Abstract
The hyaluronan-mediated motility receptor (HMMR/Rhamm) is overexpressed in numerous tumor types, including acute lymphoid leukemia and acute myeloid leukemia (AML). Several studies have reported the existence of T-cell responses directed against HMMR in AML patients that are linked to better clinical outcome. Therefore, we explored the use of HMMR-specific TCRs for transgenic expression in lymphocytes and their in vivo impact on HMMR+ solid tumors and disseminated leukemia. We obtained TCRs via an in vitro priming approach in combination with CD137-mediated enrichment. Recipient lymphocytes expressing transgenic TCR revealed the specific tumor recognition pattern seen with the original T cells. Adoptive transfer experiments using a humanized xenograft mouse model resulted in significantly retarded solid tumor outgrowth, which was enhanced using IL-15–conditioned, TCR-transgenic effector memory cells. These cells also showed an increased potency to retard the outgrowth of disseminated AML, and this was further improved using CD8-enriched effector memory cells. To define a safe clinical setting for HMMR-TCR gene therapy, we analyzed transgenic T-cell recognition of hematopoietic stem cells (HSCs) and found on-target killing of HLA-A2+ HSCs. Our findings clearly limit the use of HMMR-TCR therapy to MHC- mismatched HSC transplantation, in which HLA-A2 differences can be used to restrict recognition to patient HSCs and leukemia.
Introduction
Acute myeloid leukemia (AML) is a rapidly progressing disease with an increased incidence in elderly patients, which limits the use of aggressive therapies. The most common treatments include risk-adapted polychemotherapy regimens,1,2 5-aza-2-deoxycytidine,3 and stem cell transplantation (SCT).4,5 The persistence of resistant tumor cells leads to relapse in a high percentage of patients. Immunotherapies, including adoptive T-cell transfer, provide an alternative approach to eliminating residual leukemia. Like adoptive transfer of tumor-infiltrating lymphocytes6 or genetically modified, peripheral blood lymphocyte (PBL)–expressing chimeric Ag receptors,7,8 adoptive transfer of patient-derived lymphocytes expressing Ag-specific transgenic TCRs (tgTCRs) has the potential to target solid and disseminated tumors.9 TCR gene therapy allows MHC-restricted, Ag-specific TCR to be isolated ahead of time and later used to modify lymphocytes in a patient-specific manner, taking into account MHC allotypes and Ag profiles of the tumor. Strategies have been described to isolate high-affinity TCRs restricted by common MHC alleles that are specific for broadly expressed tumor-associated Ags10–13 ; however, the use of humanized mouse models to evaluate TCR-transgenic lymphocyte responses in vivo await development.
In addition to well-known leukemia-associated Ags such as survivin, Bcl-2, and PRAME, the hyaluronan-mediated motility receptor (HMMR/Rhamm) has been considered as a potential target for TCR gene therapy of AML and acute lymphoid leukemia (ALL). HMMR is highly expressed during embryogenesis and neural crest formation, but its expression is limited to the testes, placenta, thymus, tonsils, and bone marrow (BM) in adults.14,15 Moreover, HMMR is broadly expressed in numerous types of tumors, including prostate and breast cancer, melanoma, and various forms of leukemia. Several studies analyzing Ag-specific immune responses in AML patients have demonstrated prolonged survival correlated with the prevalence of HMMR-specific T cells.16–19 The use of HMMR-derived peptides for antitumor vaccination resulted in strong HMMR-specific immune responses but failed to cure disease.20–22 Transfer of large numbers of T cells expressing higher-affinity tgTCRs specific for HMMR might overcome this limitation.
In the present study, we describe the development of such designer lymphocytes expressing tgTCRs specific for HMMR, starting with the generation of allorestricted, HMMR-specific T cells through and ending with the assessment of TCR-transgenic PBLs in a humanized mouse model. We used CD137-based enrichment23,24 as an MHC multimer-independent method to isolate allorestricted, HMMR-specific T cells. We selected the TCRs from one HMMR-specific clone to generate TCR-transgenic PBLs. First, we characterized the function of TCR-transgenic lymphocytes in vitro and confirmed that they had the same specificity as the original clone. The epitope of HMMR seen by the TCRs and its MHC restriction by HLA-A2 were defined. TCR-transgenic lymphocytes were then assessed for their capacity to infiltrate and retard solid tumor growth in a NOD/SCID IL-2Rgnull (NSG) mouse model. Initial studies directed us to further optimize the phenotype of TCR-transgenic lymphocytes used for adoptive transfer. By inducing an IL-15–dependent effector memory T-cell (TEM) phenotype within the TCR-transgenic populations, we improved tumor killing in vitro and tumor outgrowth in vivo. To characterize the role of optimized tgTCR on disseminated leukemia, we used luciferase-based tracking to assess tumor load in vivo. We found that injection of TCR-transgenic, HMMR-specific lymphocytes led to a significant reduction of tumor burden in the settings of both solid tumor and disseminated leukemia. These findings support the further pursuit of adoptive cell therapy using TCR-transgenic, HMMR-specific lymphocytes in selected clinical settings.
Methods
Induction of HMMR-specific T cells and enrichment via CD137
An in vitro dendritic cell (DC) priming approach was used to generate human allorestricted, HMMR-specific T cells as a source of higher-affinity TCRs.10,11 Mature DCs (mDCs) of an HLA-A*02:01:01:01− donor were simultaneously loaded with ivt-RNA encoding HLA-A*02:01:01:01 and HMMR as the MHC allotype and tumor-associated Ag of interest, respectively. In vitro priming of autologous CD8-enriched T cells was initiated on day 0 using RNA-loaded autologous mDCs at a 10:1 ratio, as described previously.11 Primed cultures were restimulated once on day 7 using mDCs prepared in the same manner (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Two weeks later, bulk cultures were restimulated with RNA-loaded mDCs that had been cryopreserved, and then activated T cells were stained with CD137-specific mAb. After 9 hours of stimulation, 35.9% of the primed cells were CD137+. These cells were subjected to magnetic bead sorting, yielding a purity of 83.0% CD137+ cells (supplemental Figure 1B). T-cell clones were generated by limiting dilution directly after enrichment and were restimulated every 2 weeks, as described previously.11 These clones were assessed in standard 4-hour chromium-release assays using target cells that were shown by flow cytometry to express HLA-A2 and HMMR (supplemental Figure 2A-B).
TCR gene transfer and cytokine treatment of TCR-transgenic PBLs
TCR sequences of HMMR-specific CTL67 and CTL150 were determined by PCR.11 The synthesized TCRβ-2A–TCRα transgene cassettes (GENEART) were integrated into MP71-PRE as described previously.11 Vector plasmids were used for the production of retroviral particles and subsequent transduction of T cells.25 T-cell transfer experiments using modified culture conditions were performed using X-Vivo 15 medium (Lonza) supplemented with 1.5 g/L N-acetyl-L-cysteine, 50mM HEPES, 2mM l-glutamine, and 10% human serum. TCR retroviral transduction was performed as described previously and cells were cultured up to day 5 with IL-2.25 Cells were then washed and resuspended in culture medium supplemented with new cytokines until being used in functional assays in vitro or adoptive transfer experiments in vivo (Table 1).
Lentiviral transduction and generation of luciferase-expressing cells
The firefly luciferase construct was cloned into the pCDH vector containing a green fluorescent protein (GFP) expression cassette under the control of the EF-1α promoter (System Biosciences). Third-generation lentiviruses were produced in 293T cells. THP-1 cells were transduced overnight in the presence of polybrene and enriched by FACS using GFP as a marker.
Mice and adoptive transfer experiments
For the melanoma model, 6- to 8-week-old NSG mice were injected subcutaneously (SC) with 4 × 105 tumor cells (mel624.38; see supplemental Methods) resuspended in Matrigel (BD Biosciences) on day 0. TCR-transgenic or mock-treated lymphocytes were adoptively transferred intravenously into recipient mice on day 1. Tumor size was measured every other day using a caliper and calculated as square millimeters (length × width). Mice were killed when the tumor surpassed a volume of 150 mm2. In the disseminated AML model, 1 × 106 luciferase-positive THP-1luc cells were injected intravenously on day 0, followed by adoptive transfer of TCR-transgenic human PBLs on day 1. IL-15 treatment, using a daily dose of 10 μg/mouse, or daily IL-2 treatment (36 μg/mouse) was given on day 1 through day 10 by IP injection.26 Mice were anesthetized by isoflurane inhalation for in vivo imaging. Luciferin (100 ng/mouse/injection; SynChem) was injected intravenously before measurement of light emission using the IVIS Lumina machine (Caliper Life Sciences). All animal experiments were approved by the local authorities according to the legal regulations for animal experiments.
In vivo model for SCT
HHD mice expressing a chimeric human HLA-A*0201 transgene27 were maintained under pathogen-free conditions. BM from femur and tibia was collected from donor mice at 10-12 weeks of age. HSC enrichment was performed using a Sca-1+ isolation kit (StemCell Technologies) according to the manufacturer's instructions. HSCs were maintained in STEMPRO medium (Invitrogen) supplemented with murine IL-3 (mIL-3; 10 ng/mL), mIL-6 (50 ng/mL), and mSCF (50 ng/mL; all PeproTech) for 24 hours in the presence of TCR/GFP–transgenic splenocytes (matched gender) at a 1:1 ratio. Recipient mice were irradiated with 360 cGy 6 hours before intravenous transfer of 2 × 106 HSC/splenocyte mixtures. Control mice received only splenocytes or no cells. Mice were kept in individual ventilated cages under sterile conditions and their drinking water was supplemented with 4 g/L of dimetridazole (Sigma-Aldrich), 15% volume sugar syrup (Apomix), and 20 mg/L of Ciprobay (Bayer). Mice were monitored daily for health status and weight.
CFU assay
CD34-enriched HSCs of healthy donors were kindly provided by I. Bigalke (GMP Unit, Helmholtz Zentrum, Munich, Germany). After thawing of HLA-A2+ and HLA-A2− samples, cells were washed twice and resuspended in IMDM (Invitrogen) supplemented with 2% FCS. TCR-transgenic lymphocytes were washed and 1 × 105 cells were mixed with 1.25 × 103 HSCs in 2.5 mL of methylcellulose-containing medium (MethoCult GF H4434; Cell Systems) and plated in duplicate in 35-mm culture dishes. Incubation was performed at 37°C and 5% CO2 for 14 days before counting of differentiated colonies. Morphological discrimination was based on criteria published previously.28
Regulatory permission
Permission was granted by the Ethics Commission of the Ludwig-Maximilians-University, Munich, Germany for the use of cells of healthy adult donors and leukemia patients for these studies. Individuals gave informed consent in accordance with the Declaration of Helsinki. The in vivo animal studies were approved by the Bavarian State authorities.
Results
Selection of T-cell clones with HMMR specificity
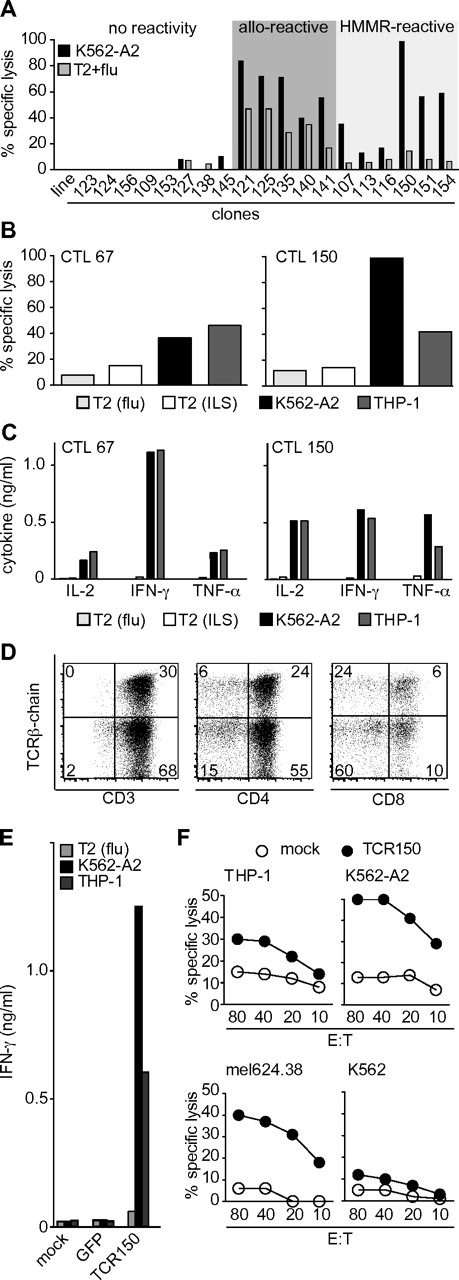
DC priming was used to generate T cells specific for HMMR. T-cell clones derived by limiting dilution from primed cultures with potential specificity for HMMR were selected based on their capacity to kill K562-A2 target cells (HLA-A2+ and HMMR+) compared with HLA-A2+ T2 cells pulsed with an irrelevant flu peptide. Cytotoxic T-lymphocytes (CTLs) were assigned to 1 of 3 groups based on patterns of reactivity: (1) no killing of either target cell, (2) alloreactivity for both target cells, and (3) potential HLA-A2–allorestricted, HMMR-specific killing of K562-A2 cells alone (Figure 1A). Selected CTLs were further tested for their capacity to kill the THP-1 AML tumor line (HLA-A2+, HMMR+) and to secrete IFN-γ in response to tumor stimulation (supplemental Figure 1C). Of 152 clones analyzed, the HMMR-reactive clones represented the highest fraction (52%), followed by 25% alloreactive and 23% nonreactive CTLs (supplemental Figure 1D). The majority of HMMR-reactive clones that recognized K562-A2 and THP-1 cells but not flu-pulsed T2 cells also secreted several Th-1–like cytokines (IL-2, IFN-γ, MIP-1α, and TNF-α) that have been linked with polyfunctionality of high-avidity T cells (supplemental Figure 1E; D.J.S., unpublished observations).29
In vitro characterization of HMMR-specific T-cell clones and TCR-transgenic lymphocytes. (A) Clones induced by in vitro priming using DCs prepared from an HLA-A2− donor pulsed with HLA-A2 and HMMR ivt-RNA. Lytic capacity (percentage specific lysis) was assessed in a standard 4-hour chromium-release assay using K562-A2 (HLA-A2+, HMMR+) cells as a positive target and HLA-A2+ T2 cells pulsed with an irrelevant peptide (flu) as a negative control. (B) Lytic capacity, shown as the percentage specific lysis by HMMR-specific CTL67 and CTL150 of THP-1 and K562-A2 and T2 cells pulsed with the flu or ILS peptide of HMMR, respectively at an effector-to-target ratio of 1:5. (C) Cytokine secretion by CTL67 and CTL150 given in nanograms per milliliter for 2 × 103 cells 24 hours after stimulation with the 4 target cells described in panel B. (D) Flow cytometry staining of TCR150-transgenic lymphocytes showing expression in CD3, CD4, and CD8 T cells (from left to right). (E) IFN-γ ELISA of 4 × 104 lymphocytes stimulated with 2 × 103 tumor cells for 24 hours. THP-1 and K562-A2 cells were used as positive stimulating cells, whereas T2 cells pulsed with flu peptide served as an HMMR− control. PBLs were transduced with TCR150 or GFP control vector. Mock PBLs served as a background control. Data are given in nanograms per milliliter. (F) Specific lysis of target cells THP-1 (A2+; HMMR+), K562-A2 (A2+; HMMR+), mel624.38 (A2+; HMMR+), and K562 (A2−; HMMR+) mediated by untransduced PBLs (mock; ○) or TCR150-transgenic PBLs (●).
In vitro characterization of HMMR-specific T-cell clones and TCR-transgenic lymphocytes. (A) Clones induced by in vitro priming using DCs prepared from an HLA-A2− donor pulsed with HLA-A2 and HMMR ivt-RNA. Lytic capacity (percentage specific lysis) was assessed in a standard 4-hour chromium-release assay using K562-A2 (HLA-A2+, HMMR+) cells as a positive target and HLA-A2+ T2 cells pulsed with an irrelevant peptide (flu) as a negative control. (B) Lytic capacity, shown as the percentage specific lysis by HMMR-specific CTL67 and CTL150 of THP-1 and K562-A2 and T2 cells pulsed with the flu or ILS peptide of HMMR, respectively at an effector-to-target ratio of 1:5. (C) Cytokine secretion by CTL67 and CTL150 given in nanograms per milliliter for 2 × 103 cells 24 hours after stimulation with the 4 target cells described in panel B. (D) Flow cytometry staining of TCR150-transgenic lymphocytes showing expression in CD3, CD4, and CD8 T cells (from left to right). (E) IFN-γ ELISA of 4 × 104 lymphocytes stimulated with 2 × 103 tumor cells for 24 hours. THP-1 and K562-A2 cells were used as positive stimulating cells, whereas T2 cells pulsed with flu peptide served as an HMMR− control. PBLs were transduced with TCR150 or GFP control vector. Mock PBLs served as a background control. Data are given in nanograms per milliliter. (F) Specific lysis of target cells THP-1 (A2+; HMMR+), K562-A2 (A2+; HMMR+), mel624.38 (A2+; HMMR+), and K562 (A2−; HMMR+) mediated by untransduced PBLs (mock; ○) or TCR150-transgenic PBLs (●).
Characterization of HMMR-reactive clones 67 and 150
Further studies were concentrated on CTL67 and CTL150, which express different TCR sequences (data not shown). CTL150 discriminated quantitatively between THP-1 and K562-A2 tumor cells, whereas CTL67 did not. CTL67 was less cytotoxic for K562-A2, but secreted more IFN-γ after tumor-cell stimulation (Figure 1B-C). These clones did not recognize the R3 (ILS) peptide of HMMR, which was reported to be immunodominant (see supplemental Methods).
We next investigated whether the expression of these TCRs as transgenes in activated PBLs would yield effector cells with specificity like the original CTLs. TCR sequences of CTL67 and CTL150 were isolated, codon optimized, and their constant regions exchanged for the murine counterparts, as described previously.11 After retroviral transduction of recipient lymphocytes, only TCR150 expression was found in transduced lymphocytes (supplemental Figure 3A).
Expression of TCR150 was found on both CD4+ and CD8+ cells by staining the murine constant region of the tgTCR, as described previously (Figure 1D).11 TCR-transgenic PBLs were tested for IFN-γ secretion after stimulation with K562-A2 and THP-1 tumor cells, and high levels of IFN-γ were detected (Figure 1E). This was dependent on tgTCR expression, because mock-transduced PBLs and PBLs transduced with a GFP control vector did not secrete IFN-γ after tumor cell stimulation. HMMR specificity was indicated by absence of IFN-γ secretion after stimulation with flu-pulsed T2 cells. Interestingly, TCR-transgenic PBLs displayed quantitative differences in the recognition of THP-1 and K562-A2 seen with the original CTLs.
TCR150-transgenic PBLs were also analyzed for their cytotoxic potential and compared with mock control PBLs. HLA-A2+ and HMMR+ double-positive cells (THP-1, K562-A2, mel624.38; supplemental Figure 2A-B) were killed by TCR150-trangenic lymphocytes (Figure 1F). K562 (HLA-A2−, HMMR+) cells were not recognized, confirming HLA-A*0201 restriction. The capacity of TCR150-trangenic PBLs to lyse tumor cells over an extended period of time was analyzed by coculturing TCR150-transgenic PBLs with the target cell lines mel624.38 (HLA-A2+, HMMR+, Tyr+) and THP-1luc (HLA-A2+, HMMR+, Tyr−) for 96 hours. As controls, target cells were cocultured with mock-treated PBLs and TCRT58-transgenic PBLs expressing a tyrosinase-specific TCR.11 Cell mixtures were analyzed by flow cytometry for surviving tumor cells based on size (mel624.38) or the GFP marker gene (THP-1luc). After incubation with mock-treated PBLs, we detected 26% mel624.38 and 42% THP-1 cells. TCR150-transgenic lymphocytes reduced these values to 4% and 10%, respectively, whereas TCRT58-trangenic PBLs reduced only the tyrosinase-positive mel624.38 cells (0%) and THP-1luc stayed at control levels (43%; supplemental Figure 3B). Therefore, TCR150-transgenic PBLs were able to specifically eliminate many more tumor cells over time compared with the 4-hour assay.
Specificity of TCR150 for HMMR
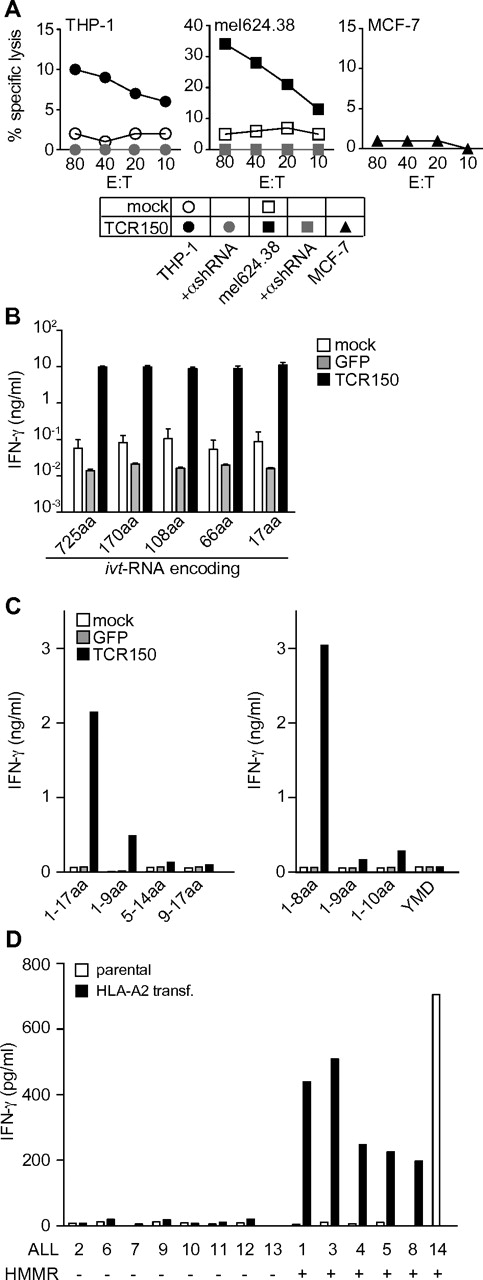
To confirm that recognition by TCR150 was indeed HMMR specific, we used a lentiviral vector to introduce shRNA specific for HMMR into HMMR-expressing target cells. The efficiency of HMMR knockdown in HMMR+ tumor lines was measured by intracellular staining for the HMMR protein compared with the HLA-A2+ human breast cancer cell line MCF-7, which is known to be HMMRlow (supplemental Figure 2A-B). THP-1 and mel624.38 showed a strong reduction of HMMR expression (supplemental Figure 2C). Therefore, this shRNA was suitable for knockdown of HMMR in target cells.
Parental THP-1 and mel624.38 cells were recognized by TCR150-transgenic PBLs to different degrees (Figure 2A). HMMRlow MCF-7 cells were not recognized. After the introduction of shRNA, treated tumor cells were no longer recognized (Figure 2A). In contrast, all 3 tumor lines were recognized by HLA-A2–alloreactive CTLs and recognition was not altered by knockdown of HMMR (data not shown). In addition, parental and shRNA-treated mel624.38 cells were recognized to similar degrees by MART-1–specific CTLs (supplemental Figure 2D). Therefore, shRNA knockdown of HMMR was specific and led to specific loss of recognition of target cells only by TCR150, confirming that this TCR was specific for HMMR.
Characterization of Ag specificity using TCR150-transgenic lymphocytes. (A) Specific lysis of parental THP-1 and mel624.38 (black symbols) or HMMR-specific shRNA-treated THP-1 or mel624.38 (gray symbols) by TCR150-transgenic PBLs, measured in a standard chromium-release assay. The HMMRlow cell line MCF-7 (black triangles) and mock PBLs with THP-1 or mel624.38 (open symbols) served as controls. (B) IFN-γ secretion (ng/mL) of HMMR-specific TCR150-transgenic, GFP-transduced, or mock-treated PBLs (2 × 105) after stimulation with autologous, HLA-A2+ mature DCs (5 × 104). DCs were pulsed with ivt-RNA encoding full-length HMMR1-725 or deletion mutants encoding aa 1-170, aa 1-108, aa 1-66, or aa 1-17, respectively. (C) IFN-γ release (ng/mL) by 2 × 105 lymphocytes (mock treated, GFP transduced, or TCR150 transgenic) after stimulation with 5 × 104 HLA-A2+ DCs, electroporated with HMMR-derived peptides. Left block depicts DCs pulsed with a long 17-mer (HMMR1-17) and corresponding nonamers; the right block shows DCs loaded with 20 μg of the peptides HMMR1-8, HMMR1-9, HMMR1-10, and irrelevant, tyrosinase-derived YMD peptide. (D) IFN-γ secretion (pg/mL) of TCR150-transgenic PBLs after stimulation with primary ALL cells. Left block shows HMMR− samples; right block depicts HMMR+ samples. All samples except ALL14 were electroporated with ivt-RNA encoding HLA-A*02:01:01:01 and expression was assessed by flow cytometry (approximately 50% positive cells; data not shown). Both HLA-A2− (parental; open bars) and transient HLA-A2+ cells (filled bars) were analyzed for stimulatory capacity in 24-hour cocultures with TCR150-transgenic PBLs at an effector-to-target ratio of 40:1 using 2 × 103 target cells.
Characterization of Ag specificity using TCR150-transgenic lymphocytes. (A) Specific lysis of parental THP-1 and mel624.38 (black symbols) or HMMR-specific shRNA-treated THP-1 or mel624.38 (gray symbols) by TCR150-transgenic PBLs, measured in a standard chromium-release assay. The HMMRlow cell line MCF-7 (black triangles) and mock PBLs with THP-1 or mel624.38 (open symbols) served as controls. (B) IFN-γ secretion (ng/mL) of HMMR-specific TCR150-transgenic, GFP-transduced, or mock-treated PBLs (2 × 105) after stimulation with autologous, HLA-A2+ mature DCs (5 × 104). DCs were pulsed with ivt-RNA encoding full-length HMMR1-725 or deletion mutants encoding aa 1-170, aa 1-108, aa 1-66, or aa 1-17, respectively. (C) IFN-γ release (ng/mL) by 2 × 105 lymphocytes (mock treated, GFP transduced, or TCR150 transgenic) after stimulation with 5 × 104 HLA-A2+ DCs, electroporated with HMMR-derived peptides. Left block depicts DCs pulsed with a long 17-mer (HMMR1-17) and corresponding nonamers; the right block shows DCs loaded with 20 μg of the peptides HMMR1-8, HMMR1-9, HMMR1-10, and irrelevant, tyrosinase-derived YMD peptide. (D) IFN-γ secretion (pg/mL) of TCR150-transgenic PBLs after stimulation with primary ALL cells. Left block shows HMMR− samples; right block depicts HMMR+ samples. All samples except ALL14 were electroporated with ivt-RNA encoding HLA-A*02:01:01:01 and expression was assessed by flow cytometry (approximately 50% positive cells; data not shown). Both HLA-A2− (parental; open bars) and transient HLA-A2+ cells (filled bars) were analyzed for stimulatory capacity in 24-hour cocultures with TCR150-transgenic PBLs at an effector-to-target ratio of 40:1 using 2 × 103 target cells.
Because CTL150 failed to specifically recognize the known HMMR-R3 peptide (ILS; Figure 1B-C), we sought to identify the epitope using an HMMR gene transfection approach: mDCs from an HLA-A2+ donor were loaded with ivt-RNA for full-length HMMR (aa 1-725) or with ivt-RNA deletion mutants encoding smaller fragments of HMMR (aa 1-170, aa1-108, aa1-66, and aa1-17). As depicted in Figure 2B, TCR150-transgenic PBLs recognized DCs expressing full-length HMMR and the 4 smaller fragments, indicating that the epitope was located in the first 17 amino acids. Overlapping peptides spanning these first 17 amino acids were electroporated into HLA-A2+ mDCs. Whereas recognition of the 17-mer peptide was confirmed, recognition of the smaller peptides was poor, although weak recognition of the first 9 amino acids was noted. Therefore, we tested 8-mer (HMMR1-8) and 10-mer (HMMR1-10) peptides and identified the 8-mer sequence (MSFPKAPL) as the corresponding HMMR epitope of TCR150 (Figure 2C).
Because recognition of primary tumor cells is a crucial factor for TCR gene therapy, we investigated whether primary tumor cells would be recognized by TCR150-transgenic PBLs. Primary AML cells of suitable quality were not available, but we could test several primary ALL cells. Unfortunately, most samples were HLA-A2−, with the exception of ALL14 (fine-typed as HLA-A*02:01:01:01). To circumvent this problem, we introduced ivt-RNA encoding HLA-A*02:01:01:01 into the ALL cells 2 hours before using them as stimulating cells. Cocultures of TCR150-transgenic PBLs with parental or HLA-A2–transfected cells showed that all ALL cells that were double-positive for HMMR (as assessed by quantitative RT-PCR; data not shown) and HLA-A2 were recognized (Figure 2D). This further confirmed the HMMR specificity and HLA-A2 restriction of TCR150.
IL-15 modulation of TCR-transgenic lymphocytes enhances cytotoxic potential
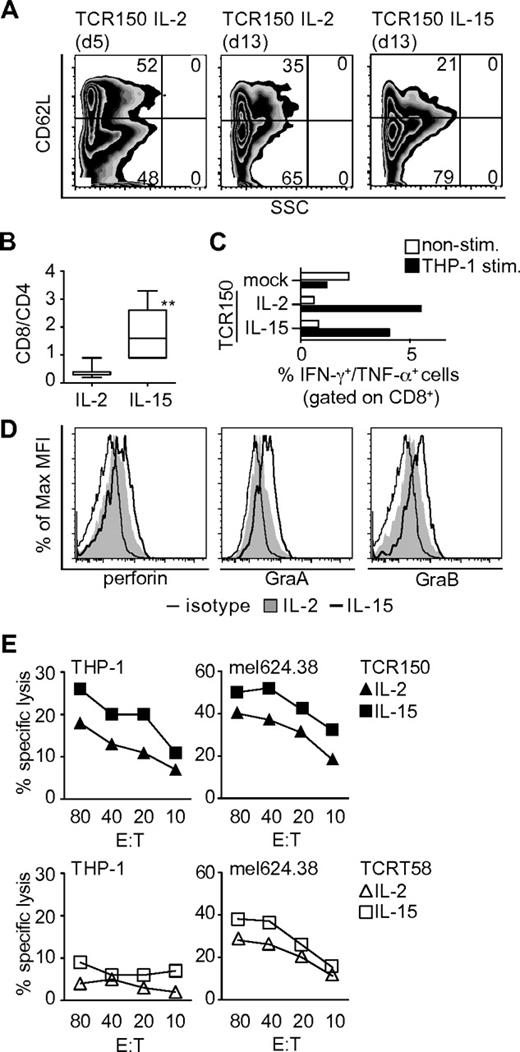
To improve in vitro function and potential survival of TCR-transgenic lymphocytes for in vivo studies, we attempted to induce memory T-cell phenotypes in TCR-transgenic PBLs. Standard cultures were initiated with IL-2 and the cytokine milieu was then changed 5 days after TCR transduction (Table 1). The phenotypes of TCR-transgenic lymphocytes were compared on days 5 and 13. CD62L expression levels were analyzed after pre-gating on CD8+/CD45RA− cells, allowing discrimination between the central-memory (TCM; CD62L+) and TEM (CD62L−) phenotypes (Figure 3A). TCR-transgenic PBLs showed equal division into the TCM and TEM phenotypes on day 5. After further culture until day 13 with IL-2 alone or with the addition of IL-15, decreases were seen in the TCM fractions, with corresponding increases in the TEM fractions. Cells exposed to IL-15–conditioned medium demonstrated a less-differentiated TEM status (CD28+, CD27+; data not shown) and yielded higher percentages of CD8+ cells compared with culture with IL-2 alone (Figure 3B).
Influence of cytokine milieu on TCR-transgenic recipient lymphocytes in vitro. (A) Surface staining of human TCR150-transgenic PBLs using human CD62L Abs on pre-gated CD8+, CD45RA− cells that were cultured over a 5-day or a 13-day period in the presence of IL-2 alone or IL-15 combined with low-dose IL-2 (IL-15). (B) Ratio of CD8+ to CD4+ T cells at day 13 after incubation with or without IL-15 (**P = .008 by Mann-Whitney test, n = 7). (C) Percentage of intracellular IFN-γ and TNF-α double-positive cells within the CD8+ T-cell fraction by flow cytometric analyses. Open bars represent nonstimulated PBLs; filled bars represent PBLs stimulated at an effector-to-target ratio of 20:1 with THP-1 cells for 6 hours. Mock-transfected PBLs were used as a background control compared with TCR150-transgenic PBLs cultured with or without IL-15. (D) Intracellular staining of perforin, granzyme A, and granzyme B in lymphocytes cultured in IL-2–containing medium (filled graph) or IL-2/IL-15–conditioned medium (open bold graph). Shattered line represents staining with isotype control. (E) Lytic capacity of TCR150-transgenic PBLs (top panel) or TCRT58-transgenic PBLs (bottom panel) cultured under IL-2 or IL-15–conditions using THP-1 and mel624.38 as target cells. Specific lysis was measured in a standard 4-hour chromium-release assay.
Influence of cytokine milieu on TCR-transgenic recipient lymphocytes in vitro. (A) Surface staining of human TCR150-transgenic PBLs using human CD62L Abs on pre-gated CD8+, CD45RA− cells that were cultured over a 5-day or a 13-day period in the presence of IL-2 alone or IL-15 combined with low-dose IL-2 (IL-15). (B) Ratio of CD8+ to CD4+ T cells at day 13 after incubation with or without IL-15 (**P = .008 by Mann-Whitney test, n = 7). (C) Percentage of intracellular IFN-γ and TNF-α double-positive cells within the CD8+ T-cell fraction by flow cytometric analyses. Open bars represent nonstimulated PBLs; filled bars represent PBLs stimulated at an effector-to-target ratio of 20:1 with THP-1 cells for 6 hours. Mock-transfected PBLs were used as a background control compared with TCR150-transgenic PBLs cultured with or without IL-15. (D) Intracellular staining of perforin, granzyme A, and granzyme B in lymphocytes cultured in IL-2–containing medium (filled graph) or IL-2/IL-15–conditioned medium (open bold graph). Shattered line represents staining with isotype control. (E) Lytic capacity of TCR150-transgenic PBLs (top panel) or TCRT58-transgenic PBLs (bottom panel) cultured under IL-2 or IL-15–conditions using THP-1 and mel624.38 as target cells. Specific lysis was measured in a standard 4-hour chromium-release assay.
The functional impact of IL-2 alone versus IL-15–supplemented conditioning was assessed in the 2 populations on day 13 by intracellular staining for IFN-γ and TNF-α (Figure 3C) and for the cytotoxic marker proteins perforin, granzyme A, and granzyme B (Figure 3D). Increased cytokine staining was only detected in CD8+ T cells after stimulation with THP-1 cells for 6 hours, indicating that TCR150-transgenic PBLs responded in a CD8-dependent manner to Ag stimulation (Figure 3C and data not shown). Moreover, the percentages of double-positive (IFN-γ+, TNF-α+) cells were similar in TCR-transgenic lymphocytes cultured under IL-2 or IL-15 conditions. Mock-transduced PBLs served as a control. In contrast to cytokines, differences were detected in intracellular staining of all 3 cytotoxins when the 2 populations were compared, with the highest staining of transgenic PBLs cultured with IL-15–conditioned medium (Figure 3D). Because IL-15–conditioned medium contained low levels of IL-2 (Table 1), we also analyzed TCR-transgenic PBLs cultured with IL-15 alone and detected reduced levels of both granzymes but not of perforin (supplemental Figure 3C).
TCR-transgenic lymphocytes were assessed for in vitro killing of THP-1 and mel624.38 target cells (Figure 3E) and K562 cells as a negative control (data not shown). Both of the HMMR+, HLA-A2+ tumor lines were recognized by TCR150-transgenic PBLs, whereas TCRT58-dependent killing was restricted to the tyrosinase-positive melanoma cell line. TCR-transgenic PBLs conditioned with the combination of IL-15 and low-dose IL-2 showed improved killing compared with IL-2 alone (Figure 3E) or IL-15 alone (supplemental Figure 3D). Therefore, low levels of IL-2 were retained in the IL-15–conditioned medium.
Targeting of solid tumors in vivo using TCR150-transgenic PBLs
To characterize the function of TCR150-transgenic lymphocytes in vivo, we analyzed adoptive transfer into NSG mice, which lack murine lymphocytes.30 The effect of TCR150-transgenic PBLs on the growth of a solid tumor cell line was assessed after SC injection of 4 × 105 mel624.38 cells in Matrigel. Human TCR150-transgenic PBLs were injected IV 24 hours later. In preliminary experiments, tumor growth in nontreated mice was first detected around day 14 after tumor inoculation (data not shown). Therefore, growth was measured on a day-to-day basis starting on day 12. The impact of TCR150-transgenic or mock control PBLs was assessed using 2 × 105 TCR+ lymphocytes, yielding a single effector cell for each 2 tumor cells (1:2) based on starting tumor cell load. TCR+ PBLs ranged from 10%-30% in all adoptive transfer experiments, and the numbers of mock-treated PBLs were matched to the highest number of injected TCR150-transgenic lymphocytes.
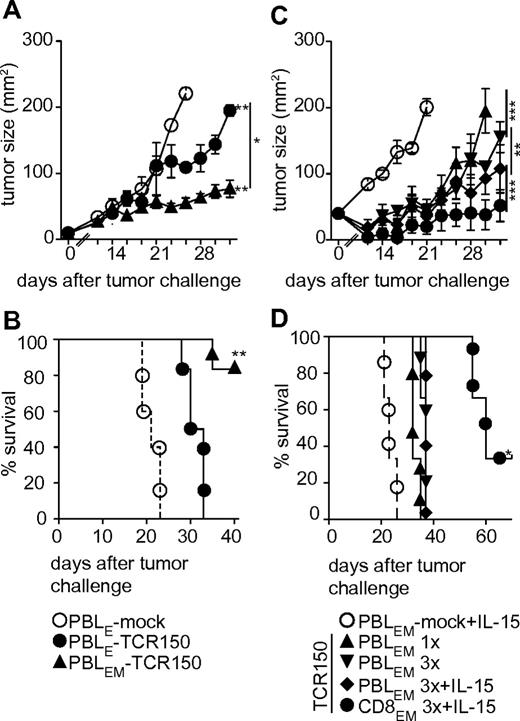
To assess in vivo capacity, TCR150-transgenic effector PBLs (PBLE) cultured only with IL-2 versus IL-15–conditioned PBLs (PBLEMs) were compared with mock PBLEs. Both populations of TCR150-transgenic lymphocytes retarded melanoma growth significantly compared with mock controls. However, the effect of TCR150-transgenic PBLEMs was significantly greater than transgenic PBLEs (Figure 4A). Retarded tumor outgrowth was also reflected in the overall survival of mice receiving PBLEMs, with significant prolongation compared with PBLEs (> 40 vs 33 days, respectively; Figure 4B). Based on these observations, further studies of melanoma used only IL-15–conditioned transgenic PBLEMs.
Potential of TCR150-transgenic lymphocytes to retard solid tumor outgrowth in vivo. (A) Tumor outgrowth (mm2) of 4 × 105 mel624.38 cells injected SC into NSG mice at day 0 followed by adoptive transfer (IV) of mock-transduced PBLs (○; n = 6), HMMR-specific TCR150-transgenic PBLEs (●; n = 6) or HMMR-specific TCR150-transgenic PBLEMs (▴; n = 6), on day 1 at a dose of 2 × 105 TCR-transgenic PBLs per mouse (**P < .01; *P < .05). A 2-way ANOVA was used for statistical analysis. (B) Percent survival of mice treated in the experiment described in Figure 3A. Data were compared using Mantel-Cox test (*P = .001). (C) Tumor outgrowth (mm2) measured every other day after initial SC injection of 4 × 105 mel624.38 cells. TCR150-transgenic PBLEMs (2 × 105) or CD8-enriched PBLEMs were injected intravenously 1 × 24 hours later (triangle arrow to top) or 3 injections were given on 3 sequential days (mock, ○; TCR150-transgenic PBLEMs, triangle arrows to bottom; TCR150-transgenic PBLEMs + IL-15, diamonds; TCR150-transgenic CD8EM + IL-15, ●). Administration of IL-15 (10 μg/mouse/d) intraperitoneally for 10 days was started 24 hours after tumor inoculation. The outgrowth was significantly retarded compared with mock treatment in the following magnitude: TCR150-transgenic TBLEMs1× < TCR150-transgenic PBLEM3× < TCR150-transgenic PBLEM3× + IL-15 < TCR150-transgenic CD8EM3× + IL-15 (**P < .01; ***P < .001 by 2-way ANOVA). (D) Survival of mice shown in panel C. Mean survival was correlated with retardation of tumor growth as follows: mock-treated PBLEMs, 23 days; TCR150-transgenic PBLEM1×, 32 days; TCR150-transgenic PBLEM3×, 37 days; TCR150-transgenic PBLEM3× + IL-15, 37 days; and TCR150-transgenic CD8EM3× + IL-15, 60 days. All treatments showed significantly prolonged survival compared with mock treatment (P < .03 by Mantel-Cox test), whereas only treatment with CD8-enriched PBLEM (3×) and IL-15 IP yielded significant prolongation compared with mice treated with TCR150-transgenic PBLEM1× (n = 6 per group; *P < .03 by Mantel-Cox test).
Potential of TCR150-transgenic lymphocytes to retard solid tumor outgrowth in vivo. (A) Tumor outgrowth (mm2) of 4 × 105 mel624.38 cells injected SC into NSG mice at day 0 followed by adoptive transfer (IV) of mock-transduced PBLs (○; n = 6), HMMR-specific TCR150-transgenic PBLEs (●; n = 6) or HMMR-specific TCR150-transgenic PBLEMs (▴; n = 6), on day 1 at a dose of 2 × 105 TCR-transgenic PBLs per mouse (**P < .01; *P < .05). A 2-way ANOVA was used for statistical analysis. (B) Percent survival of mice treated in the experiment described in Figure 3A. Data were compared using Mantel-Cox test (*P = .001). (C) Tumor outgrowth (mm2) measured every other day after initial SC injection of 4 × 105 mel624.38 cells. TCR150-transgenic PBLEMs (2 × 105) or CD8-enriched PBLEMs were injected intravenously 1 × 24 hours later (triangle arrow to top) or 3 injections were given on 3 sequential days (mock, ○; TCR150-transgenic PBLEMs, triangle arrows to bottom; TCR150-transgenic PBLEMs + IL-15, diamonds; TCR150-transgenic CD8EM + IL-15, ●). Administration of IL-15 (10 μg/mouse/d) intraperitoneally for 10 days was started 24 hours after tumor inoculation. The outgrowth was significantly retarded compared with mock treatment in the following magnitude: TCR150-transgenic TBLEMs1× < TCR150-transgenic PBLEM3× < TCR150-transgenic PBLEM3× + IL-15 < TCR150-transgenic CD8EM3× + IL-15 (**P < .01; ***P < .001 by 2-way ANOVA). (D) Survival of mice shown in panel C. Mean survival was correlated with retardation of tumor growth as follows: mock-treated PBLEMs, 23 days; TCR150-transgenic PBLEM1×, 32 days; TCR150-transgenic PBLEM3×, 37 days; TCR150-transgenic PBLEM3× + IL-15, 37 days; and TCR150-transgenic CD8EM3× + IL-15, 60 days. All treatments showed significantly prolonged survival compared with mock treatment (P < .03 by Mantel-Cox test), whereas only treatment with CD8-enriched PBLEM (3×) and IL-15 IP yielded significant prolongation compared with mice treated with TCR150-transgenic PBLEM1× (n = 6 per group; *P < .03 by Mantel-Cox test).
We next investigated whether multiple injections of PBLEMs would enhance tumor control. Mice were given 3 injections (days 1-3 after tumor inoculation) of mock or TCR150-transgenic PBLEMs. This resulted in stronger initial retardation of tumor outgrowth (Figure 4C) but did not significantly prolong survival (Figure 4D). More profound effects were measured when IL-15 was added intraperitoneally for 10 days after the first PBLEM injection, but survival was still not improved significantly. In contrast, significant prolongation of survival was achieved when TCR150-transgenic PBLEMs were enriched for CD8+ cells before adoptive transfer (Mantel-Cox test, P = .03). Transfer of CD8EM combined with IL-15 intraperitoneally for 10 days gave the best tumor retardation and overall mean survival of 60 days, compared with 37 days for multiple injections of PBLEMs with or without IL-15 and 32 days for single injection of PBLEMs with or without IL-15 and mean survival of 23 days for mice receiving mock PBLEMs.
TCR150-transgenic PBLEMs limit outgrowth of disseminated leukemia
Because we observed that IL-15–conditioned PBLEMs expressing TCR150 showed improved control of HMMR+ melanoma, we also tested their impact on control of disseminated leukemia cells. The firefly luciferase gene was introduced into THP-1 AML cells by lentiviral transduction and cells were enriched to 100% purity for in vivo tracking. We injected 1 × 106 THP-1luc cells intravenously into NSG mice, which received 4 × 105 TCR150-transgenic PBLEMs intravenously 24 hours later. As controls, we used similar numbers of mock-treated or irrelevant TCRT58-transgenic PBLEMs because THP-1 cells are tyrosinase negative, yielding 2 effector cells per 5 cells (2:5) based on starting leukemia inoculums. The tumor growth was assessed weekly starting at day 7 after tumor inoculation. In controls, we detected leukemic cells in the femurs of mice 14-21 days after leukemia injection (data not shown). This was followed by rapid spread, causing lesions in kidneys, liver, spine, and, in rare cases, lungs and ovaries. Approximately 10-15 days after tumor detection, mice displayed paralysis of the hind legs, at which point they were removed from the study. The overall survival time of mice treated with control TCRT58-transgenic PBLEMs was 31 days, compared with 35 days for mock-treated PBLEMs. In contrast, a single dose of TCR150-transgenic PBLEMs was sufficient to retard tumor outgrowth and prolong survival to a mean of 52 days (supplemental Figure 4A-B). Whereas treatment with HMMR-specific PBLEMs prevented all signs of hind-leg paralysis in all mice, animals were removed from the study over time because of lesions in kidneys and liver.
We also investigated whether CD8+EM cells given as multiple injections would further improve leukemia control. Adoptive transfer of TCR150-transgenic CD8EM cells for 3 days at a dose of 4 × 105 cells had no further impact on tumor burden or survival (60 days; Figure 5A-C) compared with a single PBL treatment (52 days; supplemental Figure 4A-B). The same was true when IL-2 was added intraperitoneally. In fact, this led to enhanced leukemia progression in individual mice (mean survival, 56 days) compared with injection of TCR150-transgenic CD8EMs alone (Figure 5A,C). This is most likely explained by expression of the IL-2 receptor (CD25) on THP-1 cells, allowing IL-2–driven proliferation (data not shown). Although intraperitoneal injection of IL-15 (days 1-10) did not affect melanoma outgrowth, it successfully contributed to controlling the outgrowth of AML cells (Figure 5A). IL-15 administration significantly prolonged the mean survival time of mice treated with 3 doses of TCR150-transgenic CD8EM cells (70 days) compared with 3 doses of CD8EM cells without IL-15 intraperitoneally (60 days; Mantel-Cox test; P = .01; Figure 5B-C). TCRT58-transgenic PBLEM and CD8EM cells served as irrelevant TCR controls, which did not significantly affect THP-1luc growth compared with mock PBLEMs (supplemental Figure 4A-E).
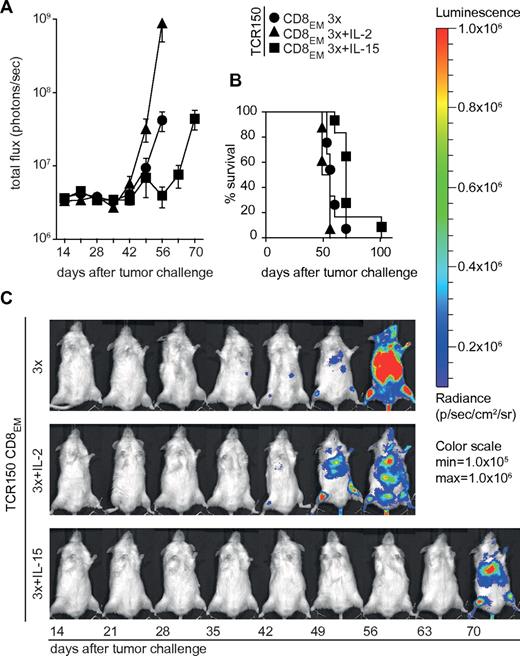
Usage of TCR150-transgenic effector-memory-like PBLs to target disseminated tumor cells in vivo. (A) After THP-1luc inoculation (1 × 106 cells/mouse), transfer of 4 × 105 CD8-enriched PBLEMs was performed on days 1-3 using TCR150-transgenic CD8EM cells. In addition, either IL-2 (36 μg/mouse/d) or IL-15 (10 μg/mouse/d) IP cytokine treatment was initiated on day 1 after tumor injection and given for 10 days. Shown is the measurement of the total flux (photons/s) as means (with SEM; n = 6 except for IL-2–treated groups n = 3) until the time point of the first dead mouse. (B) Survival of mice shown in panel C. Statistical calculation using the Mantel-Cox test indicated a significantly prolonged survival when mice received TCR150-transgenic CD8EM (mean CD8EM-TCRT58, 37.2 days, see supplemental Figure 4; mean CD8EM-TCR150, 62 days; *P < .03). Addition of cytokine intraperitoneally with 3 injections of TCR150-CD8EM showed a significant impact of IL-15 (70 days) compared with no cytokine (60 days; P = .01) or IL-2 administration (56 days; P = .003). (C) Representative examples of serial images of mice described in panels A and B.
Usage of TCR150-transgenic effector-memory-like PBLs to target disseminated tumor cells in vivo. (A) After THP-1luc inoculation (1 × 106 cells/mouse), transfer of 4 × 105 CD8-enriched PBLEMs was performed on days 1-3 using TCR150-transgenic CD8EM cells. In addition, either IL-2 (36 μg/mouse/d) or IL-15 (10 μg/mouse/d) IP cytokine treatment was initiated on day 1 after tumor injection and given for 10 days. Shown is the measurement of the total flux (photons/s) as means (with SEM; n = 6 except for IL-2–treated groups n = 3) until the time point of the first dead mouse. (B) Survival of mice shown in panel C. Statistical calculation using the Mantel-Cox test indicated a significantly prolonged survival when mice received TCR150-transgenic CD8EM (mean CD8EM-TCRT58, 37.2 days, see supplemental Figure 4; mean CD8EM-TCR150, 62 days; *P < .03). Addition of cytokine intraperitoneally with 3 injections of TCR150-CD8EM showed a significant impact of IL-15 (70 days) compared with no cytokine (60 days; P = .01) or IL-2 administration (56 days; P = .003). (C) Representative examples of serial images of mice described in panels A and B.
HMMR+ HSCs are recognized by TCR150-transgenic lymphocytes
Microarray data and quantitative RT-PCR analysis by us and others showed the expression of HMMR in BM samples of healthy donors (data not shown).14,15 Further studies of separated cells demonstrated HMMR expression in the fraction of CD34+ HSCs (Dr P. Greenberg, Fred Hutchinson Cancer Research Center, Seattle, WA, personal e-mail communication, August 2011). Whereas major hematopoietic toxicity was not reported in the HMMR vaccination studies of AML patients, the greater functional potential of TCR-transgenic lymphocytes might cause such toxicity. To assess such impacts on HSCs, we established an in vivo reconstitution model system using HHD mice expressing HLA-A2 as donors and recipients. This was possible because the 8-mer peptide recognized by TCR150 is identical in the murine HMMR protein (data not shown). Lethally irradiated mice were reconstituted with syngeneic Sca-1+–enriched HSCs precultured with syngeneic murine TCR150-transgenic or GFP-transduced splenocytes for 24 hours. After intravenous transfer of 1 × 106 HSCs, mice were monitored for signs of radiation sickness, such as weight loss and ruffled fur. Mice receiving no HSCs or HSCs precultured with TCR150-transgenic splenocytes were sickly and required removal from study at day 6. In contrast, mice receiving HSCs precultured with GFP-transduced T cells remained healthy over a period of 70 days (Figure 6A and data not shown). We then performed in vitro CFU assays of human CD34+ HSCs in the presence of human TCR150-transgenic PBLs to confirm on-target specificity. Mock-treated lymphocytes served as controls. We observed fewer colonies of differentiated cells emerging from HLA-A2+ HSCs compared with HLA-A2− HSCs, demonstrating the HLA-A2–restricted recognition of human HSCs and direct on-target toxicity. Normal differentiation of HSC samples was seen with mock-treated PBLs, but HSCs exposed to TCR150-transgenic PBLs led to decreases in all differentiated colonies (Figure 6B).
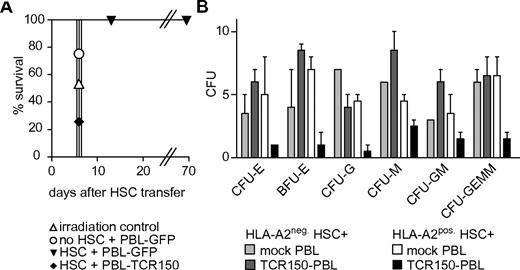
TCR150-transgenic PBL recognition of HLA-A2+ HSCs. (A) In vivo reconstitution of pre-irradiated HHD mice with Sca-1+–enriched HHD HSCs. Isolated stem cells were cocultured with GFP- or TCR150-transgenic lymphocytes at an effector-to-target ratio of 1:1 before transfer (1 × 106 stem cells intravenously). As controls, mice were injected with GFP-transduced splenocytes alone or no cells. Mice were monitored for signs of radiation syndrome and were removed from the study when weight loss was greater than 30%. (B) CFU assay using CD34+-enriched human HSCs from either HLA-A2+ or HLA-A2− healthy donors as target cells. 1.25 × 104 HSCs were cocultured with 1 × 105 TCR150-transgenic or mock-treated PBLs. After 2 weeks, duplicate cultures were analyzed for the development of differentiated cell colonies. CFU-E indicates CFUs erythroid; BFU-E, burst-forming-units erythroid; CFU-G, CFUs granulocyte; CFU-M, CFUs macrophage; CFU-GM, CFUs granulocyte and macrophage; and CFU-GEMM, CFUs granulocyte, erythroid, macrophage, and megakaryocyte.
TCR150-transgenic PBL recognition of HLA-A2+ HSCs. (A) In vivo reconstitution of pre-irradiated HHD mice with Sca-1+–enriched HHD HSCs. Isolated stem cells were cocultured with GFP- or TCR150-transgenic lymphocytes at an effector-to-target ratio of 1:1 before transfer (1 × 106 stem cells intravenously). As controls, mice were injected with GFP-transduced splenocytes alone or no cells. Mice were monitored for signs of radiation syndrome and were removed from the study when weight loss was greater than 30%. (B) CFU assay using CD34+-enriched human HSCs from either HLA-A2+ or HLA-A2− healthy donors as target cells. 1.25 × 104 HSCs were cocultured with 1 × 105 TCR150-transgenic or mock-treated PBLs. After 2 weeks, duplicate cultures were analyzed for the development of differentiated cell colonies. CFU-E indicates CFUs erythroid; BFU-E, burst-forming-units erythroid; CFU-G, CFUs granulocyte; CFU-M, CFUs macrophage; CFU-GM, CFUs granulocyte and macrophage; and CFU-GEMM, CFUs granulocyte, erythroid, macrophage, and megakaryocyte.
Discussion
The selection of a well-defined target Ag and its expression pattern is crucial for immunotherapeutic approaches using adoptive T-cell transfer. Several studies have indicated that HMMR is overexpressed in many tumor types, including prostate and breast cancer, melanoma, and chronic and acute leukemias.16–18,31,32 In vaccination studies using an immunogenic HMMR-derived peptide (ILS), patients developed a potent immune response that was correlated with clinical benefit, whereas no signs of severe toxicity were observed.22,33 On this basis, we elected to explore the development of HMMR-specific T cells for TCR gene therapy in AML, because over 70% of AML patients show a significant overexpression of this protein.22
The priming of allorestricted, HMMR-specific T cells allowed us to isolate T-cell clones from healthy donors.11 To overcome the limitation of T-cell isolation by peptide-derived multimers, restricted to a single known epitope, we used T-cell enrichment via the CD137 activation marker. Other studies described use of CD137 to enrich Ag-specific T cells or to deplete alloreactive T cells.23,24 With our approach, we isolated many HMMR-specific CTLs that recognized HMMR+, HLA-A2+ tumor cell lines while ignoring control T2 cells (HLA-A2+) pulsed with irrelevant peptide. Clones CTL67 and CTL150 with different TCRs were selected for further studies. Both of these CTLs failed to recognize HMMR-R3 peptide (ILS), which is reported to be particularly immunogenic and dominated immune responses in AML patients, in contrast to ILS-specific T cells, which are rare in healthy individuals.21
Using optimized11 TCR sequences of CTL150 for retroviral transfer into recipient lymphocytes, we detected strong surface expression of TCR150. TCR150-transgenic PBLs showed the same specificity pattern as the original CTL150, which was HLA-A2 restricted. Moreover, TCR150-transgenic PBLs recognized freshly isolated cells from ALL patients and this was correlated with HLA-A2 and HMMR expression. Furthermore, shRNA-mediated knockdown demonstrated that tumor-cell recognition was dependent on HMMR. By introducing deletion mutants of HMMR into HLA-A2+ DCs, the epitope seen by TCR150 was mapped to the N-terminal 17 amino acids. The final 8-mer epitope was defined using synthetic peptides. Interestingly, this peptide (MSFPKAPL) does not display classic anchor residues for HLA-A*02:01:01:01 binding, nor does it have the more common length of 9 amino acids. These results demonstrated the utility of priming T cells using full-length proteins and isolating T cells in a multimer-independent manner to uncover unpredictable immunogenic peptides of HMMR.
After confirming the specificity and function of TCR150-transgenic lymphocytes in vitro, we assessed their impact in vivo using humanized NSG mice, which lack murine T, B, and natural killer cells.30 This strain is known to be a suitable host for the engraftment of both solid and disseminated human tumors.30,34 We used a human melanoma cell line injected SC in Matrigel to analyze solid tumor control. TCR-transgenic PBLs were given intravenously 24 hours after tumor inoculation. A single injection of TCR150+ PBLs was sufficient to retard melanoma outgrowth, significantly prolonging overall survival. These observations were surprising, because the numbers of TCR150-transgenic PBLs compared with tumor cells was very low, particularly when compared with other studies performing intratumoral injection of much higher cell numbers or by pre-incubating tumor cells with effector cells in vitro before adoptive transfer.35,36
Several modifications further improved these results. Culture conditions were modified to include IL-15 conditioning for expansion of TCR-transgenic lymphocytes. This yielded more CD8+ T cells of the less-differentiated TEM phenotype (CD8+, CD45RA−, CD62L−, CD27+, and CD28+). These CD8+ TEM cells showed superior cytotoxin expression and better killing of tumor cells in vitro. Adoptive transfer of such TEM cells using either single or triple injections improved delay of melanoma outgrowth. Additional tumor control was achieved using CD8-enriched TEM cells. The best overall survival was observed in mice receiving triple injections of TCR150-transgenic CD8+EM cells and delivery of IL-15 (IP) for 10 days. This regime may provide the best conditions for survival of human TCR150-transgenic CD8+EM cells in NSG mice. In other studies, the persistence of engineered PBLs in NSG mice ranged from 10-20 days35 and was increased by selecting TCM cells or by in vivo administration of human IL-15 after adoptive transfer of lymphocytes.37,38 It may not be necessary to provide exogenous IL-15 in human patients if they are preconditioned with lympho-depleting regimes to increase the available levels of IL-15.
To address TCR150-mediated control of disseminated human leukemia, we focused on IL-15–conditioned PBLEMs for adoptive transfer. A single injection of TCR150-transgenic PBLEMs significantly retarded AML outgrowth compared with PBLEMs transduced with an irrelevant TCR. Maximal delay was achieved using triple injections of CD8+EM cells together with IL-15 IP for 10 days, with some mice surviving 2-3 times longer than control mice.
To determine the setting in which TCR150-transgenic cells could be applied clinically, we used an additional in vivo mouse model to address hematopoietic toxicity based on known HMMR expression in BM. Fortunately, the 8-mer peptide of human HMMR seen by TCR150 is identical in the murine HMMR protein. Therefore, HHD mice, which express a chimeric human HLA-A*02:01:01:01 transgene, could be used to study HSC toxicity in vivo.27 Reconstitution with HHD HSCs precultured with syngeneic splenocytes expressing TCR150 failed to rescue lethally irradiated HHD mice. Direct on-target toxicity for human HSCs was confirmed by coculturing human CD34+ cells with human TCR150-transgenic PBLs. All differentiated hematopoietic lineages were strongly reduced when the HSCs were derived from HLA-A2+ donors, whereas no negative impact was seen on HLA-A2− HSCs, demonstrating a direct, HLA-A2–restricted on-target hematopoietic toxicity.
These in vitro and in vivo studies demonstrated that TCR150 gene therapy can only be used safely in the clinical setting of HLA-mismatched SCT in which the patient is HLA-A2+ and the donor is HLA-A2−. In that scenario, TCR150-transgenic PBLs are able to attack residual leukemia and remove residual patient-derived HSCs. Although this clinical situation is limited, the medical need is great because many AML and ALL patients fail to achieve complete remission after allogeneic SCT.39 The increasing use of HLA-haploidentical donors for SCT would provide a clinical setting to analyze the potential of TCR150-transgenic PBLEMs to prolong survival in high-risk patients who fail to clear their leukemia after HLA-A2–mismatched SCT.40–43
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Slusarski, N. Hömberg, and V. Groiss for excellent technical support, and E. Simpson for providing helpful suggestions about the experimental approach and the manuscript.
This work was supported by grants from the German Research Foundation (SFB-TR36) and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer.
Authorship
Contribution: S.S. designed and performed all of the experiments and drafted the manuscript; I.J. established the luciferase-tagged AML cells and the in vivo imaging technology; S.W. and B.M. developed the T-cell separation strategy and helped with T-cell priming; M.L. performed transduction of splenocytes; L.S. and W.U. provided the HSC transfer technology; M.H.M.H. provided the shRNA; D.J.S. and B.F. designed the experimental concept and prepared the final manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dolores J. Schendel, Institute of Molecular Immunology, Helmholtz Zentrum München, Marchioninistrasse 25, 81377 Munich, Germany; e-mail: schendel@helmholtz-muenchen.de.
References
Author notes
D.J.S. and B.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal