was confirmed by performing complete blood cell counts before intravital experiments. Similar to the global Jam-A knockout, we found enhanced thrombus growth and size in mice recipient of Jam-Agt/gt hematopoietic cells compared with the mice receiving Jam-Awt/wt cells (Figure 2B, supplemental Video 2). The initial rate of thrombus formation was not much different, possibly because of differences in the severity of the injury. However, a minor contribution of endothelial Jam-A cannot be ruled out at this time. These results strongly suggest that Jam-A expressed on platelets is responsible for the negative regulation of thrombosis.
In vivo thrombosis is enhanced in the absence of Jam-A. (A) Fluorescence images of cremaster muscle of Jam-Awt/wt and Jam-Agt/gt mice on in vivo laser-induced injury. Quantitation of peak fluorescence and the thrombus size was analyzed using Intelligent Imaging software (n = 40 injuries). (B) In vivo laser-induced injury assay as in panel A using chimeric mice generated by lethally irradiated Jam-Awt/wt mice receiving BM transplantation from Jam-Agt/gt mice (n = 22 injuries). Quantitation of the data analyzed using Intelligent Imaging software.
In vivo thrombosis is enhanced in the absence of Jam-A. (A) Fluorescence images of cremaster muscle of Jam-Awt/wt and Jam-Agt/gt mice on in vivo laser-induced injury. Quantitation of peak fluorescence and the thrombus size was analyzed using Intelligent Imaging software (n = 40 injuries). (B) In vivo laser-induced injury assay as in panel A using chimeric mice generated by lethally irradiated Jam-Awt/wt mice receiving BM transplantation from Jam-Agt/gt mice (n = 22 injuries). Quantitation of the data analyzed using Intelligent Imaging software.
Absence of Jam-A accelerates pulmonary thromboembolism
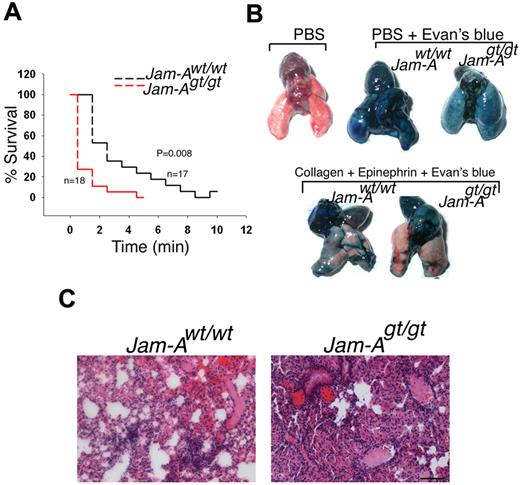
Finally, in a collagen/epinephrine-induced pulmonary thromboembolism model, a model of thrombosis in which platelet activation is the most prominent feature,25,31 we found that Jam-Agt/gt mice succumb significantly faster than Jam-Awt/wt mice (Figure 3A, P = .008). We also found that the pulmonary circulation was obstructed to a greater extent in Jam-Agt/gt mice as indicated by increased exclusion of Evans blue dye from the lungs compared with Jam-Awt/wt mice (Figure 3B). Histologic examination of lung sections showed numerous microemboli in addition to larger emboli in Jam-Agt/gt mice than Jam-Awt/wt (Figure 3C). Taken together, these results show that in vivo thrombotic function is enhanced in the absence of Jam-A via a platelet-specific mechanism.
Absence of Jam-A accelarate pulmonary thromboembolism. (A) Survival curve of Jam-Awt/wt and Jam-Agt/gt mice after induction of pulmonary thromboembolism (n = 18). (B) Representative images of lung isolated from Jam-Awt/wt and Jam-Agt/gt injected with PBS or PBS and Evans blue or a mixture of collagen and epinephrine solution. (C) H&E-stained sections of lungs.
Absence of Jam-A accelarate pulmonary thromboembolism. (A) Survival curve of Jam-Awt/wt and Jam-Agt/gt mice after induction of pulmonary thromboembolism (n = 18). (B) Representative images of lung isolated from Jam-Awt/wt and Jam-Agt/gt injected with PBS or PBS and Evans blue or a mixture of collagen and epinephrine solution. (C) H&E-stained sections of lungs.
Jam-A–deficient platelets hyperaggregate
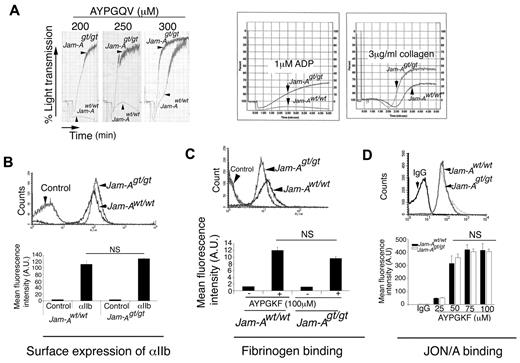
To more clearly define the precise role of JAM-A in platelet function, we performed ex vivo platelet aggregation studies. We found that a lack of Jam-A on platelet surfaces renders platelets hyperreactive to agonists such as the PAR4 agonist peptide (AYPGQV), ADP, and collagen, suggesting a defect in a pathway of platelet activation that is common to multiple agonists (Figure 4A). Interestingly, this defect was pronounced at a low dose of AYPGQV, which was overcome by an increased dose. This hyperaggregation response is not due to increased surface expression of integrin αIIbβ3 in platelets from Jam-Agt/gt mice because its level was comparable with Jam-Awt/wt (Figure 4B).
Ablation of Jam-A results in hyperaggregability. (A) Representative aggregation tracings of platelets from Jam-Awt/wt and Jam-Agt/gt mice induced by AYPGQV (PAR 4 peptide), ADP, or collagen as indicated. Experiments were performed at least 3 times independently. (B) Surface expression of integrin αIIbβ3 on Jam-Awt/wt and Jam-Agt/gt platelets as analyzed by flow cytometry. (C) FITC-labeled Fg binding to Jam-Awt/wt and Jam-Agt/gt platelets stimulated with 100μM AYPGKF and analyzed by flow cytometry. (D) Representative flow cytometric histogram of JON/A binding to Jam-Awt/wt and Jam-Agt/gt platelets stimulated with AYPGKF. Quantitation of mean fluorescence intensity of JON/A binding on stimulation with various concentrations of AYPGKF from 3 independent experiments.
Ablation of Jam-A results in hyperaggregability. (A) Representative aggregation tracings of platelets from Jam-Awt/wt and Jam-Agt/gt mice induced by AYPGQV (PAR 4 peptide), ADP, or collagen as indicated. Experiments were performed at least 3 times independently. (B) Surface expression of integrin αIIbβ3 on Jam-Awt/wt and Jam-Agt/gt platelets as analyzed by flow cytometry. (C) FITC-labeled Fg binding to Jam-Awt/wt and Jam-Agt/gt platelets stimulated with 100μM AYPGKF and analyzed by flow cytometry. (D) Representative flow cytometric histogram of JON/A binding to Jam-Awt/wt and Jam-Agt/gt platelets stimulated with AYPGKF. Quantitation of mean fluorescence intensity of JON/A binding on stimulation with various concentrations of AYPGKF from 3 independent experiments.
Integrin inside-out signaling is normal in the absence of Jam-A
To assess whether inside-out signaling induced by agonists results in augmentation of the activation of the fibrinogen receptor (integrin αIIbβ3), we determined FITC-fibrinogen–binding to integrin αIIbβ3 on platelet stimulation with agonists. We found that the Fg-binding to activated integrin αIIbβ3 was not enhanced in Jam-Agt/gt platelets (Figure 4C). This was further confirmed by the binding of Ab JON/A, which is specific for the activated conformation of αIIbβ3 on mouse platelets.32 JON/A binding in response to varying concentrations of AYPGKF was comparable in both Jam-Awt/wt and Jam-Agt/gt platelets (Figure 4D).
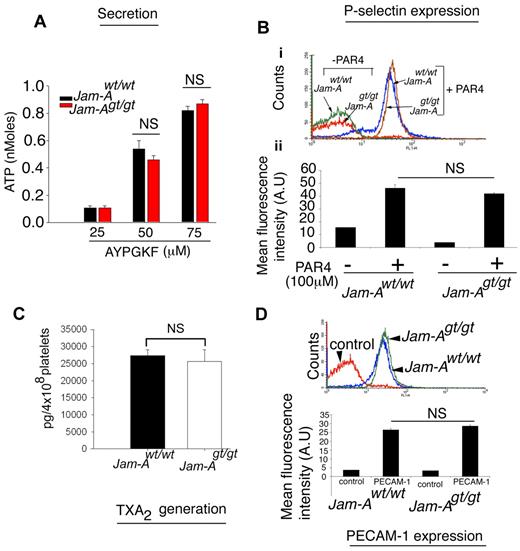
To determine whether the hyperaggregation observed in Jam-Agt/gt platelets is due to enhanced granular secretion, we measured ATP release as a measure of dense granule exocytosis and P-selectin exposure on the platelet surface as a marker of α-granule release. Neither was affected by the absence of Jam-A (Figure 5A-B). To determine whether agonist-induced TxA2 generation was affected, we analyzed TxB2, a stable analog of TxA2. We found no difference in thrombin-induced TxA2 generation in platelets from Jam-Agt/gt compared with Jam-Awt/wt mice (Figure 5C). We also found no difference in the surface expression of PECAM-1 (Figure 5D), the latter being measured because of the evidence that loss of PECAM-1 also produces a gain of function in platelets.33
The granular secretion is normal in Jam-Agt/gt platelets. (A) ATP secretion from Jam-Awt/wt and Jam-Agt/gt platelets stimulated by various concentrations of AYPGKF. Quantitation of experiments performed 3 times independently. (B) Flow cytometric histogram of P-selectin exposure on Jam-Awt/wt and Jam-Agt/gt platelets stimulated with 100μM AYPGKF for 10 minutes. Quantitation of mean fluorescence intensity normalized over control IgG from at least 3 independent experiments. (C) Quantitation of thrombin (0.1 U/mL)–induced TxA2 generation in Jam-Awt/wt and Jam-Agt/gt platelets (n = 3). (D) Flow cytometric histogram of PECAM-1 expression on Jam-Awt/wt and Jam-Agt/gt platelets. Quantitation of mean fluorescence intensity was normalized over control IgG from at least 3 independent experiments.
The granular secretion is normal in Jam-Agt/gt platelets. (A) ATP secretion from Jam-Awt/wt and Jam-Agt/gt platelets stimulated by various concentrations of AYPGKF. Quantitation of experiments performed 3 times independently. (B) Flow cytometric histogram of P-selectin exposure on Jam-Awt/wt and Jam-Agt/gt platelets stimulated with 100μM AYPGKF for 10 minutes. Quantitation of mean fluorescence intensity normalized over control IgG from at least 3 independent experiments. (C) Quantitation of thrombin (0.1 U/mL)–induced TxA2 generation in Jam-Awt/wt and Jam-Agt/gt platelets (n = 3). (D) Flow cytometric histogram of PECAM-1 expression on Jam-Awt/wt and Jam-Agt/gt platelets. Quantitation of mean fluorescence intensity was normalized over control IgG from at least 3 independent experiments.
Lack of Jam-A results in augmented platelet spreading
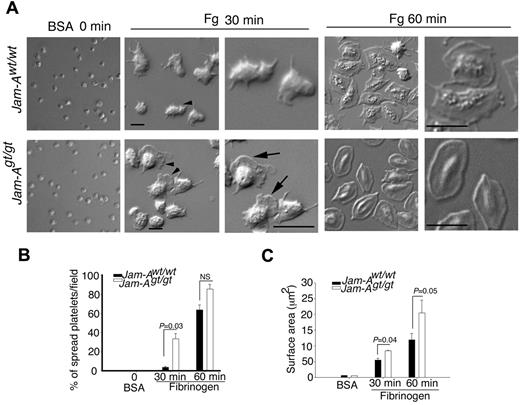
Because these results suggest that agonist-induced inside-out signaling leading to integrin activation occurs normally in Jam-Agt/gt platelets, we next investigated whether the hyperreactivity that they displayed in vitro and in vivo is because of enhanced integrin-dependent outside-in signaling. To test this hypothesis, we measured platelet spreading on immobilized fibrinogen, a process primarily regulated by integrin-dependent outside-in signaling. Platelets exposed to BSA remained discoid (Figure 6A). When exposed to immobilized fibrinogen, Jam-Awt/wt platelets showed only adhesion and filopodia formation by 30 minutes, whereas Jam-Agt/gt platelets showed distinct lamellipodia formation (Figure 6A) and the number of spread platelet population was significantly higher in Jam-Agt/gt than Jam-Awt/wt platelets. Although by 60 minutes the majority of both Jam-Awt/wt and Jam-Agt/gt platelets spread completely (Figure 6A-B), the surface area of the spread Jam-Agt/gt platelets was significantly greater compared with Jam-Awt/wt platelets at both time points (Figure 6C). These results clearly suggest that loss of Jam-A results in enhanced platelet spreading.
Absence of Jam-A promotes platelet spreading on immobilized Fg. (A) Representative DIC images of Jam-Awt/wt and Jam-Agt/gt platelets spread on immobilized Fg. BSA was used as a control. Zoomed views are indicated with arrowheads. (B) Quantification of the percentage of fully spread platelet on immobilized Fg compared with BSA. At least 100 individual platelets per view in triplicate for each time point were analyzed. Data shown are the quantification from at least 3 independent experiments. (C) Platelet surface area from panel A. Quantitation of > 50 platelets in a given view in triplicate were analyzed from at least 3 independent experiments.
Absence of Jam-A promotes platelet spreading on immobilized Fg. (A) Representative DIC images of Jam-Awt/wt and Jam-Agt/gt platelets spread on immobilized Fg. BSA was used as a control. Zoomed views are indicated with arrowheads. (B) Quantification of the percentage of fully spread platelet on immobilized Fg compared with BSA. At least 100 individual platelets per view in triplicate for each time point were analyzed. Data shown are the quantification from at least 3 independent experiments. (C) Platelet surface area from panel A. Quantitation of > 50 platelets in a given view in triplicate were analyzed from at least 3 independent experiments.
Clot retraction is enhanced in the absence of Jam-A
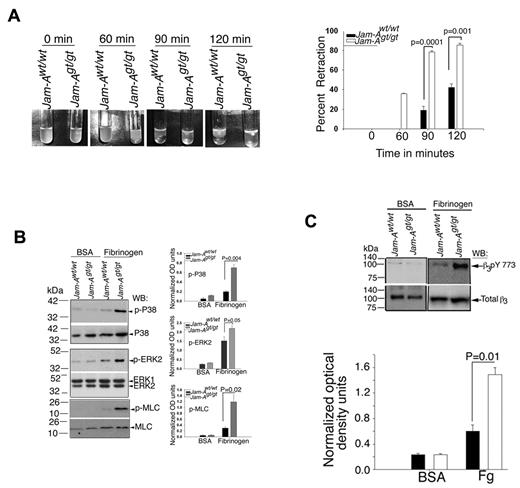
We next evaluated clot retraction, a process known to be regulated in part by outside-in signaling in platelets.34,35 Clot retraction rate in Jam-Agt/gt platelets was significantly enhanced compared with Jam-Awt/wt platelets (Figure 7A). In Jam-Awt/wt platelets the retraction started after 1 hour and reached about 50% by 2 hours, whereas in Jam-Agt/gt platelets the ∼ 40% retraction occurred before 1 hour and was almost complete by 1.5 hours (Figure 7A). When evaluating the signaling events at the molecular level, we found that activation of the signaling pathway regulating platelet contractile processes was augmented in Jam-Agt/gt platelets, as outside-in signaling-induced activation of Erk2, p38 MAP kinase, and myosin light chain kinase was enhanced (Figure 7B). Finally, we examined β3Y773 phosphorylation, an indicator of outside-in signaling, in platelets exposed to immobilized fibrinogen and found that phosphorylation of β3Y773 is significantly increased in Jam-Agt/gt platelets compared with Jam-Awt/wt platelets (Figure 7C, P = .01). Taken together, our results indicate that Jam-A normally attenuates outside-in signaling and that the failure to do so in the knockout mouse results in a gain of function because of augmented outside-in signaling.
Ablation of Jam-A results in enhanced integrin outside-in signaling. (A) Photographs of clot retraction using washed Jam-Awt/wt and Jam-Agt/gt platelets. Quantitation of the percentage of clot retraction from 3 independent experiments. (B) Western blots of protein lysates from Jam-Awt/wt and Jam-Agt/gt platelets exposed for 1 hour to immobilized Fg or BSA and probed with anti-phospho-specific p38 or anti-p38, or anti-phospho-specific Erk2 or anti-Erk2, or anti-phospho–specific myosin light chain or anti–myosin light chain. Quantitation of normalized optical density of 3 independent experiments. (C) Western blots of protein lysates from panel A probed using anti-phospho–specific β3Y773 or anti-β3. Quantitation of normalized optical density of 3 independent experiments.
Ablation of Jam-A results in enhanced integrin outside-in signaling. (A) Photographs of clot retraction using washed Jam-Awt/wt and Jam-Agt/gt platelets. Quantitation of the percentage of clot retraction from 3 independent experiments. (B) Western blots of protein lysates from Jam-Awt/wt and Jam-Agt/gt platelets exposed for 1 hour to immobilized Fg or BSA and probed with anti-phospho-specific p38 or anti-p38, or anti-phospho-specific Erk2 or anti-Erk2, or anti-phospho–specific myosin light chain or anti–myosin light chain. Quantitation of normalized optical density of 3 independent experiments. (C) Western blots of protein lysates from panel A probed using anti-phospho–specific β3Y773 or anti-β3. Quantitation of normalized optical density of 3 independent experiments.
Discussion
The process of hemostasis is tightly regulated by both positive and negative regulators of platelet activation. Although much is known about the molecules involved in the platelet activation process, little is known about the molecules involved in the inhibitory process. Here we show that JAM-A acts as a negative regulator of platelet activation and hence the process of hemostasis and thrombosis. Our results identify a novel function for JAM-A in suppressing platelet activation as opposed to its previously thought stimulatory role.
JAM-A was originally characterized as a receptor for a stimulatory mAb F11 (mAb F11).6 It was therefore believed that JAM-A may be involved in platelet activation by physiologic agonists. However, it was later shown that activation of platelets by mAb F11 requires crosslinking of JAM-A and FcγRIIA receptor and that the FcγRIIA receptor is responsible for the signaling that leads to platelet activation.7 Subsequently, several reports claimed that JAM-A is involved in platelet activation.9-11 However, in these reports mAb F11 was used as the agonist to stimulate platelets instead of using physiologic agonists.9-11 The role of JAM-A in agonist-induced platelet activation was, however, never investigated.
Because mAb F11 can cross-link JAM-A and FcγRIIA and a recent report implicated FcγRIIA as one of the regulators of outside-in signaling in platelets, it was felt that JAM-A might affect platelet activation by blocking FcγRIIA.36 However, the fact that murine platelets do not express FcγRIIA, and that the loss of JAM-A results in a gain of function in murine platelets, suggests that JAM-A exerts its effect through a mechanism independent of FcγRIIA.
JAM-A may inhibit outside-in signaling through integrin by regulating activation of downstream components such as c-Src, Syk, or FAK activation.37 It may also recruit a protein tyrosine phosphatase such as SHP1 or SHP2, as in the case of ITIM receptors such as PECAM-1,38 or it may recruit a scaffolding protein such as NHERF-1, as shown in the case of ESAM, a CTX family member,24 or recruit a negatively regulating kinase such as C-terminal Src kinase, Csk.37
Several members of the CAM family have been show to exert an inhibitory effect on platelet function.39 These include PECAM-1, CEACAM1, and ESAM; all are expressed on the platelet surface and are involved in homotypic interactions.40-42 Genetic ablation of either of these proteins results in enhanced thrombus formation in vivo.24,43,44 PECAM-1 and CEACAM1 both contain 2 ITIMs within their cytoplasmic domains and recruit protein tyrosine phosphatases such as SHP1 and SHP2 through which they exert their inhibitory effect.45,46 Therefore, it was felt that JAM-A might also exert the inhibitory effect through a similar ITIM-dependent mechanism. However, the JAM-A cytoplasmic domain contains 2 tyrosine residues, which do not resemble the ITIM motif, thus ruling out the ITIM-based mechanism. Furthermore, platelet function induced by collagen, ADP, and thrombin are all affected in the absence of JAM-A, whereas PECAM-1 and CEACAM1 primarily affect platelet collagen receptor, GPVI/FcγR-dependent signaling.47 Thus, it is more likely that the inhibitory effect exerted by JAM-A is through a mechanism independent of ITIM. Interestingly, ESAM, which is also a member of the CTX family of cell adhesion molecules, like JAM-A, also lacks ITIM and genetic deletion of ESAM exhibits enhanced thrombus formation in vivo24 as well. Thus, JAM-A may function through similar mechanism as that of ESAM. However, lack of ESAM results in delayed clot retraction whereas lack of JAM-A enhances clot retraction.24 These findings suggest that JAM-A may be functioning through an as yet undefined mechanism.
Our results indicate that the agonist-induced inside-out signaling leading to integrin activation is unaffected in the absence of Jam-A. However, earliest events of outside-in signaling through the activated integrin such as β3 phosphorylation are significantly enhanced in the absence of Jam-A. This suggests that JAM-A may interfere in fibrinogen binding to the activated integrin. It has been shown that JAM-A interacts with integrin αvβ3 on endothelial cells and with integrin αLβ2 on leukocytes.16,48 It is therefore conceivable that JAM-A may exert its negative effect on platelet function by interacting with integrin αIIbβ3. In fact, it has been shown that JAM-A may associate with platelet integrin αIIbβ3.10 However, it needs to be noted that the absence of JAM-A does not enhance fibrinogen or JON/A binding to the activated integrin, suggesting that JAM-A may not interfere with Fg binding to the activated integrin. It was interesting to find that a defect in integrin outside-in signaling regulates platelet aggregation. This is not surprising because deletion of the C-terminal RGT motif of the β3 cytoplasmic domain in mice or introduction of the myristoylated RGT peptide have been shown to attenuate aggregation.49,50 Further experimentations are ongoing to establish the molecular mechanism of the negative regulation of platelet integrin αIIbβ3 function by JAM-A.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peter Millili for assisting in flowcytometry and Sharmila Chatterjee for H&E staining, as well as Kushal Naik for proofreading the final manuscript.
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants HL57630 and HL63960, National Center for Research Resources grants 5P20 RR015588-10 and 2P20 RR016472-11 (U.P.N.), and NHLBI grant HL40387 (L.F.B.).
National Institutes of Health
Authorship
Contribution: M.U.N. performed experiments, analyzed data, and wrote the manuscript; T.J.S. performed experiments and analyzed data; L.F.B. interpreted data and helped write the manuscript; and U.P.N. interpreted data and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulhas P. Naik, Department of Biological Sciences, University of Delaware, 309 Wolf Hall, 105 The Green, Newark, DE 19716; e-mail: unaik@udel.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal