Abstract
Diamond Blackfan anemia (DBA) is an inherited bone marrow failure syndrome characterized by red cell aplasia and congenital anomalies. A predisposition to cancer has been suggested but not quantified by case reports. The DBA Registry of North America (DBAR) is the largest established DBA patient cohort, with prospective follow-up since 1991. This report presents the first quantitative assessment of cancer incidence in DBA. Among 608 patients with 9458 person-years of follow-up, 15 solid tumors, 2 acute myeloid leukemias, and 2 cases of myelodysplastic syndrome were diagnosed at a median age of 41 years in patients who had not received a bone marrow transplant. Cancer incidence in DBA was significantly elevated. The observed-to- expected ratio for all cancers combined was 5.4 (P < .05); significant observed-to-expected ratios were 287 for myelodysplastic syndrome, 28 for acute myeloid leukemia, 36 for colon carcinoma, 33 for osteogenic sarcoma, and 12 for female genital cancers. The median survival was 56 years, and the cumulative incidence of solid tumor/leukemia was approximately 20% by age 46 years. As in Fanconi anemia and dyskeratosis congenita, DBA is both an inherited bone marrow failure syndrome and a cancer predisposition syndrome; cancer risks appear lower in DBA than in Fanconi anemia or dyskeratosis congenita. This trial was registered at www.clinicaltrials.gov as #NCT00106015.
Introduction
Diamond Blackfan anemia (DBA; Online Mendelian Inheritance in Man [OMIM] 105650) is a rare inherited bone marrow failure syndrome (IBMFS) characterized by red cell aplasia and congenital anomalies.1-4 There is wide variability in clinical presentations, family history, and response to therapy.5 Case reports describe DBA patients with cancer; however, no quantitative assessment of cancer risk has been published to date. A predisposition to both acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) was suggested by early reports6 and summarized in a systematic review of 970 DBA cases by Shimamura and Alter.1 In addition to 15 cases with AML or MDS, there were 19 cases with solid tumors, including osteogenic sarcoma7 in 6 patients. The published cases with cancer were characterized by a young age at diagnosis and generally poor outcomes.
Although literature cases provide important clues, it is not possible to quantify specific cancer risks, because reporting of neoplastic events may be biased, and the denominator representing the total DBA patient population at risk is not available from case reports or small series. The Diamond Blackfan Anemia Registry of North America (DBAR) is the largest known cohort of DBA patients, with > 600 patients enrolled and approximately 20 years of follow-up. This database was used to systematically classify the types of cancer and patient ages to quantify the cumulative incidence and hazard rates. Using this study, we also compared cancer risks in DBA with other comparably analyzed cancer-associated IBMFS, namely, Fanconi anemia (FA; OMIM 227650)8-11 and dyskeratosis congenita (DC; OMIM 127550).8,12
Methods
The Diamond Blackfan Anemia Registry
The Diamond Blackfan Anemia Registry was established in 1991 to enable a comprehensive assessment of the clinical epidemiology and pathophysiology of DBA. The DBAR is a voluntary registry, and patients are enrolled after informed consent is obtained in accordance with the Declaration of Helsinki. This study was approved by the North Shore–Long Island Jewish Institutional Review Board and other participating institutions.
Patients are enrolled either through self-referral or from cooperating hematologists throughout North America. The patients and their parents and physicians complete a detailed questionnaire on enrollment in the DBAR. The information is reviewed by the principal investigator and colleagues and verified by use of medical records requested from each patient. All enrolled patients and/or their physicians are contacted yearly for updates. For the present analysis, we used telephone or e-mail to ascertain vital status and cancer experience as of August 2010. In patients for whom a neoplasm was reported, pathology reports and medical records were obtained from hospitals and physicians, with the assistance of the patient/family, to verify the diagnosis. Of the 24 cancers reported to the DBAR, 14 were verified with pathology/physician reports; the remainder were self-reported.
Statistical methods
A competing-risks approach was used to estimate cause-specific hazard functions and cumulative incidence curves for DBA, as described previously.9,10,13 For these analyses, bone marrow (hematopoietic stem cell) transplantation (BMT), development of a solid tumor (ST), development of AML, and death caused by complications of DBA were considered competing adverse events, that is, the first occurrence of any of these events was counted as the initial complication of the disorder. In brief, the cause-specific hazards describe the annual risk by age of each competing adverse event among persons who have not yet experienced any adverse event. Cumulative incidence curves describe the cumulative proportion of patients by age who have developed each adverse event type as an initial complication of the syndrome. MDS was analyzed separately as a noncompeting risk.
To quantify cancer incidence for each specific type of neoplasm in DBA patients relative to the general population, the observed number of neoplastic cases that occurred before transplantation was compared with the expected number (O/E ratio), after accounting for age, sex, and birth cohort, based on the experience of the US Surveillance, Epidemiology, and End Results Program (SEER 9).14 P < .05 was considered statistically significant.
Results
Data were analyzed from 608 patients who enrolled in the DBAR from November 1991 through August 2010 (Table 1). These patients contributed 9458 person-years of follow-up. There were 306 males. The median age at diagnosis of DBA was 3 months (range 0-30 years), and at last follow-up, it was 18 years (range < 1-69 years). Ethnicity/race was available for 71% of the DBAR patients: 70% were white, 9.5% were Latino, and roughly equal numbers of patients had black, Asian, Jewish, or mixed ancestry. Sixty patients had died, and 62 had received a bone marrow transplant.
Demographics of DBAR participants
| . | Total . | STs* . | Leukemia . | MDS . | No cancer or MDS . |
|---|---|---|---|---|---|
| No. of cases | 608 | 15 | 2 | 4 (includes 1 ST and 1 AML) | 589 |
| Male-female ratio | 306:302 | 5:10 | 2:0 | 3:1 | 298:291 |
| Birth years | 1941-2009 | 1941-1987 | 1954, 1958 | 1952-2007 | 1941-2009 |
| Person-years | 9458 | 501 | 90 | 116 | ND |
| Age at diagnosis of DBA | 3 mo (birth-30 y) | 3 mo (birth-29 y) | 3 mo, unstated | 8 mo (2 mo-1 y) | 3 mo (birth-30 y) |
| Age at event or last follow-up | 18 (< 1-69) | 41 (13-64) | 44, 46 | 32 (2-50) | 18 (< 1-69) |
| No. who died | 60 | 6 | 2 | 2 | 50 |
| Age at death | 18 (1 mo-64 y) | 33 (14-64) | 45, 46 | 18, 46 | 18 (1 mo-64 y) |
| No. who had BMT | 62 | 0 | 1 | 1 | 60 |
| Age at BMT | 8 (1-45) | 45 | 2 | 8 (1-45) |
| . | Total . | STs* . | Leukemia . | MDS . | No cancer or MDS . |
|---|---|---|---|---|---|
| No. of cases | 608 | 15 | 2 | 4 (includes 1 ST and 1 AML) | 589 |
| Male-female ratio | 306:302 | 5:10 | 2:0 | 3:1 | 298:291 |
| Birth years | 1941-2009 | 1941-1987 | 1954, 1958 | 1952-2007 | 1941-2009 |
| Person-years | 9458 | 501 | 90 | 116 | ND |
| Age at diagnosis of DBA | 3 mo (birth-30 y) | 3 mo (birth-29 y) | 3 mo, unstated | 8 mo (2 mo-1 y) | 3 mo (birth-30 y) |
| Age at event or last follow-up | 18 (< 1-69) | 41 (13-64) | 44, 46 | 32 (2-50) | 18 (< 1-69) |
| No. who died | 60 | 6 | 2 | 2 | 50 |
| Age at death | 18 (1 mo-64 y) | 33 (14-64) | 45, 46 | 18, 46 | 18 (1 mo-64 y) |
| No. who had BMT | 62 | 0 | 1 | 1 | 60 |
| Age at BMT | 8 (1-45) | 45 | 2 | 8 (1-45) |
All ages are median (range) in years unless otherwise stated.
DBA indicates Diamond Blackfan anemia; DBAR, Diamond Blackfan Anemia Registry; ND, not done; and BMT, bone marrow (stem cell) transplantation.
STs in patients who had not received a BMT.
Seventeen patients who had not received a BMT had 1 or more cancers, including 15 with ST and 2 with AML, for a crude rate of cancer of 3%. The median age at presentation of the first cancer was 41 years (range 13-64 years). The STs included 3 adenocarcinomas of the colon, 2 osteogenic sarcomas, 2 breast cancers, 2 squamous cell carcinomas (1 oral and 1 vaginal), and 1 patient each with non-Hodgkin lymphoma, soft tissue sarcoma, uterine cancer, cervical cancer, testicular cancer, choroid meningioma of the lung, and melanoma (Table 2). There were 2 patients with AML and 4 with MDS; 1 patient with MDS progressed to AML and is counted in each of these categories. Another patient (patient no. 0438-200) had 2 successive STs and MDS: breast cancer at age 43 years, adenocarcinoma of the colon at age 49 years, and MDS at age 51 years. Two patients with posttransplantation neoplasms were excluded from analysis; 1 had rectal cancer 15 years after BMT, and 1 had osteogenic sarcoma 2 years after BMT. Another patient with basal cell carcinoma was also excluded because no comparative data are available in the SEER database for this diagnosis.
Neoplasms in patients from the DBAR
| DBAR UPIN . | Cancer diagnosis . | Sex . | Age at diagnosis, y . | DBA status at cancer diagnosis . | Outcome . | Gene . |
|---|---|---|---|---|---|---|
| 0009-200 | Adenocarcinoma of the colon | M | 43 | Unknown | Alive | Not tested |
| 0300-104 | Adenocarcinoma of the colon | F | 34 | TD | Died | RPS19 |
| 0438-200 | Adenocarcinoma of the colon | F | 49 | SD | Alive | Negative |
| 0143-200 | Osteogenic sarcoma | M | 22 | TD, A, rhGH | Died, sepsis | Not tested |
| 0354-200 | Osteogenic sarcoma | F | 13 | TD, A | Died, metastatic disease | Not tested |
| 0185-200 | Soft tissue sarcoma | M | 30 | TD | Died, metastatic disease | Not tested |
| 0438-200 | Breast cancer | F | 43 | SD | Alive | Negative |
| 0458-200 | Breast cancer | F | 34 | Remission | Alive | RPS19 |
| 0416-102 | Uterine cancer | F | 64 | Never treated | Died, metastatic disease | RPS19 |
| 0534-200 | Cervical cancer | F | 27 | TD | Alive | RPS19 |
| 0109-101 | Testicular cancer | M | 62 | Remission | Alive | RPL35a |
| 0024-200 | Choroid meningioma of lung | F | 21 | TD | Alive | RPS19 |
| 0245-002 | SCC oral | F | 69 | TD, Steroids | Died | RPL11 |
| 0245-102 | SCC vaginal | F | 45 | Tx with chemotherapy only | Alive | RPL11 |
| 0025-102 | Melanoma | F | 50 | On no treatment | Alive | RPL5 |
| 0365-200 | Non-Hodgkin lymphoma | M | 41 | TD | Alive | RPL5 |
| 0364-101 | AML | M | 45 | On no treatment | Died, sepsis | Negative |
| 0387-200 | AML | M | 44 | SD | Died, chemotherapy/BMT, PD | Not tested |
| 0100-200 | MDS | M | 17 | TD | Died, sepsis | Not tested |
| 0308-200 | MDS | M | 2 | SD | Alive | Not tested |
| 0364-101 | MDS | M | 45 | On no treatment | Died (progressed to AML) | Negative |
| 0438-200 | MDS | F | 51 | TD, on azathioprine | Alive | Negative |
| 0200-200* | Osteogenic sarcoma | M | 4 | TD, s/p BMT, rhGH | Died, metastatic disease | Not tested |
| 0260-200* | Rectal cancer | F | 28 | s/p BMT | Alive | Not tested |
| 0277-101* | Basal cell cancer | M | 30 | SD | Alive | Not tested |
| DBAR UPIN . | Cancer diagnosis . | Sex . | Age at diagnosis, y . | DBA status at cancer diagnosis . | Outcome . | Gene . |
|---|---|---|---|---|---|---|
| 0009-200 | Adenocarcinoma of the colon | M | 43 | Unknown | Alive | Not tested |
| 0300-104 | Adenocarcinoma of the colon | F | 34 | TD | Died | RPS19 |
| 0438-200 | Adenocarcinoma of the colon | F | 49 | SD | Alive | Negative |
| 0143-200 | Osteogenic sarcoma | M | 22 | TD, A, rhGH | Died, sepsis | Not tested |
| 0354-200 | Osteogenic sarcoma | F | 13 | TD, A | Died, metastatic disease | Not tested |
| 0185-200 | Soft tissue sarcoma | M | 30 | TD | Died, metastatic disease | Not tested |
| 0438-200 | Breast cancer | F | 43 | SD | Alive | Negative |
| 0458-200 | Breast cancer | F | 34 | Remission | Alive | RPS19 |
| 0416-102 | Uterine cancer | F | 64 | Never treated | Died, metastatic disease | RPS19 |
| 0534-200 | Cervical cancer | F | 27 | TD | Alive | RPS19 |
| 0109-101 | Testicular cancer | M | 62 | Remission | Alive | RPL35a |
| 0024-200 | Choroid meningioma of lung | F | 21 | TD | Alive | RPS19 |
| 0245-002 | SCC oral | F | 69 | TD, Steroids | Died | RPL11 |
| 0245-102 | SCC vaginal | F | 45 | Tx with chemotherapy only | Alive | RPL11 |
| 0025-102 | Melanoma | F | 50 | On no treatment | Alive | RPL5 |
| 0365-200 | Non-Hodgkin lymphoma | M | 41 | TD | Alive | RPL5 |
| 0364-101 | AML | M | 45 | On no treatment | Died, sepsis | Negative |
| 0387-200 | AML | M | 44 | SD | Died, chemotherapy/BMT, PD | Not tested |
| 0100-200 | MDS | M | 17 | TD | Died, sepsis | Not tested |
| 0308-200 | MDS | M | 2 | SD | Alive | Not tested |
| 0364-101 | MDS | M | 45 | On no treatment | Died (progressed to AML) | Negative |
| 0438-200 | MDS | F | 51 | TD, on azathioprine | Alive | Negative |
| 0200-200* | Osteogenic sarcoma | M | 4 | TD, s/p BMT, rhGH | Died, metastatic disease | Not tested |
| 0260-200* | Rectal cancer | F | 28 | s/p BMT | Alive | Not tested |
| 0277-101* | Basal cell cancer | M | 30 | SD | Alive | Not tested |
DBA indicates Diamond Blackfan anemia; DBAR, Diamond Blackfan Anemia Registry; UPIN, unique patient identification number; M, male; F, female; TD, transfusion dependent; SD, steroid dependent; A, androgen therapy; rhGH, recombinant human growth hormone; SCC, squamous cell carcinoma; s/p BMT, status post bone marrow (stem cell) transplantation; Tx, transfusion; and PD, progressive disease.
Excluded from analysis.
Cancer occurred in patients who were in hematologic remission or had received a variety of treatments for their anemia. Eight patients were transfusion dependent at the time of their cancer diagnosis. Two patients were steroid dependent at the time they developed AML or MDS. Patient no. 0438-200 was steroid dependent at the time of development of breast cancer and continued taking steroids when she was diagnosed with adenocarcinoma of the colon. This patient became transfusion dependent before, and likely as a consequence of, her diagnosis with MDS. Two patients were in hematologic remission (defined as maintaining an adequate hemoglobin level for > 6 months without corticosteroids or red cell transfusions3 ) when they presented with cancer. Four patients had never been treated for anemia; 1 of these patients became transfusion dependent while undergoing chemotherapy. The DBA treatment status of 1 patient at the time of cancer diagnosis was unknown.
Because DBA causative genes were described years after the start of the DBAR, genotyping of the earlier enrolled patients was infrequent. Among the 18 patients with cancer, 10 had a mutation in a ribosomal protein gene known to be associated with DBA. Three of the 4 most common genotypes3 were represented: 5 with RPS19; 2 with RPL11 (mother and daughter); 2 with RPL5; and 1 with RPL35a. Two patients were wild type for the known DBA genes. Seven patients have not been tested for the known genetic mutations; 5 of these patients are deceased (Table 2).
The relative risk of all cancers (excluding MDS) in DBA was increased significantly (5.4-fold) compared with the general population. The O/E ratios were 36.2 for adenocarcinoma of the colon, 32.6 for sarcoma, 12 for female genital cancer, 27.9 for AML, and 287 for MDS (Table 3).
Observed cancers, O/E ratios, and 95% CIs
| Cancer type . | No. of observed cancers* . | O/E Ratio . | 95% CI . |
|---|---|---|---|
| Events with significant O/E ratios | |||
| All cancers | 18* | 5.4 | 3.2-8.6 |
| Colon (adenocarcinoma) | 3 | 36.2 | 7.5-105.8 |
| Bones (osteogenic) | 2 | 32.6 | 4.0-117.7 |
| Female genital† | 3 | 12.0 | 2.5-35.1 |
| AML‡ | 2 | 27.9 | 3.4-100.9 |
| MDS‡ | 4 | 287.0 | 77.2-734.7 |
| Events with nonsignificant O/E ratios | |||
| Oral cavity | 1 | 15.9 | 0.4-88.3 |
| Soft tissue sarcoma | 1 | 9.8 | 0.3-54.8 |
| Lung | 1 | 8.3 | 0.2-46.4 |
| Testis | 1 | 8.3 | 0.2-46.1 |
| Non-Hodgkin lymphoma | 1 | 5.7 | 0.1-31.7 |
| Melanoma | 1 | 4.5 | 0.1-25.3 |
| Breast | 2 | 4.1 | 0.5-14.9 |
| Cancer type . | No. of observed cancers* . | O/E Ratio . | 95% CI . |
|---|---|---|---|
| Events with significant O/E ratios | |||
| All cancers | 18* | 5.4 | 3.2-8.6 |
| Colon (adenocarcinoma) | 3 | 36.2 | 7.5-105.8 |
| Bones (osteogenic) | 2 | 32.6 | 4.0-117.7 |
| Female genital† | 3 | 12.0 | 2.5-35.1 |
| AML‡ | 2 | 27.9 | 3.4-100.9 |
| MDS‡ | 4 | 287.0 | 77.2-734.7 |
| Events with nonsignificant O/E ratios | |||
| Oral cavity | 1 | 15.9 | 0.4-88.3 |
| Soft tissue sarcoma | 1 | 9.8 | 0.3-54.8 |
| Lung | 1 | 8.3 | 0.2-46.4 |
| Testis | 1 | 8.3 | 0.2-46.1 |
| Non-Hodgkin lymphoma | 1 | 5.7 | 0.1-31.7 |
| Melanoma | 1 | 4.5 | 0.1-25.3 |
| Breast | 2 | 4.1 | 0.5-14.9 |
All data shown are significant at P < .05.
Eighteen cancers in 17 individuals. One person had breast cancer, colon cancer, and MDS at ages 43, 49, and 51 years.
Female genital included cervix, uterus, and vagina. Respective O/E ratios were 11.27 (0.29-62.78), 14.2 (0.36-79.14), and 270.81 (6.86-1508.83).
MDS is not included in the cancers. One patient had MDS followed by AML and is counted in both groups. A second patient is referred to above, with breast, colon, and MDS.
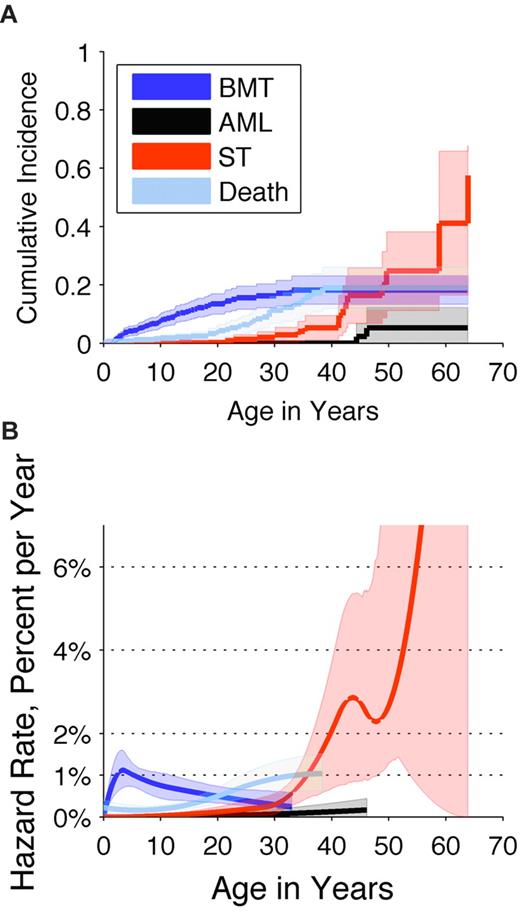
The cumulative incidence of adverse outcomes (excluding MDS) was determined by a competing-risk analysis (Figure 1A). By age 30 years, no patients had developed AML, 3% had an ST, 16% had undergone a BMT, and 11% had died. By age 46 years, 5% had developed AML, 16% had an ST, 18% had undergone a BMT, and 19% had died. The overall cumulative incidence of cancer (excluding MDS) was 22% by age 46 years. Cause-specific hazard rates were as follows: BMT occurred at a peak rate of approximately 1% per year; deaths caused by complications of DBA occurred at a rate of approximately 1% per year; the rate of AML slowly increased beginning at age 40 years; and the rate of STs increased rapidly beginning at age 30 years (Figure 1B).
Cumulative incidence and annual hazard rate of competing adverse events by age in patients with Diamond Blackfan anemia. Adverse events included BMT (dark blue), AML (black), STs (red), or death not caused by AML, ST, or BMT (light blue). (A) Cumulative incidence by age (cumulative percentage experiencing each event as initial cause of failure) and 95% CIs (shaded areas). (B) Annual hazard rates (incidence rate per year among subjects who are still susceptible) and 95% CIs (shaded areas).
Cumulative incidence and annual hazard rate of competing adverse events by age in patients with Diamond Blackfan anemia. Adverse events included BMT (dark blue), AML (black), STs (red), or death not caused by AML, ST, or BMT (light blue). (A) Cumulative incidence by age (cumulative percentage experiencing each event as initial cause of failure) and 95% CIs (shaded areas). (B) Annual hazard rates (incidence rate per year among subjects who are still susceptible) and 95% CIs (shaded areas).
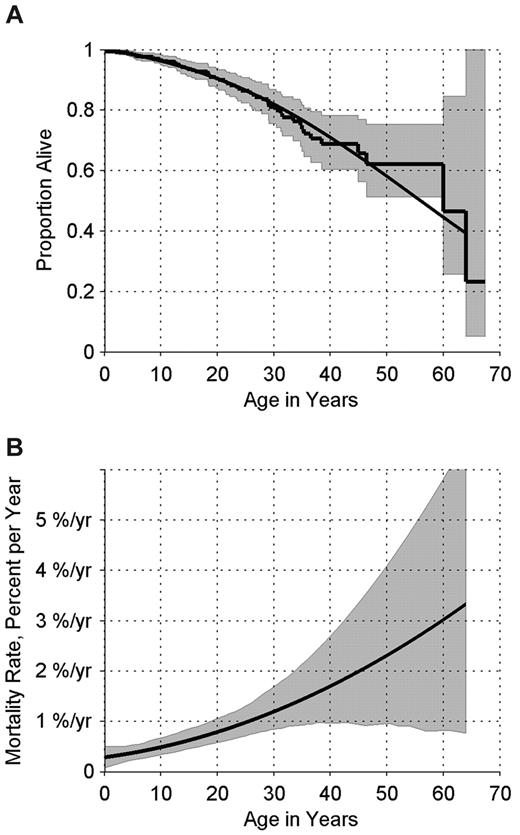
The median overall survival for all patients was 56 years (95% confidence interval [CI] 49-62 years; Figure 2A). The risk of death of any cause increased from approximately 1 in 200 per year among patients < 20 years of age to approximately 1 in 100 per year among patients in their 20s (Figure 2B). The data are limited but suggest overall mortality rates of approximately 1.5% per year in patients ≥ 30 years of age, with very broad confidence limits. Ten of the patients with cancer/MDS are currently alive. Four deaths were caused by metastatic/progressive disease and 3 by sepsis. The cause of death in 2 patients was unknown (Table 2).
Survival experience of patients in the DBAR. (A) Kaplan-Meier survival curve (step function), with 95% CI, and smoothed survival curve, estimating the probability of surviving to each age. (B) Percent dying of any cause per year, by age, and 95% CI.
Survival experience of patients in the DBAR. (A) Kaplan-Meier survival curve (step function), with 95% CI, and smoothed survival curve, estimating the probability of surviving to each age. (B) Percent dying of any cause per year, by age, and 95% CI.
Discussion
The present analysis provides, for the first time, clarity regarding the spectrum and incidence of cancer in DBA, incorporating approximately 20 years of patient accrual and prospective follow-up in the DBAR. Overall, we identified that the rate of any ST or leukemia was 5.4-fold higher in DBA patients than expected in a demographically matched comparison with the general population. Furthermore, specific tumors had significantly elevated incidence ratios for MDS, AML, adenocarcinoma of the colon, osteogenic sarcoma, and female genital cancer. Indeed, there were more STs than hematologic malignancies.
The strengths of the DBAR cohort include active accrual and follow-up for nearly 20 years, enrollment of the largest group of patients with DBA, and confirmation of the cancer diagnosis from pathology reports in 14 cases. Limitations of the DBAR include no verification of the cancer self-reports in 10 patients. In addition, the DBAR is voluntary, and there could have been biased enrollment of older individuals with cancer or concerns about cancer or of patients with more severe manifestations of DBA, including a different cancer risk. Furthermore, the number of cancers is small, and genotype information is currently available on only a subset of patients, which limits our ability to assess genotype-phenotype associations.
Unfortunately, the available clinical data suggest that cancer treatment in DBA patients is difficult at best. Some of the cancer deaths in patients followed up by the DBAR occurred because of disease progression, whereas others occurred because of toxicity of treatment. Some patients who received chemotherapy had prolonged neutropenia that led to septic deaths. For other patients, the prolonged period of neutropenia led to chemotherapy delay and eventual disease progression with development of metastatic disease. Optimal treatment strategies for DBA patients with cancer are an urgent and critical future research area.
DBA now joins 2 other IBMFS cancer predisposition syndromes, FA and DC, with quantified hematologic and ST risks. The first studies to review and quantify cancer in IBMFS involved FA and included 4 diverse cohorts,8-11 all of which indicated that the most frequent STs were head and neck and gynecologic squamous cell carcinoma, and the most common hematologic malignancy was AML. In FA, the O/E ratios for all neoplasms ranged from 39 to 71. Although caution is called for when one compares O/E ratios across cohorts with different demographics by age and sex, both the FA cohorts and the DBAR participants are quite young and have similar proportions of males and females. DC also was found to have an elevated O/E ratio (11 for all sites combined),8,12 and the sites that occurred in excess were similar to those for FA. In DBA, the O/E ratio for all sites was 5.4, which was significant but lower than in the other 2 IBMFS. Thus, the present analysis does suggest that the risk of cancer is elevated but lower in DBA than in FA and DC.
The cumulative incidence of ST and AML in DBA was approximately 20% by the mid-40s compared with approximately 30% in both FA and DC by this age; however, these estimates reflect different competing risks in the different syndromes. The cumulative incidence of cancer in FA would be even higher were it not for the correspondingly high risk of early competing events (eg, BMT).11 In contrast, patients with DC do not have a high rate of early BMT, and thus, the cumulative incidence of cancer in this group appears similar to FA. As in FA, patients with DBA have an early risk of BMT or death due to complications. The risk of STs in nontransplanted patients begins to rise in DBA starting at approximately 30 years of age, whereas the rise appears earlier in FA and DC, at approximately age 20 years. AML and ST occur with equal frequency in patients with FA, whereas in DC and DBA, the most common cancers are STs.
The present data strongly suggest that patients with DBA should receive appropriate counseling and surveillance for neoplastic complications beginning much earlier than the general population. Unfortunately, the small numbers and diversity of cancers, as well as the inability to define specific cancer genotypes, make it difficult to recommend specific cancer screening. Other cancer risks may emerge as the DBAR matures. Special attention should be given to blood per rectum and bone findings, because these may identify the most frequently occurring neoplasms in DBA patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to the DBA patients, their parents, and their physicians for contributing data to the DBA Registry.
This research was supported in part by grants from the National Heart, Lung, and Blood Institute (R01 HL 079571-07; A.V., E.A., and J.M.L.), the Centers for Disease Control and Prevention (A.V.), the Pediatric Cancer Foundation (J.M.L.), and the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (P.S.R. and B.P.A.).
National Institutes of Health
Authorship
Contribution: A.V. and P.S.R. take primary responsibility for this paper; A.V., B.P.A., and J.M.L. designed the DBAR; P.S.R. performed the statistical analysis; A.V., E.A., and J.M.L reviewed patients' charts; E.A. transferred files to the DBAR database; and A.V., P.S.R., E.A., B.P.A., and J.M.L. participated in the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrianna Vlachos, MD, Cohen Children's Medical Center, Pediatric Hematology/Oncology and Stem Cell Transplantation, 269-01 76th Ave, Rm 255, New Hyde Park, NY 11040; e-mail: avlachos@nshs.edu.