Abstract
For decades, hematopoietic stem cells (HSCs) were thought to be a homogeneous population of cells with flexible behavior. Now a new picture has emerged: The HSC compartment consists of several subpopulations of HSCs each with distinct, preprogrammed differentiation and proliferation behaviors. These programs are epigenetically fixed and are stably bequeathed to all daughter HSCs on self-renewal. HSCs within each subset are remarkably similar in their self- renewal and differentiation behaviors, to the point where their life span can be predicted with mathematical certainty. Three subsets can be distinguished when HSCs are classified by their differentiation capacity: myeloid-biased, balanced, and lymphoid-biased HSCs. The relative number of the HSC subsets is developmentally regulated. Lymphoid-biased HSCs are found predominantly early in the life of an organism, whereas myeloid-biased HSCs accumulate in aged mice and humans. Thus, the discovery of distinct subpopulations of HSCs has led to a new understanding of HCS aging. This finding has implications for other aspects of HSC biology and applications in re-generative medicine. The possibility that other adult tissue stem cells show similar heterogeneity and mechanisms of aging is discussed.
Introduction
Hematopoietic stem cells (HSCs) were the first adult stem cells used successfully to treat and cure patients. Therefore, HCSs are paradigmatic for regenerative medicine, the branch of medicine that seeks to harness the power of stem cells to repair damaged tissues. Extensive experimental analyses of HSCs were performed in the middle of the last century, and successful clinical HSC transplants were reported around the same time.1 With such a long history, one might assume that the biology of HSCs is well understood and their application as clinical tools is well standardized. Yet, the last years have shown that there are still some surprises in store that compel us to reevaluate what we think we know about HSCs. One of these surprises was the discovery that the HSC compartment consists of distinct and separable subsets of HSCs with largely preprogramed differentiation and self-renewal behaviors.
It has long been appreciated that the hematopoietic system is very heterogeneous, consisting of many different types of myeloid and lymphoid cells. Mature cells are generated through a complex series of differentiation steps where a committed HSC gives rise to progenitors, which produce precursors, which ultimately generate mature cells. It has now become apparent that HSCs themselves are heterogeneous. Data accumulated during the last decade have coalesced into the understanding that the adult HSC compartment consists of a limited number of distinct subsets of HSCs. Each subset has its own epigenetically fixed differentiation and self-renewal program.2-9 Thus, the hematopoietic system is not sustained by a single type of HSCs with flexible behavior. Rather, the HSC compartment consists of a mixture of several types of HSCs (Figure 1). Each subset of HSCs is distinguished by different self-renewal capacity,3 has distinct differentiation programs,2 and probably has different requirements for proliferation.5,7 These different types of HSCs coexist in adult BM, and each subset maintains its own stable differentiation and proliferation program. Consequently, any manipulations could affect different types of HSCs in different ways. This clearly has consequences for the understanding of the biology of HSCs. New concepts about the mechanisms that control HSC self-renewal and differentiation are needed.
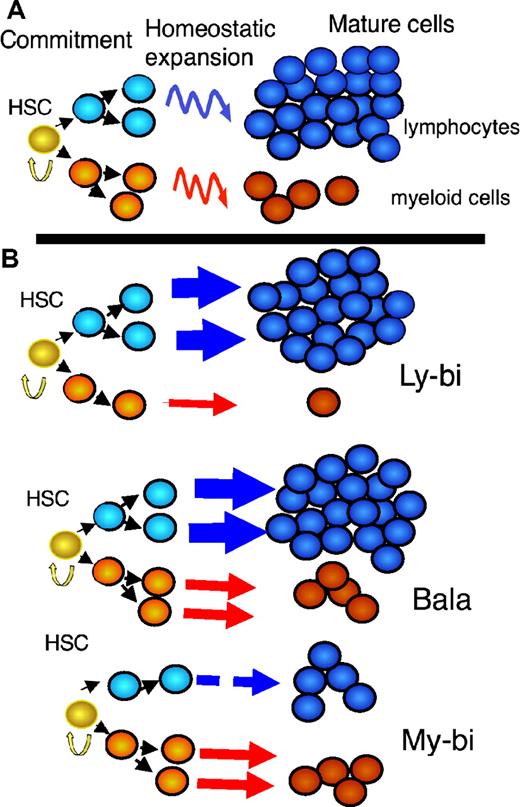
A new view of the hematopoietic differentiation tree. (A) The traditional model: A homogeneous population of HSCs drives the hematopoietic system. This implies that all HSCs in the population will react in the same way to identical extrinsic stimuli. On commitment, each HSC generates the same number of myeloid (red) and lymphoid (blue) progenitors. Homeostatic mechanisms (curved arrows) adjust myeloid and lymphoid cells to the typical ratios seen in blood. (B) The new model: Several populations of HSCs each with distinct, stable differentiation programs drive the hematopoietic system. The thickness of the arrows corresponds to the ability of the HSCs to generate progeny of the indicated type. Straight arrows indicate that the ratios of lymphoid to myeloid precursors are reflected in the periphery. My-bi HCSs produce few lymphocytes but standard levels of myeloid cells. The reverse is true for Ly-bi HSCs; they generate few myeloid cells but standard levels of lymphocytes. Bala HCSs generate more lymphocytes than myeloid cells (at least in mouse) and are called balanced because their output resembles the average output of mature cells from all HSC subsets together. All HSCs have self-renewal capacity (yellow arrow), and all HSCs give rise to all types of mature cells and thus are true multipotent stem cells.
A new view of the hematopoietic differentiation tree. (A) The traditional model: A homogeneous population of HSCs drives the hematopoietic system. This implies that all HSCs in the population will react in the same way to identical extrinsic stimuli. On commitment, each HSC generates the same number of myeloid (red) and lymphoid (blue) progenitors. Homeostatic mechanisms (curved arrows) adjust myeloid and lymphoid cells to the typical ratios seen in blood. (B) The new model: Several populations of HSCs each with distinct, stable differentiation programs drive the hematopoietic system. The thickness of the arrows corresponds to the ability of the HSCs to generate progeny of the indicated type. Straight arrows indicate that the ratios of lymphoid to myeloid precursors are reflected in the periphery. My-bi HCSs produce few lymphocytes but standard levels of myeloid cells. The reverse is true for Ly-bi HSCs; they generate few myeloid cells but standard levels of lymphocytes. Bala HCSs generate more lymphocytes than myeloid cells (at least in mouse) and are called balanced because their output resembles the average output of mature cells from all HSC subsets together. All HSCs have self-renewal capacity (yellow arrow), and all HSCs give rise to all types of mature cells and thus are true multipotent stem cells.
A brief history of stochastic, deterministic, and instructive models of HSC heterogeneity
How did we overlook for such a long time that adult HSCs are largely preprogrammed in their differentiation and self-renewal behavior? We contend that there are 2 major reasons: (1) Tedious and costly analyses on the clonal level are necessary to detect HSC heterogeneity; and (2) our thinking about HSC behavior is largely built on concepts and hypotheses developed to fit data from the analysis of myeloid progenitors. The elegant simplicity of these hypotheses has led to a resistance of the field to accept different, perhaps messier, insights into HSC biology.
As most adult stem cells, HSCs are defined by mutipotent differentiation and extensive self-renewal capacity. Traditionally, all HSCs were thought to be born with the same proliferation and differentiation capacity, resulting in a homogeneous population of cells.10 On commitment to differentiation, an HSC was thought to differentiate into one lymphoid and one myeloid restricted progenitor11 (Figure 1A). This was not consistent with the observed ratios of lymphoid to myeloid cells in blood and other hematopoietic organs. Therefore, homeostatic mechanisms acting on more mature cells (progeny of the HSCs) were invoked to explain how the ratio of myeloid to lymphoid cells transitioned from the earliest differentiation steps to the mature cell compartments. This was a simple and elegant model that was supported by several experimental observations. For example, it is well established that the size of mature cell populations can be regulated by homeostasis, diet, and disease.12,13 However, compelling evidence for the concept of HSC homogeneity was lacking. Unfortunately, this led to a very unproductive period in HSC research when contentious debates were fought about which methods enriched for the “real” HSC and the “homogeneity” of the resulting populations.
There were early signs that the old model of a homogeneous HSC was overly simplistic. CFU assays both in vivo and in vitro allowed the first glimpse into the heterogeneity of primitive hematopoietic cells. It is now clear that most cells that are detected by the CFU assays are not HSCs but rather myeloid-restricted progenitors and precursors. Nevertheless, data from these assays, particularly from the spleen CFU (CFU-s) assay, led to the formulation of important concepts that inform our thinking to this day.
CFU-s differ in size, in the time at which they can be detected after transplant, in the number of myeloid lineages per colony, and in how many secondary colonies are generated from primary colonies.10,14,15 Much effort has been exerted to reconcile the concept of a homogeneous population of HSCs/CFU-s with the experimentally observed heterogeneity. Till et al hypothesized that self-renewal rates of HSCs were controlled by stochastic mechanisms.14 Heterogeneity would then be generated because a HSC would randomly decide to differentiate or to self-renew. There is solid support for stochastic differentiation among myeloid progenitors,16 but support for stochastic HSC behavior comes mostly from in silico modeling.17-19 Accumulating data demonstrating programmed HSC behaviors2,20 required adjustments of such models toward more deterministic approaches.21
An alternative model to explain the generation of heterogeneity in immature cell populations was the Generation Age Hypothesis,15 which postulated that all HSCs are born with equal self-renewal and differentiation capacity. However, at each division, an HSC loses self-renewal capacity (referred to as primitiveness). Heterogeneity in the HSC/progenitor compartment is then generated by the number of divisions that each HSC had undergone. Although not explicitly stated, this hypothesis assumes that HSCs are predictable (deterministic) if it were possible to deduce the number of previous cell divisions.
A third, interrelated idea held that all stem cell decisions were instructed by extrinsic signals.22,23 According to this concept, HSCs would encounter different extrinsic signals, which then would instruct HSCs to acquire distinct characteristics. Inherent in this model is that HSC differentiation and self-renewal could be guided by extrinsic signals. This was an attractive idea because it promised that HSCs could be readily optimized for clinical applications. To some extent, that promise has materialized. For example, cytokine-mediated HSC mobilization has revolutionized HSC transplantation. However, there is little evidence that extrinsic signals instruct HSC differentiation or self-renewal in vivo. Rather, extrinsic signals appear to be permissive. An elegant example for this concept was found when increasing the number of HSC niches increased the number of HSCs.24,25 Unfortunately, the promise of harnessing HSC self-renewal for ex vivo expansion has not fully materialized. Although numerous conditions have been identified that can maintain HSCs or modestly expand them,26,27 so far it has been a challenge to achieve clinically meaningful HSC expansion ex vivo. However, a recent report that an aryl hydrocarbon receptor antagonists can significantly expand human HSCs offers fresh hope.28
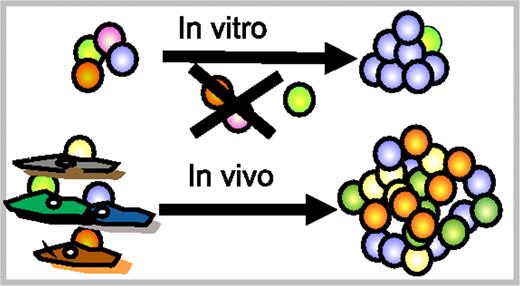
Perhaps a solution to the enigma of inefficient ex vivo HSC expansion lies in the heterogeneity of HSCs. All types of HSCs can self-renew in vivo, albeit to different extents. However, in vivo HSCs self-renew in specific niches that might provide different milieus. Indeed, the stromal cell compartment is heterogeneous in its ability to interact with HSCs.26 Different subsets of HSCs could well have distinct requirements for self-renewal, and conditions that expand one subset might be deleterious for other subsets of HSCs (Figure 2). There is evidence that different subsets of HSCs respond differently to the same cytokine. For example, very low doses of TGF-β stimulated myeloid-biased (My-bi) but suppressed lymphoid-biased (Ly-bi) HSC proliferation.5 Along the same line, culture in stem cell factor, IL-11, and fetal tyrosine kinase receptor ligand 2 selected for Ly-bi HSCs and depleted My-bi HSCs.7 Thus, we may have to define optimal conditions for each HSC subset to obtain optimal ex vivo expansion. This would also assure that a transplant would contain all types of HSCs necessary for a healthy hematopoietic system.
Different HSCs may have different requirements for survival/self-renewal. Top: All HSCs in a culture experience the same stimuli. These may expand a subset of HSCs (blue), leave another subset untouched (green), but could be detrimental for other (purple, orange) HSCs. The outcome is a modest expansion of a subset of HSCs. Bottom: In vivo, HSCs reside in niches that might provide selective stimuli to different HSC subsets. This leads to optimal self-renewal conditions for each subset of HSCs. Ex vivo HSC expansion could depend on finding distinct conditions for different HSC subsets.
Different HSCs may have different requirements for survival/self-renewal. Top: All HSCs in a culture experience the same stimuli. These may expand a subset of HSCs (blue), leave another subset untouched (green), but could be detrimental for other (purple, orange) HSCs. The outcome is a modest expansion of a subset of HSCs. Bottom: In vivo, HSCs reside in niches that might provide selective stimuli to different HSC subsets. This leads to optimal self-renewal conditions for each subset of HSCs. Ex vivo HSC expansion could depend on finding distinct conditions for different HSC subsets.
Limited heterogeneity of the HSC compartment
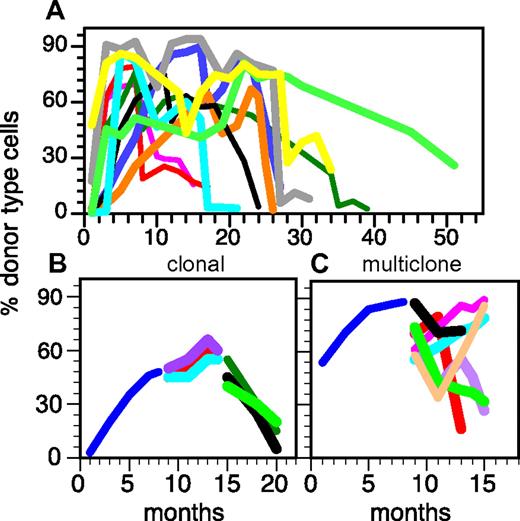
Direct data on HSC heterogeneity were obtained after transplants of limiting numbers of HSCs or of clonally marked HSCs into ablated hosts. The differentiation capacity of each HSC is read out by measuring donor-type cells in the periphery over time. Self-renewal capacity is revealed by the ability of the original HSC to create daughter HSCs that can repopulate secondary hosts. These experiments revealed extensive heterogeneity in the HSC compartment in all species tested.4,17,29-38 HSCs differed in self-renewal capacity, differentiation potential, how fast mature cells appeared in blood, how long they generated mature progeny, how many mature cells were generated, and what types of mature cells were produced.39 Together, these features control the rate and extent with which an HSC generates mature cells after transplantation (referred to as repopulation kinetics). Figure 3A exemplifies the heterogeneity in repopulation kinetics seen after transplants of limiting numbers of HSCs.4 When a large number of individual HSCs is examined, the repopulation kinetics appear to be random at first glance. However, a closer inspection reveals two surprisingly ordered features. First, a mathematical analysis showed that all HSCs can be grouped into maximally 16 of 54 possible subsets based on similarities of the repopulation kinetics (Figure 3B).4 The probability of finding new types of HSCs, not covered in the 16 groups, is exceedingly small. Thus, the heterogeneity of the HSC compartment is limited and can be traced back to a modest number of HSC subsets with distinct behaviors. Similar conclusions were drawn based on HSC behavior in cats17 and a smaller study in mice.40 These HSC subsets are stable throughout the life span of the organisms, although the number of HSCs in the different groups changes with age. The discrete subsets that are found in the adult HSC stem cell compartment are not consistent with hypotheses that predict the generation of diversity in the adult HSC compartment. Rather, the generation of HSC diversity must be initiated during the development of the hematopoietic system.
The heterogeneity of the HSC compartment derives from a limited number of distinct HSC classes. (A) HSC activity in several individual, clonally repopulated mice was followed for 7 months generating 3 segments for each repopulation kinetic. Shown are the percent donor type cells in blood measured at the indicated time points. (B) Distinct subsets of HSCs can be revealed when the repopulation kinetics are classified by symbolic analysis. Similarities between kinetics were quantified by Hamming distance.81 The squares represent the 54 possible HSC groups as defined by the 3 segments of the kinetics and a high (↑) versus low (↓) output of mature cells. Black squares represent the HSC groups actually found. The figures below the square are examples of the HSC subsets actually found. + indicates increase in mature cells over time; −, decrease; and ∼, no change. For details see Sieburg et al.4,81 This research was originally published in Blood.4
The heterogeneity of the HSC compartment derives from a limited number of distinct HSC classes. (A) HSC activity in several individual, clonally repopulated mice was followed for 7 months generating 3 segments for each repopulation kinetic. Shown are the percent donor type cells in blood measured at the indicated time points. (B) Distinct subsets of HSCs can be revealed when the repopulation kinetics are classified by symbolic analysis. Similarities between kinetics were quantified by Hamming distance.81 The squares represent the 54 possible HSC groups as defined by the 3 segments of the kinetics and a high (↑) versus low (↓) output of mature cells. Black squares represent the HSC groups actually found. The figures below the square are examples of the HSC subsets actually found. + indicates increase in mature cells over time; −, decrease; and ∼, no change. For details see Sieburg et al.4,81 This research was originally published in Blood.4
Finite life span of all HSCs
The second strikingly ordered feature shared by all HSCs is that each HSC clone at some point ceased generating mature cells (Figure 4A). An HSC clone is composed of all daughter HSCs and mature cells generated from a single mother HSC. Thus, the life span of a clone is determined by how many daughter HSCs a mother HSC can generate (self-renewal) and by the differentiation capacity of each HSC in the clone. The life spans of individual HSC clones differed markedly, ranging from a few months to almost 5 years (Figure 4A).41 Even HSC clones that were only transplanted once proceeded with the same finite, ballistically shaped kinetics through their life span as did HSC clones that were serially transplanted. This argues against the interpretation that limited life span could be an artifact of diluting HSCs during serial transplants.42
The heterogeneity of life spans in the HSC compartment is derived from HSC clones with preprogrammed self-renewal capacity. (A) Single donor type HSCs were transplanted into individual hosts. Daughter HSCs were serially transplanted into multiple secondary, tertiary and, if possible, quaternary host to follow the HSC clones over extended periods.3 All HSCs have a limited life span, although the life span varies. (B) Representation of the serial transplants of an individual HSC demonstrating the synchronicity of HSCs within a clone. Note the similarities of the repopulation kinetics in the secondary hosts and the simultaneous extinction of all daughter HSCs in all tertiary hosts. Additional examples can be found in Sieburg et al.41 The behavior of daughter HSC contrasts with the extensive heterogeneity of secondary repopulation kinetics after a multiclonal graft (C). This representation exemplifies a multiclonal graft (2 × 105 BM cells) originally injected into a single ablated host. Blue line represents donor type cells in blood after the primary graft; and different colored lines, donor type cells in blood in secondary and tertiary hosts. This research was originally published in Blood.3,4
The heterogeneity of life spans in the HSC compartment is derived from HSC clones with preprogrammed self-renewal capacity. (A) Single donor type HSCs were transplanted into individual hosts. Daughter HSCs were serially transplanted into multiple secondary, tertiary and, if possible, quaternary host to follow the HSC clones over extended periods.3 All HSCs have a limited life span, although the life span varies. (B) Representation of the serial transplants of an individual HSC demonstrating the synchronicity of HSCs within a clone. Note the similarities of the repopulation kinetics in the secondary hosts and the simultaneous extinction of all daughter HSCs in all tertiary hosts. Additional examples can be found in Sieburg et al.41 The behavior of daughter HSC contrasts with the extensive heterogeneity of secondary repopulation kinetics after a multiclonal graft (C). This representation exemplifies a multiclonal graft (2 × 105 BM cells) originally injected into a single ablated host. Blue line represents donor type cells in blood after the primary graft; and different colored lines, donor type cells in blood in secondary and tertiary hosts. This research was originally published in Blood.3,4
In striking contrast to the overall diversity of the HSC compartment, all daughter HSCs within a clone shared the same life span. Daughter HSCs (derived from the same mother HSC) proceeded with roughly the same kinetics of repopulation in serial transplants (Figure 4B). Thus, clonally derived daughter HSCs behaved very similar to each other, even when physically separated in different hosts. Because daughter HSCs in different hosts cannot communicate with each other, the number of HSCs in the environment cannot determine the self-renewal capacity and life span of an HSC clone. Rather, HSCs must have an intrinsic program that limits their life span. The program(s) that fix the life span, together with the memory of the life span of the mother and grandmother HSCs, is inherited by all daughter HSCs and, in turn, passed on to the granddaughters. These programs are so precise that the life span of individual HSC clones can be predicted with mathematical certainty.41 It is tempting to speculate that a limited life span is a hallmark of a normal HSC, whereas an unlimited life span characterizes a transformed HSC.
The mechanisms that synchronize HSCs and assure inheritance of their programs to daughter HSCs remain enigmatic. Similarly, what imbues different HSCs with different life spans is not well understood. Could HSCs with a short life span simply be derived from HSCs with long life spans as would be predicted by the Generation Age Hypothesis? A model where HSCs lose self-renewal with each division predicts a continuous distribution of HSC subsets. The discrete subsets that we identified in the HSC compartment are not consistent with this hypothesis. Could the environment, perhaps through functionally different niches, create HSCs with different life spans? Possibly, but the stability of the clonal programs in separate hosts argues strongly for HSC intrinsic mechanisms for the control of life span. Overall, the data argue that the adult HSC compartment does not create new diversity. Thus, clonal heterogeneity is probably generated in early development. What molecular mechanisms could control HSC life span? Numerous HSC-intrinsic and -extrinsic molecules have been identified that contribute to the self-renewal of HSCs.11 For example, several members of the homeobox (Hox) family of genes have been shown to expand HSCs intrinsically.43 Overexpression of Dickkopf in the environment inhibited intrinsic wnt signaling leading to HSC exhaustion.44 However, HSCs with different life spans are found in the same, inbred animal. Unless one assumes an unprecedented hypermutation process specifically in HSCs that does not affect the mature progeny, genetic mechanisms cannot account for differences in HSC life span. This points to epigenetic mechanisms for the generation and maintenance of HSC heterogeneity. Indeed, modulation of the epigenetic program of HSCs has major effects on their self-renewal and differentiation capacity.45-48
Programmed lineage potential of HSCs
Not only are the self-renewal capacities of HSCs programmed, but also their differentiation capacities. As touched on earlier, HSCs can be divided into 3 different subsets based on their differentiation potential. We defined these HSC subsets operationally by comparing the output of myeloid and lymphoid cells with that seen in an unmanipulated mouse.3 Although every HSC subset generates all of the cellular heterogeneity of the hematopoietic system, the ratios of myeloid to lymphoid cells differ measurably. Balanced (Bala) HSCs generate roughly the same ratio of lymphoid to myeloid cells as seen in the blood of an unmanipulated mouse. My-bi HSCs produce a lower ratio of lymphoid to myeloid cells and Ly-bi HSCs a higher ratio (Figure 1B) compared with Bala HSCs. All 3 subsets of HSCs have extensive self-renewal capacity, albeit to different extents.3 Because of their extensive ability to self-renew and their multipotent differentiation potential, the 3 subsets of HSCs are bona fide HSCs and together comprise the complete HSC compartment.
The first evidence suggesting the existence of lineage-biased HSCs came from the analysis of retrovirally marked HSCs. Jordan and Lemischka30 identified HSCs with a skewed differentiation program that was stably inherited to daughter HSCs. Subsequently, Sudo et al reported that the differentiation program of HSCs isolated as Lin−ckit+Sca-1+CD34− (LSKCD34−) from aged, but not from young, BM was skewed toward the myeloid lineage.49 These HSCs were named myeloid-dominant, as the authors thought that they represented a preleukemic, age-damaged population of HSCs. When we found HSCs with a myeloid skewed differentiation program in young mice, we called them myeloid-biased to emphasize that these are normal, healthy HSCs. Additional support for the existence of HSC subsets with myeloid-biased differentiation programs came from the analysis of HSCs that survive low-dose irradiation50 and of HSCs with a very long latency period after transplantation.8,51 Lastly, My-bi HSCs9 and population of cells that might represent Ly-bi HSC52 were found in human BM. Thus, the mouse studies accurately predicted human HSC biology. Subsequently, lineage-biased HSCs have received additional names, including α cells,7 and myeloid-predominant.8 Different criteria for classifying Bala HSCs have also been proposed.7
Despite the plethora of names, there is now a fairly strong consensus about the biology of lineage-biased HSCs. We and others3,5 showed that lineage skewing does not result from the overproduction of myeloid or lymphoid cells. Rather, My-bi HSCs produce normal levels of myeloid cells but have an attenuated ability to generate lymphocytes. This is evident on the level of the earliest precursors. My-bi HSCs produce few common lymphoid progenitors but normal levels of myeloid progenitors. Conversely, Ly-bi HSCs generate normal levels of lymphoid but only low levels of myeloid progenitors. A hallmark of My-bi, Ly-bi, and Bala HSCs is that their differentiation potential is stably inherited through many rounds of self-renewal. Thus, the differentiation program is epigenetically fixed on the level of the HSCs.
When a Ly-bi and a My-bi HSC compete directly, their lineage bias is exaggerated. For example, in a mixture of Ly-bi and My-bi HSCs, few My-bi HSC-derived lymphocytes are seen. Similarly, myeloid cells derived from the Ly-bi HSC are almost undetectable in the periphery.5,53 Thus, in competition, Ly-bi HSCs could mistakenly be classified as lymphoid-restricted progenitors and My-bi HSCs as myeloid-restricted progenitors. This might also explain startling observations in the human system. When virally marked BM cells were examined after gene therapy trials, strong lineage bias was observed. This was interpreted to mean that the hematopoietic system is driven by lineage-restricted progenitors rather than HSCs.52 However, the extensive self-renewal capacity and the ability to give rise to all mature cells set lineage-biased HSCs apart from progenitors.
It has now become possible to prospectively enrich the different HSC subsets: My-bi HSCs express higher levels of CD150 than Ly-bi HSCs.5,6,8 The ability to enrich the CD150-defined HSC subsets greatly facilitated the characterization of these rare cells. It became possible to examine genes differentially expressed between these HSC subsets, which in turn has led to the identification of distinct conditions for the activation of CD150+ and CD150− HCSs.5,6 Yet, the CD150 populations are by no means pure, and HSC phenotypes notoriously change when cells are manipulated.32,54 This seems to be also a concern for CD150 expression. For example, CD150+ cells regenerate both CD150+ and CD150− cells after transplantation. However, in functional assays, we have not seen My-bi HSCs turn into Ly-bi HSCs. Moreover, the CD150− population also contains very short-lived HSCs. Because lymphocytes have a longer life span than myeloid cells, the mature cells derived from a dying HSC appear to be artificially skewed toward the lymphoid lineage. The ability to self-renew and to repopulate a secondary host distinguishes graft failure from Ly-bi HSCs. Unfortunately, these criteria have not been consistently applied to the characterization of sorted cell populations. Hopefully, refined purification schemes will lead to better enrichments of the HSC subsets. This would make it possible to elucidate the epigenetic mechanisms that endow HSCs with their distinct, stable differentiation and self-renewal capacity.
HSC composition predicted by myeloid skewed blood
The HSC differentiation programs appear to have a more immediate effect on the composition of the mature cells in the periphery than previously thought (Figure 1B). This becomes very clear when the HSC subsets and mature cells are compared during development and aging. Young adult mouse blood contains approximately 80% lymphocytes and 20% myeloid cells. When mice age (> 16 months), their blood turns distinctively myeloid. This is seen in most mice of the strain DBA/2 (D2) and in some mice of the strain C57BL/6 (B6).53 The variability of age-related effects in B6 mice might be an artifact of the long life span of these mice. In our hands, even 22-month-old B6 are relatively youthful, whereas D2 mice of the same age are distinctly old. We found that the myeloid bias in blood correlates with the status of the HSC compartment. In both strains of mice, Ly-bi HSCs compose the majority of HSCs in the young. In both strains, Ly-bi HSCs are lost with aging and My-bi HSCs increase. However, on average, My-bi HSCs in aged B6 do not compose more than 40% of the HSC compartment, and the number of My-bi HSCs appears to be variable in different animals.55 This could explain why some, but not all, older B6 have myeloid biased blood. In contrast, the HSC compartment in aged D2 mice consists of almost 80% My-bi HSCs, and most animals show a corresponding increase of myeloid cells in blood. Thus, the composition of the HSC compartment is probably reflected by the composition of the mature cell compartment.
Aging of the HSC compartment
Whether HSCs age has long been debated. On one hand, at least some HSCs have sufficient self-renewal capacity to outlive the original host. On the other hand, HSCs isolated from aged individuals show many differences to HSCs isolated from young BM. HSCs isolated from young and aged donors have been reported to differ in cell surface phenotype, self-renewal, and differentiation capacity.56-59 These apparently opposing views were reconciled when we53 and later others showed that most of the “aged” behavior of HSCs could be fully explained by an accumulation of My-bi HSCs in the aged HSC compartment of both mice5,6 and humans.9 We directly compared My-bi HSCs isolated from young and aged mice and found them to be equivalent in self-renewal and differentiation potential.53 My-bi HSCs isolated from either young or aged sources show a delayed onset of repopulation and a differentiation program skewed toward the myeloid lineages. A cursory inspection of My-bi HSCs from young and aged sources did not reveal significant differences in life span. However, in a carefully executed study, Dykstra et al recently examined a very large number of CD150+ HSC clones and reported that CD150+ cells from aged mice have less self-renewal capacity and home less efficiently to BM than CD150+ cells isolated from young animals.55 This deficit can be attributed largely to HSCs with very low repopulation capacity and very low lymphoid output. These HSCs/progenitors exist in both young and aged environments but are enriched in aged mice.55 This raises 2 interesting possibilities: (1) These low repopulating HSCs/progenitors are aged HSCs. They are at the end of their life span but for unknown reasons persist for a long time as weak, myeloid-skewed cells. (2) The low repopulating HSCs are another class of HSCs that accumulates in the aged environment without having changed their properties. That would make the low repopulating cells a special subset of My-bi HSCs, albeit with distinct self-renewal and differentiation potentials. It remains to be tested whether these low repopulating HSCs are identical when isolated from young or aged donors. Overall, the existing data suggest that the aging of the organism has at best a modest effect on the behavior of individual HSCs. However, age does affect the composition of the HSC compartment because Ly-bi HSCs are lost. Thus, the overall function of the hematopoietic system is changed during aging.
We do not yet know why the composition of the HSC compartment shifts during organismal aging. At least 2 mechanism can be envisioned: (1) We postulated the concept of a lazy HSC, where My-bi HSCs have a slower turnover and therefore survive longer than other types of HSC.56 (2) My-bi HSCs might have a higher self-renewal capacity than other types of HSC, leading to clonal dominance at the end of the organism's life. In either case, one would expect a longer life span and a concurrent enrichment of My-bi HSC in the aged organism (Figure 5)
Developmental regulation of the HSC compartment. Each HSC has a limited life span, and the production of mature cells found in blood follows a ballistic curve from the start to the end of their life. Early in the life of the organism, Ly-bi (blue lines) are more frequent than My-bi (red) HSCs. On average, My-bi HSCs have a longer life span than other subsets of HSCs, leading to an accumulation of My-bi and a loss of Ly-bi HSCs as the organism ages. The bar above the graph shows how the HSC composition is reflected in the ratios of lymphoid to myeloid cells in blood. Because of the differences in self-renewal, My-bi HSCs will preferentially (but not exclusively) outlive the host as has been shown in serial transplants.3
Developmental regulation of the HSC compartment. Each HSC has a limited life span, and the production of mature cells found in blood follows a ballistic curve from the start to the end of their life. Early in the life of the organism, Ly-bi (blue lines) are more frequent than My-bi (red) HSCs. On average, My-bi HSCs have a longer life span than other subsets of HSCs, leading to an accumulation of My-bi and a loss of Ly-bi HSCs as the organism ages. The bar above the graph shows how the HSC composition is reflected in the ratios of lymphoid to myeloid cells in blood. Because of the differences in self-renewal, My-bi HSCs will preferentially (but not exclusively) outlive the host as has been shown in serial transplants.3
HSC “aging,” a paradigm for other adult stem cells?
There is evidence that the adult stem cells in other tissues are heterogeneous. Similarly, changes in the differentiation programs of stem cells in other tissues in young and aged organisms have been documented. Thus, it is tempting to speculate that aging in multiple organs is associated with a shift in the stem cell compartment rather than an aging of the stem cells itself. This would make stem cell heterogeneity a new paradigm for the understanding of tissue maintenance and aging. This would open venues to new approaches to prevent and repair age-related stem cell deficiencies. The following is a brief summary of evidence for stem cell heterogeneity in adult tissues.
Mesenchymal stem cells
Mesenchymal stem cells give rise to fibroblasts, skeletal muscle cells, bone, and cartilage. Mesenchymal stem cells from aged persons show decreased osteoblast and fibroblast development accompanied by increased adipogenesis.60 Maijenburg et al showed that the mesenchymal stem cell compartment can be dissected into 3 phenotypically distinct subpopulations.61 A population of CD271Bright mesenchymal stem cells with a strong potential for generating fibroblasts was found to be highly represented in fetal and young human BM but was depleted in elderly humans. Thus, the clonal composition of the mesenchymal stem cell compartment shifts during aging, and the altered representation of mesenchymal stem cells subsets led to a changed output of differentiated progeny. Thus, mesenchymal stem cells mirror HSC heterogeneity and aging.
Cardiac stem cells
The existence of cardiac stem cells is still debated.62,63 Yet, clonal marking studies identified a multipotent stem cell that can generate cardiomyocytes, fibroblasts, and endothelial cells.64 There is evidence that cardiac stem cells are active in the elderly albeit at a lower efficiency than in young humans.65 At least some populations of cardiac stem cells exist early in development but not in adults,66 indicating that cardiac stem cells are heterogeneous and that the composition of the stem cell compartment is developmentally regulated.
Neural stem cells
Neural stem cells are multipotent and self-renewing cells, which can give rise to all the different subtypes of glia and neurons.67,68 Although neurogenesis persists throughout adulthood, the rate at which new neurons are generated dramatically declines as the animal becomes older. Neural stem cells in aged mice tend to give rise to more astrocytes than neurons compared with their younger counterparts.69 This is reminiscent of the My-bi HSC in the hematopoietic system. However, whether neural stem cells are composed of different subsets, and whether the composition of such putative subsets changes with aging awaits clonal analysis. This can now be addressed because beautiful clonal approaches are possible in the brain.70
Speculations about implications for regenerative medicine
HSCs have a long track record in regenerative medicine. The identification of distinct subsets of HSCs suggests that one could selectively expand one or the other subset to suit different applications. One could envision that myelopenia would be selectively treated by the infusion of My-bi HSCs. Because My-bi HSCs tend to generate mature cells slowly,3 these HSCs could be activated, perhaps by short-term exposure to low levels of TGF-β.5 Infusion of My-bi HSCs will lead to durable production of myeloid cells, but one would expect a lower probability of GVHD because of the low levels of lymphocytes produced. Conversely, for the treatment of lymphopenia, transplants of Ly-bi HSCs might be considered. Ly-bi HSCs would rapidly provide lymphocytes and some myeloid cells. The short life span of most Ly-bi HSCs would limit the potential for long-term side effects. Analogous approaches could be envisioned for other types of tissue specific stem cells.
Another area where Ly-bi HSCs could be useful might be to ameliorate age-related immune defects. Ly-bi HSCs are lost in the aged HSC compartment in both mice and humans,9,53 and their loss probably contributes to the attenuated immunity found in many elderly. The sluggish immune response in the aged results in decreased immune surveillance and a corresponding increase in cancers. Similarly, the elderly are more susceptible to infectious diseases, such as influenza, compared with young populations.71 At the same time, vaccination is less effective in the aged than in the young. Thus, the attenuated immune system contributes to morbidity and mortality in the aged. Previous attempts to revive the immune system in the aged have focused on stimulating precursors and mature cells.72-74 This has met with limited success. Restoring youthful Ly-bi HSCs is likely to rejuvenate the immune system in the elderly. Currently, it is difficult to foresee HSC transplants as a feasible treatment of age-related immune defects. However, if we would better understand the biology of the different subsets of HSCs, it could be possible to delay or prevent the loss of youthful HSCs in situ.
Embryonic or induced
Whereas HSCs are accessible, other adult tissue stem cells are more difficult to procure. The promise of regenerative medicine is that embryonic stem (ES) cells or induced pluripotent stem (iPS) cells can be sources for all types of tissue stem cells. A holdup in the application of these pluripotent stem cells derives from difficulties to differentiate them into equivalents of adult tissue stem cells that engraft damaged tissues.75 The difficulties and surprises in generating adult stem cells from ES cells are cogently exemplified by the attempts to generate HSCs. Although conditions have been defined to derive myeloid cells from ES and iPS cells,76,77 generating lymphoid potential has been challenging. Engrafting, multipotent HSCs can be generated from ES cells after overexpression of HoxB4 or Cdx4.78 However, the differentiation potential of even these ES cell-derived HSCs was heavily skewed toward the myeloid lineage in vivo. Even more pronounced myeloid skewing has been observed from HSCs derived from human ES or iPS cells.79,80 It is tempting to speculate that ES cells give rise predominantly to a primitive form of My-bi HSCs. If most adult stem cell compartments are also heterogeneous, is it possible that the ES/iPS cultures preferentially generate certain stem cell subsets and not others? Could we improve the efficacy of stem cell replacement therapy by tweaking the in vitro conditions to support the generation of more complete stem cell populations? Hopefully, we will eventually understand the in vivo conditions that lead to the emergence of stem cell subsets during development. These insights then could be translated into improved methods for generating adult stem cells.
Acknowledgments
The authors thank Dr Ellen Rothenberg (California Institute of Technology) for suggesting “biased” to name myeloid-biased and lymphoid-biased HSCs.
This work was supported by the National Institutes of Health (grants DDK48015 and AG023197). J.M.B. was the recipient of a California Institute for Regenerative Medicine (CIRM) Bridges award.
National Institutes of Health
Authorship
Contribution: All authors wrote parts of the manuscript and edited the whole manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christa E. Muller-Sieburg, Sanford-Burnham Institute for Medical Research, 10901 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: cmuller@burnham.org.