Abstract
Approximately one-third of patients with an indication for hematopoietic cell transplantation (HCT) have an HLA-matched related donor (MRD) available to them. For the remaining patients, a matched unrelated donor (MUD) is an alternative. Prior studies comparing MRD and MUD HCT provide conflicting results, and the relative efficacy of MRD and MUD transplantation is an area of active investigation. To address this issue, we analyzed outcomes of 2223 adult acute myelogenous leukemia patients who underwent allogeneic HCT between 2002 and 2006 (MRD, n = 624; 8/8 HLA locus matched MUD, n = 1193; 7/8 MUD, n = 406). The 100-day cumulative incidence of grades B-D acute GVHD was significantly lower in MRD HCT recipients than in 8/8 MUD and 7/8 MUD HCT recipients (33%, 51%, and 53%, respectively; P < .001). In multivariate analysis, 8/8 MUD HCT recipients had a similar survival rate compared with MRD HCT recipients (relative risk [RR], 1.03; P = .62). 7/8 MUD HCT recipients had higher early mortality than MRD HCT recipients (RR, 1.40; P < .001), but beyond 6 months after HCT, their survival rates were similar (RR, 0.88; P = .30). These results suggest that transplantation from MUD and MRD donors results in similar survival times for patients with acute myelogenous leukemia.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 4093.
Disclosures
The authors, the Associate Editor Martin S. Tallman, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare outcomes of graft-versus-host disease (GVHD) in adult AML patients who underwent allogeneic HCT with matched related donors (MRD), 8/8 HLA locus matched unrelated donor (MUD), and 7/8 HLA locus MUD, based on a cohort study using the CIBMTR database.
Compare survival outcomes in adult AML patients who underwent allogeneic HCT with MRD, 8/8 HLA locus matched MUD, and 7/8 MUD, based on a cohort study using the CIBMTR database.
Compare relapse rates and leukemia-free survival outcomes of GVHD in adult AML patients who underwent allogeneic HCT with MRD, 8/8 HLA locus matched MUD, and 7/8 MUD, based on a cohort study using the CIBMTR database.
Release date: April 26, 2012; Expiration date: April 26, 2013
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a life-saving treatment for many patients with hematologic malignancies. Ideally, HCT is performed using cells collected from an HLA-identical sibling. The probability of any single sibling being HLA-matched is 25% and, given the average family size in North America, approximately one-third of patients will have a matched related donor (MRD). For the remaining two-thirds of patients, approximately half will have an HLA-matched unrelated donor (MUD).1,2 Understanding how transplantation outcomes are influenced by donor source is a critical component of the therapeutic decision-making process.
Many advances in MUD HCT have occurred over the past 20 years.3 We now know that high-resolution HLA typing and matching are critical for success.4 Peripheral blood stem cells (PBSCs) have largely replaced BM as the most utilized graft source in North America.3 Whether PBSCs are preferable is currently the subject of a large-scale, multicenter, phase 3 clinical trial that has completed enrollment, with initial results presented in December 2011 (BMT CTN Study 0201).5 In addition to these trends, the age of MUD HCT recipients has increased dramatically, primarily because of the introduction of reduced-intensity conditioning (RIC) regimens. The median recipient age was 39 years in 1996-1998, with fewer than 1% of patients older than 60 years versus a median age of 47 years in 2003-2006, with 12% older than 60 years.3 Most importantly, survival rates have improved significantly. Two-year survival after MUD HCT for adults with acute myelogenous leukemia (AML) was approximately 25% in 1987-1998, but was 40% in 2003-2006 (P < .0001).3 Corresponding survival rates for adults with acute lymphoblastic leukemia were 23% and 40% (P < .0001), respectively.3
Despite these advances, some health care decision makers have concerns regarding MUD HCT as a viable treatment option. In 2010, the state of Arizona denied adults (≥ 21 years of age) Medicaid coverage for MUD HCT for any indication.6 After further review, this decision was reversed in April 2011.7 However, the passage and subsequent reversal of this legislation highlight the current uncertainty regarding the relative risks and benefits of MUD HCT and the potential for faulty decision making without sufficient comparative effectiveness data. At the time of the initial Arizona decision, the available studies comparing outcomes of MRD and MUD yielded conflicting results, with some reporting inferior survival or disease-free survival with MUD HCT,8-16 and others reporting similar survival rates.17-25 In the setting of RIC HCT, because of the theoretical potential for increased GVL effects with MUD transplantation compared with MRD, a few experts actually advocated preferentially for MUDs over MRDs.26
Because the success of MUD HCT is significantly influenced by the degree of high-resolution HLA matching,4 and because advances in supportive care influence outcomes,3 a comparison of the safety and efficacy of MUD versus MRD HCT in a recently treated cohort of patients is important to best inform decision-making. Because of biologic differences that might affect this comparison, these comparative studies are best done in a disease-specific manner. The present study is a comparative effectiveness cohort study using the Center for International Blood & Marrow Transplant Research (CIBMTR) database to compare outcomes after MRD HCT versus 8/8 HLA-matched MUD HCT (high-resolution matched at HLA-A, HLA-B, HLA-C, and HLA-DRB1) and 7/8 HLA-matched MUD HCT for the most common indication for allogeneic HCT in adults: AML.
Methods
Data source
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program comprising a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR's capacity as a public health authority under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) privacy rule. Additional details regarding the data source are described elsewhere.27 All subjects whose data were included in this study provided institutional review board–approved consent in accordance with the Declaration of Helsinki to participate in the CIBMTR research database and to have their data included in observational research studies.
Patient selection
The patient population consisted of adult patients (≥ 21 years of age) with AML undergoing allogeneic HCT in the United States between 2002 and 2006 who had comprehensive data reported to the CIBMTR. A total of 2223 patients fulfilling the inclusion criteria were identified; of these, 624 received MRD transplantations, 1193 received 8/8 HLA-matched MUD transplantations, and 406 received 7/8 HLA-matched MUD transplantations. Patients whose grafts were depleted of T cells ex vivo were excluded. Patients with M3 AML, patients receiving cord blood transplantations, patients receiving HLA-mismatched or nonsibling-related donor transplantations, and patients receiving identical twin transplantations were also excluded.
Study end points and definitions
The primary outcome studied was survival. Patients were considered to have an event at time of death from any cause; survivors were censored at last contact. Relapse was defined by hematologic criteria, and transplantation-related mortality (TRM) was considered a competing event. TRM was defined as death without evidence of leukemia recurrence; relapse was considered a competing event. Leukemia-free survival (LFS) was defined as the time to treatment failure (death or relapse). For relapse, TRM, and LFS, patients alive in continuous complete remission were censored at last follow-up. Time to engraftment was calculated as the time from transplantation to achieving the first of 3 consecutive days with an absolute neutrophil count > 500/mm3. Acute GVHD was graded using the IBMTR Severity Index.28 Chronic GVHD was diagnosed by standard criteria.29 For engraftment and GVHD, death without the event was considered a competing event. Cytogenetic abnormalities were classified according to the Southwest Oncology Group/Eastern Cooperative Oncology Group cytogenetic classification system.30
Statistical analysis
All end points were assessed through 3 years after HCT, with excellent follow-up data available to that time point. TRM, relapse, engraftment, acute GVHD, and chronic GVHD were estimated as cumulative incidences, taking into account competing risks. Probabilities of survival and LFS were calculated using the Kaplan-Meier estimator with variance estimated by the Greenwood formula. Survival curves were compared using the log-rank test. Multivariate analyses were conducted to identify and adjust for independent predictors of TRM, relapse, LFS, and survival other than donor type. The proportional hazards model was built by forcing the main effect variable (MRD vs 8/8 MUD vs 7/8 MUD using HLA-identical siblings as the reference group) into the model. Backward elimination with a criterion of P < .05 for retention was used to select a final model. The following variables were analyzed for their prognostic value on each of the outcomes: patient characteristics (age, sex, race, and Karnofsky performance score [KPS]), disease characteristics (WBC count at diagnosis, extramedullary disease presentation, therapy-related AML, AML arising from antecedent myelodysplastic syndrome (MDS), cytogenetics at diagnosis, and disease status at transplantation), and transplantation-related factors (donor age, donor-recipient sex match, donor-recipient CMV serology, conditioning regimen intensity, stem cell source, GVHD prophylaxis regimen, and use of antithymocyte globulin). The proportional hazards assumption was assessed for each variable using a time-dependent covariate approach. For survival, the 7/8 MUD group had a time-varying effect that violated the proportionality assumption. Therefore, the comparison for this group was divided into early (< 6 months) and late (> 6 months) posttransplantation time periods. The 6-month cut point was selected using a maximum likelihood method to identify the optimal cut point; using this cut point, the proportionality assumption held. Two-way interactions were checked between each selected variable and the main effect variable and no significant interactions were detected. Adjusted 3-year LFS and survival probabilities were estimated through the direct adjusted survival curves estimation method.31 SAS Version 9.1 software (SAS Institute) was used in all analyses.
Results
Patients
Baseline characteristics of the population are summarized in Table 1. Median follow-up times for surviving patients were 57, 42, and 45 months for the MRD, 8/8 MUD, and 7/8 MUD donor groups, respectively. MUD recipients were younger than MRD recipients. MUD donors were also younger than MRD donors. Most patients in all 3 groups were white, but the MRD group had a higher proportion of nonwhite recipients. MUD recipients were more likely to be female. A higher proportion of MUD recipients had poor-risk cytogenetics and they were more likely to have advanced disease status at transplantation. MUD transplantation was more likely than MRD transplantation to be done with RIC regimen but less likely to use PBSC grafts. Unrelated donors were more likely to be male and more likely to be CMV-negative than related donors. Higher proportions of MUD recipients received tacrolimus-based GVHD prophylaxis and antithymocyte globulin.
Baseline characteristics of 2223 AML patients who underwent allogeneic HCT from 2002-2006
| Variable . | MRD . | 8/8 MUD . | 7/8 MUD . | P* . |
|---|---|---|---|---|
| Size, n | 624 | 1193 | 406 | |
| Centers, n | 62 | 99 | 84 | |
| Median age, y (range) | 52 (21-76) | 51 (21-75) | 48 (21-75) | < .001‡ |
| Age group, y, n (%) | < .001 | |||
| 20-29 | 54 (9) | 146 (12) | 67 (17) | |
| 30-39 | 74 (12) | 142 (12) | 70 (17) | |
| 40-49 | 165 (26) | 308 (26) | 93 (23) | |
| 50-59 | 229 (37) | 372 (31) | 113 (28) | |
| ≥ 60 | 102 (16) | 225 (19) | 63 (16) | |
| Sex, n (%) | .04 | |||
| Male | 356 (57) | 618 (52) | 226 (56) | |
| Female | 265 (42) | 574 (48) | 180 (44) | |
| Missing | 3 (< 1) | 1 (< 1) | 0 | |
| KPS, n (%) | < .001 | |||
| ≥ 90 | 382 (61) | 700 (59) | 231 (57) | |
| < 90 | 214 (34) | 336 (28) | 129 (32) | |
| Missing | 28 (4) | 157 (13) | 46 (11) | |
| Race, n (%) | < .001 | |||
| White | 528 (85) | 1134 (95) | 359 (88) | |
| Black | 38 (6) | 21 (2) | 27 (7) | |
| Asian | 18 (3) | 12 (1) | 5 (1) | |
| Other—American Indian/Native Hawaiian | 23 (4) | 6 (1) | 4 (1) | |
| Missing | 17 (3) | 20 (2) | 11 (3) | |
| AML disease status at transplantation, n (%) | < .001 | |||
| Primary induction failure | 91 (15) | 204 (17) | 65 (16) | |
| CR1 | 337 (54) | 547 (46) | 150 (37) | |
| CR2 | 97 (16) | 261 (22) | 109 (27) | |
| First relapse | 80 (13) | 173 (15) | 79 (19) | |
| Missing | 19 (3) | 8 (1) | 3 (1) | |
| Cytogenetics, n (%) | < .001 | |||
| Unknown | 29 (5) | 75 (6) | 31 (8) | |
| Normal | 288 (46) | 403 (34) | 127 (31) | |
| Good | 34 (5) | 80 (7) | 27 (7) | |
| Intermediate | 88 (14) | 134 (11) | 56 (14) | |
| Poor | 158 (25) | 394 (33) | 135 (33) | |
| Tested–not evaluated metaphases | 10 (2) | 46 (4) | 7 (2) | |
| Not tested | 17 (3) | 61 (5) | 23 (6) | |
| WBC count at diagnosis, n (%) | .30 | |||
| < 7.6 × 109/L | 258 (41) | 550 (46) | 180 (44) | |
| > 7.6 × 109/L | 296 (47) | 515 (43) | 175 (43) | |
| Missing | 70 (11) | 128 (11) | 51 (13) | |
| Therapy-related AML, n (%) | < .001 | |||
| No | 572 (92) | 1121 (94) | 378 (93) | |
| Yes | 35 (6) | 72 (6) | 25 (6) | |
| Missing | 17 (3) | 0 | 3 (1) | |
| Preexisting MDS, n (%) | < .001 | |||
| No | 426 (68) | 879 (74) | 309 (76) | |
| Yes | 130 (21) | 313 (26) | 95 (23) | |
| Missing | 68 (11) | 1 (< 1) | 2 (< 1) | |
| Extramedullary disease, n (%) | < .001 | |||
| No | 570 (91) | 1122 (94) | 387 (95) | |
| Yes | 39 (6) | 68 (6) | 16 (4) | |
| Missing | 15 (2) | 3 (< 1) | 3 (1) | |
| Median interval between diagnosis and transplantation, mo (range) | 5 (1-142) | 6 (1-133) | 7 (1-114) | |
| Median interval from CR1 to transplantation for patients transplanted in CR1, mo (range) | 3 (1-25) | 3 (1-24) | 4 (1-14) | |
| Median CR1 duration (only patients transplanted in CR2), mo (range) | 10 (1-53) | 11 (1-110) | 11 (1-100) | |
| Median donor age, y (range) | 50 (7-85) | 34 (19-61) | 37 (19-60) | < .001‡ |
| Donor age group, y, n (%) | < .001 | |||
| < 20 | 16 (3) | 14 (1) | 2 (< 1) | |
| 20-29 | 35 (6) | 406 (34) | 92 (23) | |
| 30-39 | 75 (12) | 427 (36) | 158 (39) | |
| 40-49 | 180 (29) | 273 (23) | 118 (29) | |
| 50-59 | 180 (29) | 71 (6) | 35 (9) | |
| > 60 | 131 (21) | 2 (< 1) | 0 | |
| Missing | 7 (1) | 0 | 1 (< 1) | |
| Sex match, n (%) | < .001 | |||
| M/M | 200 (32) | 436 (37) | 146 (36) | |
| M/F | 132 (21) | 369 (31) | 99 (24) | |
| F/M | 155 (25) | 182 (15) | 80 (20) | |
| F/F | 132 (21) | 205 (17) | 81 (20) | |
| Missing | 5 (1) | 1 (< 1) | 0 | |
| CMV match, n (%) | < .001 | |||
| +/+ | 258 (41) | 202 (17) | 96 (24) | |
| +/− | 71 (11) | 86 (7) | 37 (9) | |
| −/+ | 146 (23) | 499 (42) | 136 (33) | |
| −/− | 130 (21) | 325 (27) | 104 (26) | |
| Not tested/inconclusive | 19 (3) | 81 (7) | 33 (8) | |
| Conditioning regimen intensity, n (%) | .003 | |||
| Traditional ablative | 335 (54) | 547 (46) | 220 (54) | |
| Reduced intensity | 135 (22) | 336 (28) | 104 (26) | |
| Nonmyeloablative | 74 (12) | 148 (12) | 36 (9) | |
| Nontraditional ablative† | 80 (13) | 162 (14) | 45 (11) | |
| Missing | 0 | 0 | 1 (< 1) | |
| Stem cell source, n (%) | < .001 | |||
| BM | 48 (8) | 328 (27) | 105 (26) | |
| Peripheral blood | 576 (92) | 865 (73) | 301 (74) | |
| GVHD prophylaxis, n (%) | < .001 | |||
| Missing | 3 (< 1) | 0 | 0 | |
| None | 14 (2) | 8 (1) | 4 (1) | |
| FK506 + MTX + other | 241 (39) | 578 (48) | 191 (47) | |
| FK506 + other | 104 (17) | 249 (21) | 79 (19) | |
| CsA + MTX + other | 124 (20) | 192 (16) | 87 (21) | |
| CsA + other | 125 (20) | 149 (12) | 39 (10) | |
| Other | 13 (2) | 17 (1) | 6 (1) | |
| Antithymocyte globulin, n (%) | < .001 | |||
| No | 565 (91) | 894 (75) | 306 (75) | |
| Yes | 59 (9) | 299 (25) | 100 (25) | |
| Year of transplantation, n (%) | ||||
| 2002 | 99 (16) | 124 (10) | 38 (9) | |
| 2003 | 89 (14) | 166 (14) | 69 (17) | |
| 2004 | 141 (23) | 275 (23) | 89 (22) | |
| 2005 | 157 (25) | 295 (25) | 106 (26) | |
| 2006 | 138 (22) | 333 (28) | 104 (26) | |
| Median follow-up of survivors, mo (range) | 57 (2-97) | 42 (6-89) | 45 (3-85) | |
| Total deaths, n | 397 | 773 | 275 |
| Variable . | MRD . | 8/8 MUD . | 7/8 MUD . | P* . |
|---|---|---|---|---|
| Size, n | 624 | 1193 | 406 | |
| Centers, n | 62 | 99 | 84 | |
| Median age, y (range) | 52 (21-76) | 51 (21-75) | 48 (21-75) | < .001‡ |
| Age group, y, n (%) | < .001 | |||
| 20-29 | 54 (9) | 146 (12) | 67 (17) | |
| 30-39 | 74 (12) | 142 (12) | 70 (17) | |
| 40-49 | 165 (26) | 308 (26) | 93 (23) | |
| 50-59 | 229 (37) | 372 (31) | 113 (28) | |
| ≥ 60 | 102 (16) | 225 (19) | 63 (16) | |
| Sex, n (%) | .04 | |||
| Male | 356 (57) | 618 (52) | 226 (56) | |
| Female | 265 (42) | 574 (48) | 180 (44) | |
| Missing | 3 (< 1) | 1 (< 1) | 0 | |
| KPS, n (%) | < .001 | |||
| ≥ 90 | 382 (61) | 700 (59) | 231 (57) | |
| < 90 | 214 (34) | 336 (28) | 129 (32) | |
| Missing | 28 (4) | 157 (13) | 46 (11) | |
| Race, n (%) | < .001 | |||
| White | 528 (85) | 1134 (95) | 359 (88) | |
| Black | 38 (6) | 21 (2) | 27 (7) | |
| Asian | 18 (3) | 12 (1) | 5 (1) | |
| Other—American Indian/Native Hawaiian | 23 (4) | 6 (1) | 4 (1) | |
| Missing | 17 (3) | 20 (2) | 11 (3) | |
| AML disease status at transplantation, n (%) | < .001 | |||
| Primary induction failure | 91 (15) | 204 (17) | 65 (16) | |
| CR1 | 337 (54) | 547 (46) | 150 (37) | |
| CR2 | 97 (16) | 261 (22) | 109 (27) | |
| First relapse | 80 (13) | 173 (15) | 79 (19) | |
| Missing | 19 (3) | 8 (1) | 3 (1) | |
| Cytogenetics, n (%) | < .001 | |||
| Unknown | 29 (5) | 75 (6) | 31 (8) | |
| Normal | 288 (46) | 403 (34) | 127 (31) | |
| Good | 34 (5) | 80 (7) | 27 (7) | |
| Intermediate | 88 (14) | 134 (11) | 56 (14) | |
| Poor | 158 (25) | 394 (33) | 135 (33) | |
| Tested–not evaluated metaphases | 10 (2) | 46 (4) | 7 (2) | |
| Not tested | 17 (3) | 61 (5) | 23 (6) | |
| WBC count at diagnosis, n (%) | .30 | |||
| < 7.6 × 109/L | 258 (41) | 550 (46) | 180 (44) | |
| > 7.6 × 109/L | 296 (47) | 515 (43) | 175 (43) | |
| Missing | 70 (11) | 128 (11) | 51 (13) | |
| Therapy-related AML, n (%) | < .001 | |||
| No | 572 (92) | 1121 (94) | 378 (93) | |
| Yes | 35 (6) | 72 (6) | 25 (6) | |
| Missing | 17 (3) | 0 | 3 (1) | |
| Preexisting MDS, n (%) | < .001 | |||
| No | 426 (68) | 879 (74) | 309 (76) | |
| Yes | 130 (21) | 313 (26) | 95 (23) | |
| Missing | 68 (11) | 1 (< 1) | 2 (< 1) | |
| Extramedullary disease, n (%) | < .001 | |||
| No | 570 (91) | 1122 (94) | 387 (95) | |
| Yes | 39 (6) | 68 (6) | 16 (4) | |
| Missing | 15 (2) | 3 (< 1) | 3 (1) | |
| Median interval between diagnosis and transplantation, mo (range) | 5 (1-142) | 6 (1-133) | 7 (1-114) | |
| Median interval from CR1 to transplantation for patients transplanted in CR1, mo (range) | 3 (1-25) | 3 (1-24) | 4 (1-14) | |
| Median CR1 duration (only patients transplanted in CR2), mo (range) | 10 (1-53) | 11 (1-110) | 11 (1-100) | |
| Median donor age, y (range) | 50 (7-85) | 34 (19-61) | 37 (19-60) | < .001‡ |
| Donor age group, y, n (%) | < .001 | |||
| < 20 | 16 (3) | 14 (1) | 2 (< 1) | |
| 20-29 | 35 (6) | 406 (34) | 92 (23) | |
| 30-39 | 75 (12) | 427 (36) | 158 (39) | |
| 40-49 | 180 (29) | 273 (23) | 118 (29) | |
| 50-59 | 180 (29) | 71 (6) | 35 (9) | |
| > 60 | 131 (21) | 2 (< 1) | 0 | |
| Missing | 7 (1) | 0 | 1 (< 1) | |
| Sex match, n (%) | < .001 | |||
| M/M | 200 (32) | 436 (37) | 146 (36) | |
| M/F | 132 (21) | 369 (31) | 99 (24) | |
| F/M | 155 (25) | 182 (15) | 80 (20) | |
| F/F | 132 (21) | 205 (17) | 81 (20) | |
| Missing | 5 (1) | 1 (< 1) | 0 | |
| CMV match, n (%) | < .001 | |||
| +/+ | 258 (41) | 202 (17) | 96 (24) | |
| +/− | 71 (11) | 86 (7) | 37 (9) | |
| −/+ | 146 (23) | 499 (42) | 136 (33) | |
| −/− | 130 (21) | 325 (27) | 104 (26) | |
| Not tested/inconclusive | 19 (3) | 81 (7) | 33 (8) | |
| Conditioning regimen intensity, n (%) | .003 | |||
| Traditional ablative | 335 (54) | 547 (46) | 220 (54) | |
| Reduced intensity | 135 (22) | 336 (28) | 104 (26) | |
| Nonmyeloablative | 74 (12) | 148 (12) | 36 (9) | |
| Nontraditional ablative† | 80 (13) | 162 (14) | 45 (11) | |
| Missing | 0 | 0 | 1 (< 1) | |
| Stem cell source, n (%) | < .001 | |||
| BM | 48 (8) | 328 (27) | 105 (26) | |
| Peripheral blood | 576 (92) | 865 (73) | 301 (74) | |
| GVHD prophylaxis, n (%) | < .001 | |||
| Missing | 3 (< 1) | 0 | 0 | |
| None | 14 (2) | 8 (1) | 4 (1) | |
| FK506 + MTX + other | 241 (39) | 578 (48) | 191 (47) | |
| FK506 + other | 104 (17) | 249 (21) | 79 (19) | |
| CsA + MTX + other | 124 (20) | 192 (16) | 87 (21) | |
| CsA + other | 125 (20) | 149 (12) | 39 (10) | |
| Other | 13 (2) | 17 (1) | 6 (1) | |
| Antithymocyte globulin, n (%) | < .001 | |||
| No | 565 (91) | 894 (75) | 306 (75) | |
| Yes | 59 (9) | 299 (25) | 100 (25) | |
| Year of transplantation, n (%) | ||||
| 2002 | 99 (16) | 124 (10) | 38 (9) | |
| 2003 | 89 (14) | 166 (14) | 69 (17) | |
| 2004 | 141 (23) | 275 (23) | 89 (22) | |
| 2005 | 157 (25) | 295 (25) | 106 (26) | |
| 2006 | 138 (22) | 333 (28) | 104 (26) | |
| Median follow-up of survivors, mo (range) | 57 (2-97) | 42 (6-89) | 45 (3-85) | |
| Total deaths, n | 397 | 773 | 275 |
CR1 indicates first complete remission; CR2, second complete remission; FK506, tacrolimus; MTX, methotrexate; and CsA, cyclosporin.
By χ2.
Unfractionated total body irradiation (TBI) > 5 Gy or fractionated TBI > 8 Gy; melphalan > 150 mg/m2; busulfan + melphalan; busulfan > 9 mg/kg.
Wilcoxon test.
GVHD and engraftment
At day 100, the cumulative incidences of engraftment were 95% or greater in all 3 groups (Table 2). At day 100, the cumulative incidences of grades B-D and grades C-D acute GVHD were higher with recipients of MUD transplantation (including 7/8 and 8/8) than with MRD transplantation (Table 2). At 1 year after transplantation, there was a higher cumulative incidence of chronic GVHD in the MUD groups, but by 3 years after transplantation, the cumulative incidences of chronic GVHD did not differ significantly among the groups (Table 2).
Univariate analysis of transplantation outcomes by donor type
| . | n . | MRD probability, % (95% CI) . | n . | 8/8 MUD probability, % (95% CI) . | n . | 7/8 MUD probability, % (95% CI) . | P* . | 8/8 MUD vs MRD, P† . | 7/8 MUD vs MRD, P† . | 7/8 MUD vs 8/8 MUD, P† . |
|---|---|---|---|---|---|---|---|---|---|---|
| ANC recovery | 621 | 1192 | 406 | |||||||
| @ 28 d | 96 (94-97) | 93 (92-95) | 92 (89-95) | .01 | .008 | .02 | .59 | |||
| @ 100 d | 97 (96-99) | 96 (94-97) | 95 (93-97) | .03 | .02 | .04 | .65 | |||
| Platelet recovery | 615 | 1183 | 398 | |||||||
| @ 60 d | 87 (85-90) | 83 (81-86) | 81 (78-85) | .02 | .02 | .01 | .40 | |||
| @ 100 d | 89 (86-91) | 85 (83-87) | 85 (81-88) | .05 | .02 | .05 | .76 | |||
| Acute GVHD grade B-D | 622 | 1192 | 406 | |||||||
| @ 100 d | 33 (29-36) | 51 (48-54) | 53 (48-58) | < .001 | < .001 | < .001 | .51 | |||
| Acute GVHD grade C-D | 623 | 1193 | 406 | |||||||
| @ 100 d | 12 (10-15) | 25 (23-28) | 31 (27-36) | < .001 | < .001 | < .001 | .01 | |||
| Chronic GVHD | 608 | 1150 | 394 | |||||||
| @ 1 y | 39 (35-43) | 45 (42-48) | 43 (38-48) | .03 | .008 | .19 | .40 | |||
| @ 3 y | 44 (40-48) | 48 (45-51) | 46 (41-51) | .29 | .11 | .43 | .63 | |||
| TRM | 604 | 1157 | 395 | |||||||
| @ 1 y | 18 (15-21) | 21 (19-23) | 32 (27-37) | < .001 | .08 | < .001 | < .001 | |||
| @ 3 y | 25 (22-29) | 28 (25-31) | 36 (32-41) | .001 | .23 | < .001 | .001 | |||
| Relapse | 604 | 1157 | 395 | |||||||
| @ 1 y | 32 (29-36) | 35 (32-37) | 27 (23-32) | .02 | .34 | .09 | .005 | |||
| @ 3 y | 39 (35-43) | 38 (35-41) | 32 (28-37) | .06 | .65 | .02 | .04 | |||
| LFS | 604 | 1157 | 395 | .09‡ | ||||||
| @ 1 y | 50 (45-54) | 44 (41-47) | 40 (36-45) | .013 | .02 | .004 | .22 | |||
| @ 3 y | 35 (31-39) | 34 (31-36) | 31 (26-35) | .37 | .46 | .16 | .35 | |||
| Survival | 624 | 1193 | 406 | .01‡ | ||||||
| @ 1 y | 55 (51-59) | 52 (50-55) | 45 (40-50) | .004 | .28 | .001 | .008 | |||
| @ 3 y | 39 (35-43) | 37 (34-40) | 34 (30-39) | .28 | .37 | .11 | .31 |
| . | n . | MRD probability, % (95% CI) . | n . | 8/8 MUD probability, % (95% CI) . | n . | 7/8 MUD probability, % (95% CI) . | P* . | 8/8 MUD vs MRD, P† . | 7/8 MUD vs MRD, P† . | 7/8 MUD vs 8/8 MUD, P† . |
|---|---|---|---|---|---|---|---|---|---|---|
| ANC recovery | 621 | 1192 | 406 | |||||||
| @ 28 d | 96 (94-97) | 93 (92-95) | 92 (89-95) | .01 | .008 | .02 | .59 | |||
| @ 100 d | 97 (96-99) | 96 (94-97) | 95 (93-97) | .03 | .02 | .04 | .65 | |||
| Platelet recovery | 615 | 1183 | 398 | |||||||
| @ 60 d | 87 (85-90) | 83 (81-86) | 81 (78-85) | .02 | .02 | .01 | .40 | |||
| @ 100 d | 89 (86-91) | 85 (83-87) | 85 (81-88) | .05 | .02 | .05 | .76 | |||
| Acute GVHD grade B-D | 622 | 1192 | 406 | |||||||
| @ 100 d | 33 (29-36) | 51 (48-54) | 53 (48-58) | < .001 | < .001 | < .001 | .51 | |||
| Acute GVHD grade C-D | 623 | 1193 | 406 | |||||||
| @ 100 d | 12 (10-15) | 25 (23-28) | 31 (27-36) | < .001 | < .001 | < .001 | .01 | |||
| Chronic GVHD | 608 | 1150 | 394 | |||||||
| @ 1 y | 39 (35-43) | 45 (42-48) | 43 (38-48) | .03 | .008 | .19 | .40 | |||
| @ 3 y | 44 (40-48) | 48 (45-51) | 46 (41-51) | .29 | .11 | .43 | .63 | |||
| TRM | 604 | 1157 | 395 | |||||||
| @ 1 y | 18 (15-21) | 21 (19-23) | 32 (27-37) | < .001 | .08 | < .001 | < .001 | |||
| @ 3 y | 25 (22-29) | 28 (25-31) | 36 (32-41) | .001 | .23 | < .001 | .001 | |||
| Relapse | 604 | 1157 | 395 | |||||||
| @ 1 y | 32 (29-36) | 35 (32-37) | 27 (23-32) | .02 | .34 | .09 | .005 | |||
| @ 3 y | 39 (35-43) | 38 (35-41) | 32 (28-37) | .06 | .65 | .02 | .04 | |||
| LFS | 604 | 1157 | 395 | .09‡ | ||||||
| @ 1 y | 50 (45-54) | 44 (41-47) | 40 (36-45) | .013 | .02 | .004 | .22 | |||
| @ 3 y | 35 (31-39) | 34 (31-36) | 31 (26-35) | .37 | .46 | .16 | .35 | |||
| Survival | 624 | 1193 | 406 | .01‡ | ||||||
| @ 1 y | 55 (51-59) | 52 (50-55) | 45 (40-50) | .004 | .28 | .001 | .008 | |||
| @ 3 y | 39 (35-43) | 37 (34-40) | 34 (30-39) | .28 | .37 | .11 | .31 |
Overall point-wise comparison.
Point-wise pairwise comparison
Log-rank test.
TRM
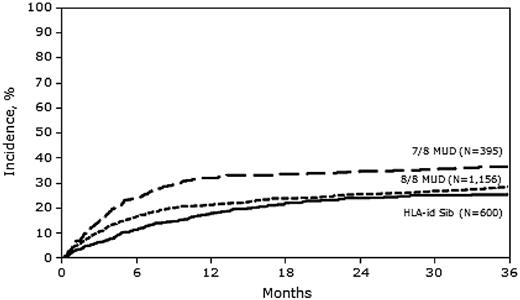
In univariate analysis, the cumulative incidences of TRM at 1 and 3 years were significantly higher in the 7/8 MUD group than in the MRD and 8/8 MUD groups (Table 2).
In multivariate analysis, the risk of TRM was similar between 8/8 MUD and MRD transplantation (hazard ratio [HR] = 1.11; 95% confidence interval [CI], 0.90-1.37), but was significantly higher with 7/8 MUD transplantation (HR = 1.48; 95% CI, 1.16-1.89) compared with MRD transplantation (Figure 1 and Table 3). Other adverse covariates that were significant in the final TRM model included: female donor into male recipient, lower KPS, advanced disease status at time of transplantation, high-risk cytogenetics, and AML arising from myelodysplastic syndrome.
Multivariate analysis for TRM, relapse, and treatment failure (inverse of LFS) in adult AML patients who underwent HLA-identical sibling (MRD) HCT or 8/8 or 7/8 MUD HCT from 2002-2006
| . | TRM, RR (95% CI)* . | P . | Relapse, RR (95% CI)† . | P . | Treatment failure (death or relapse), RR (95% CI)‡ . | P . |
|---|---|---|---|---|---|---|
| 8/8 MUD vs MRD | 1.11 (0.90-1.37) | 0.31 | 0.93 (0.78-1.09) | .37 | 0.98 (0.86-1.12) | .77 |
| 7/8 MUD vs MRD | 1.48 (1.16-1.89) | 0.001 | 0.78 (0.63-0.98) | .03 | 1.02 (0.86-1.20) | .77 |
| 7/8 MUD vs 8/8 MUD | 1.33 (1.09-1.62) | 0.004 | 0.84 (0.69-1.03) | .10 | 1.04 (0.91-1.20) | .55 |
| . | TRM, RR (95% CI)* . | P . | Relapse, RR (95% CI)† . | P . | Treatment failure (death or relapse), RR (95% CI)‡ . | P . |
|---|---|---|---|---|---|---|
| 8/8 MUD vs MRD | 1.11 (0.90-1.37) | 0.31 | 0.93 (0.78-1.09) | .37 | 0.98 (0.86-1.12) | .77 |
| 7/8 MUD vs MRD | 1.48 (1.16-1.89) | 0.001 | 0.78 (0.63-0.98) | .03 | 1.02 (0.86-1.20) | .77 |
| 7/8 MUD vs 8/8 MUD | 1.33 (1.09-1.62) | 0.004 | 0.84 (0.69-1.03) | .10 | 1.04 (0.91-1.20) | .55 |
Other significant factors include KPS, disease status at transplantation, cytogenetic findings at diagnosis, preceding MDS, and female donor into male recipient.
Other significant factors include disease status at transplantation, cytogenetic findings at diagnosis, WBC at diagnosis, and female donor into male recipient.
Other significant factors include KPS, disease status at transplantation, cytogenetic findings at diagnosis, and preceding MDS.
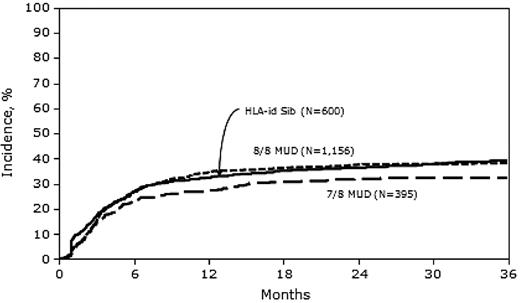
Relapse
In univariate analysis, the 1-year cumulative incidence of relapse was significantly lower in the 7/8 MUD group than in the MRD and 8/8 MUD groups (Table 2). In multivariate analysis, the risk of relapse was similar between MRD and 8/8 MUD transplantation (HR = 0.93; 95% CI, 0.78-1.09), but significantly lower with 7/8 MUD transplantation (HR = 0.78; 95% CI, 0.63-0.98) compared with MRD transplantation (Figure 2 and Table 3). Other covariates that were significant in the final relapse model included: female donor into male recipient (which was associated with lower relapse risk), advanced disease status at transplantation, high-risk cytogenetics, and higher WBC count at diagnosis, all of which were associated with higher relapse risk.
LFS
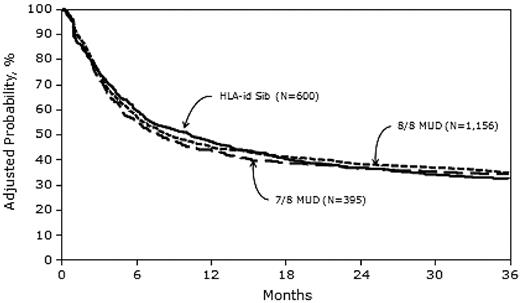
In univariate analysis, the 1-year probability of LFS was higher in the MRD group than in the MUD groups, but at 3 years, the LFS probability did not differ significantly among the 3 study groups (Table 2).
In multivariate analysis, the risks of treatment failure (death or relapse, the inverse of LFS) were similar with 8/8 MUD transplantation (HR = 0.98; 95% CI, 0.86-1.12) and with 7/8 MUD transplantation (HR = 1.02; 95% CI, 0.86-1.20) compared with MRD transplantation (Table 3). Other adverse covariates that were significant in the final LFS model include: lower KPS, advanced disease status at transplantation, high-risk cytogenetics, and AML arising from myelodysplastic syndrome.
Three-year probabilities of LFS, adjusted for other significant variables in the multivariate models, were 32% (95% CI, 29-36), 35% (95% CI, 32-37), and 34% (95% CI, 30-39) after MRD, 8/8 MUD, and 7/8 MUD transplantation, respectively (Figure 3).
Survival
In univariate analysis, the 1-year probability of survival was higher in the MRD group than in the 7/8 MUD group, but at 3 years, the survival probability did not differ significantly among the 3 study groups (Table 2).
In multivariate analysis, the risk of mortality with 8/8 MUD transplantation was similar to the risk with MRD transplantation (HR = 1.03; 95% CI, 0.90-1.17). The relative risk of mortality for 7/8 MUD transplantation patients versus MRD transplantation differed according to the posttransplantation time period. The risk was higher with 7/8 MUD transplantation in the first 6 months after HCT (HR = 1.40; 95% CI, 1.15-1.70), but was similar thereafter (HR = 0.88; 95% CI, 0.69-1.12; Table 4). Other adverse covariates that were significant in the final survival model included: age greater than 50 years, lower KPS, advanced disease status at transplantation, and high-risk cytogenetics.
Multivariate analysis for survival in adult AML patients who underwent HLA-identical sibling (MRD) HCT or 8/8 or 7/8 MUD HCT from 2002-2006 in the United States
| . | Death, RR (95 % CI) . | P . |
|---|---|---|
| 8/8 MUD vs MRD | 1.03 (0.90-1.17) | .62 |
| 7/8 MUD vs MRD | ||
| Early (≤ 6 mo after HCT) | 1.40 (1.15-1.70) | < .001 |
| Late (> 6 mo) | 0.88 (0.69-1.12) | .30 |
| 7/8 MUD vs 8/8 MUD | ||
| Early (≤ 6 mo after HCT) | 1.35 (1.13-1.62) | < .001 |
| Late (> 6 mo) | 0.85 (0.68-1.07) | .17 |
| . | Death, RR (95 % CI) . | P . |
|---|---|---|
| 8/8 MUD vs MRD | 1.03 (0.90-1.17) | .62 |
| 7/8 MUD vs MRD | ||
| Early (≤ 6 mo after HCT) | 1.40 (1.15-1.70) | < .001 |
| Late (> 6 mo) | 0.88 (0.69-1.12) | .30 |
| 7/8 MUD vs 8/8 MUD | ||
| Early (≤ 6 mo after HCT) | 1.35 (1.13-1.62) | < .001 |
| Late (> 6 mo) | 0.85 (0.68-1.07) | .17 |
Factors that were significant in the final model include recipient age, KPS, disease status at transplantation, and cytogenetic findings at diagnosis.
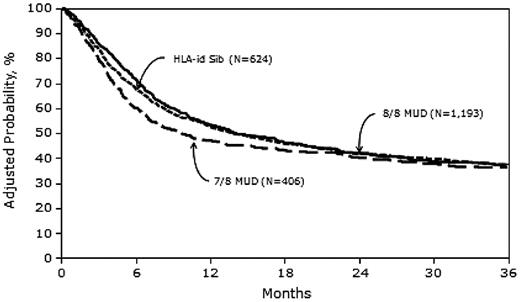
Three-year probabilities of survival, adjusted for other significant variables in the multivariate models, were 37% (95% CI, 33-41), 37% (95% CI, 34-40), and 36% (95% CI, 31-41) after MRD, 8/8 MUD, and 7/8 MUD transplantation, respectively (Figure 4 and Table 5).
Adjusted probability of overall survival in 2223 adult AML patients by donor type.
Adjusted probability of overall survival in 2223 adult AML patients by donor type.
Adjusted probability of survival in adult AML patients who underwent HLA-identical sibling (MRD) HCT or 8/8 or 7/8 matched unrelated donor (MUD) HCT at 6 mo, 1 y, 2 y, and 3 y
| . | MRD, % (95% CI) . | 8/8 MUD, % (95% CI) . | 7/8 MUD, % (95% CI) . | 8/8 MUD vs MRD, P* . | 7/8 MUD vs MRD, P* . | 7/8 MUD vs 8/8 MUD, P* . |
|---|---|---|---|---|---|---|
| @ 6 mo | 71 (67-75) | 67 (65-70) | 60 (55-64) | .09 | < .001 | .005 |
| @ 1 y | 53 (49-57) | 52 (50-55) | 47 (42-51) | .73 | .04 | .04 |
| @ 2 y | 42 (38-46) | 42 (39-44) | 40 (35-44) | .90 | .51 | .52 |
| @ 3 y | 37 (33-41) | 37 (34-40) | 36 (31-41) | .97 | .76 | .71 |
| . | MRD, % (95% CI) . | 8/8 MUD, % (95% CI) . | 7/8 MUD, % (95% CI) . | 8/8 MUD vs MRD, P* . | 7/8 MUD vs MRD, P* . | 7/8 MUD vs 8/8 MUD, P* . |
|---|---|---|---|---|---|---|
| @ 6 mo | 71 (67-75) | 67 (65-70) | 60 (55-64) | .09 | < .001 | .005 |
| @ 1 y | 53 (49-57) | 52 (50-55) | 47 (42-51) | .73 | .04 | .04 |
| @ 2 y | 42 (38-46) | 42 (39-44) | 40 (35-44) | .90 | .51 | .52 |
| @ 3 y | 37 (33-41) | 37 (34-40) | 36 (31-41) | .97 | .76 | .71 |
Point-wise pairwise comparison.
Causes of death
Table 6 summarizes the reported causes of death by donor type. The most common cause of death in all 3 groups was leukemia relapse. There were somewhat higher proportions of deaths from infection and/or GVHD in the MUD groups.
Causes of death in adult AML patients who underwent HLA-identical sibling (MRD) HCT or 8/8 or 7/8 MUD HCT
| Cause, n (%) . | MRD . | 8/8 MUD . | 7/8 MUD . |
|---|---|---|---|
| Missing | 6 (1.5) | 7 (< 1) | 3 (1) |
| Graft rejection | 1 (< 1) | 8 (1) | 0 |
| Infection | 39 (10) | 109 (14) | 55 (20) |
| Interstitial pneumonitis | 7 (2) | 23 (3) | 10 (4) |
| ARDS | 4 (1) | 20 (3) | 2 (< 1) |
| GVHD | 44 (11) | 98 (13) | 44 (16) |
| Primary disease | 216 (54) | 362 (47) | 102 (37) |
| Organ failure | 40 (10) | 63 (8) | 26 (9) |
| Secondary malignancy | 5 (1) | 5 (< 1) | 1 (< 1) |
| Hemorrhage | 9 (2) | 10 (1) | 2 (< 1) |
| Accidental death | 1 (< 1) | 1 (< 1) | 0 |
| Vascular | 2 (< 1) | 4 (< 1) | 3 (1) |
| Toxicity | 0 | 25 (3) | 5 (2) |
| Other cause | 23 (6) | 38 (5) | 22 (8) |
| Total | 397 | 773 | 275 |
| Cause, n (%) . | MRD . | 8/8 MUD . | 7/8 MUD . |
|---|---|---|---|
| Missing | 6 (1.5) | 7 (< 1) | 3 (1) |
| Graft rejection | 1 (< 1) | 8 (1) | 0 |
| Infection | 39 (10) | 109 (14) | 55 (20) |
| Interstitial pneumonitis | 7 (2) | 23 (3) | 10 (4) |
| ARDS | 4 (1) | 20 (3) | 2 (< 1) |
| GVHD | 44 (11) | 98 (13) | 44 (16) |
| Primary disease | 216 (54) | 362 (47) | 102 (37) |
| Organ failure | 40 (10) | 63 (8) | 26 (9) |
| Secondary malignancy | 5 (1) | 5 (< 1) | 1 (< 1) |
| Hemorrhage | 9 (2) | 10 (1) | 2 (< 1) |
| Accidental death | 1 (< 1) | 1 (< 1) | 0 |
| Vascular | 2 (< 1) | 4 (< 1) | 3 (1) |
| Toxicity | 0 | 25 (3) | 5 (2) |
| Other cause | 23 (6) | 38 (5) | 22 (8) |
| Total | 397 | 773 | 275 |
ARDS indicates acute respiratory distress syndrome.
Discussion
The recent passage and subsequent reversal of legislation in Arizona that eliminated coverage of MUD transplantation for adult Medicaid patients highlighted significant uncertainty about the safety and efficacy of unrelated donor transplantation.6,7 In the present study, we compared transplantation outcomes after MRD, 8/8 MUD, and 7/8 MUD HCT in adults with AML in contemporary practice. We chose to study AML because it is the most common indication for which allogeneic HCT is performed,32 and because it is a disease in which patients with intermediate- and high-risk cytogenetics were demonstrated to benefit from MRD transplantation over nontransplantation therapy in single studies30,33-35 and in pooled analyses of prospective clinical trials.36,37
Our sample included 2223 patients; 28% received MRD HCT, 54% received 8/8 MUD HCT, and 18% received 7/8 MUD HCT. Despite higher rates of acute GVHD in both MUD groups compared with MRD HCT recipients (Table 2), neither 3-year overall survival nor 3-year LFS rates differed significantly among the 3 groups (Tables 3 and 4). There was higher early mortality in the first 6 months after HCT in the 7/8 MUD versus the MRD group; however, the 3-year LFS and 3-year survival rates were comparable (Figures 3 and 4).
The observed increased risk of acute and chronic GVHD in both MUD groups compared with MRD HCT recipients is important. Several studies have shown that health care costs are significantly higher with the development of acute GVHD,38,39 and others have shown that quality of life is negatively affected by acute and chronic GVHD.40,41 Although our analysis demonstrated no difference in survival, a broader end point that also considers costs and quality of life could show MRD transplantation as having advantages over MUD transplantation. Most patients do not have the option of choosing either a related or unrelated donor, but these factors may be important considerations for those who do have the option.
Several studies recently compared unrelated donor transplantation to HLA-identical sibling transplantation.16,22,27 There are important differences between these studies and ours. Ringdén et al conducted a registry-based analysis to determine whether MUD HCT is associated with a greater GVL effect than HLA-identical sibling HCT, and concluded that these effects were similar and that, among patients without GVHD, survival was better with related donors.27 That study compared patients undergoing 8/8 MUD HCT with those receiving MRD HCT; it did not include patients receiving 7/8 MUD HCT. It also included patients with AML, acute lymphoblastic leukemia, and chronic myeloid leukemia (CML), whereas our study was purposely limited to only AML patients. The Ringdén study spanned the years from 1995-2004 and only included patients receiving myeloablative conditioning, whereas ours assessed transplantation data from 2002-2006 and included both myeloablative and reduced-intensity conditioning. In their analysis, Ringdén et al included acute and chronic GVHD as time-dependent covariates to evaluate their impact on transplantation outcomes. Because the relationship between donor source and GVL was not our primary focus, our study did not consider acute or chronic GVHD as covariates; this is important, because to do so might have obscured differences in survival resulting from differences in GVHD-related deaths. Furthermore, the purpose of our study was to aid clinical decision-making, and it is not possible to know which patients will and will not develop GVHD when the decision to proceed to HCT is made.
Woolfrey et al conducted a single-center retrospective analysis of 1448 patients who underwent HCT between 1992 and 2008 with myeloablative conditioning from either an HLA-identical sibling (n = 885) or a 10/10 MUD (n = 563), for intermediate- or high-risk hematologic malignancies that included AML, myelodysplasia, CML, idiopathic myelofibrosis, and myeloproliferative neoplasms.16 Consistent with our findings, that study found no differences between HCT using identical sibling donors and 10/10 MUD in survival or LFS for high-risk hematologic malignancies and those receiving BM as the graft type. However, for those patients with intermediate-risk disease and those receiving PBSCs, 10/10 MUD HCT was associated with higher TRM and lower survival.16 In our analysis, we tested for interactions between the donor type and all other covariates and did not observe an association. Possible explanations include differences in how disease risks were defined, inclusion of only patients with AML, and the fact that approximately 40% of our population received RIC regimens (which would lower TRM). In addition, approximately 80% of patients in the present study received PBSCs compared with 50% in the study by Woolfrey et al,16 which may limit the power of our analysis to discern differences based on graft source.
In a multicenter prospective study, 236 standard risk patients with acute leukemia (74%), myelodysplasia (8%), and CML (18%) underwent allogeneic HCT with myeloablative conditioning from 2000-2003 from either an MRD (n = 181) or a 10/10 MUD (n = 55).22 Again, despite differences in characteristics and trial design compared with our study, neither mortality (MUD vs MRD, HR = 1.08; 95% CI, 0.64-1.81) nor LFS (HR = 1.09; 95% CI, 0.69-1.73) differed significantly among the donor groups.22
Our results suggest that when an HLA-identical sibling donor is not available for an adult patient with AML who is otherwise a candidate for HCT, an 8/8 or 7/8 MUD may be used with the expectation of similar rates of TRM, LFS, and survival at 3 years. Even with this expanded use, as many as one-third of patients will not have a donor source and alternative donors such as haploidentical donors and cord blood stem cells may need to be considered.
We believe that the results of the present study demonstrate that an important clinical question (ie, the suitability of unrelated donors for an accepted HCT indication) about an expensive treatment (HCT) can be addressed by a federally funded outcomes registry (Center for International Blood and Marrow Transplant Research [CIBMTR]) that is charged with systematic data collection and analysis to inform policy makers. The use of resources such as the CIBMTR database allows policy decisions to be formulated in a way that ensures efficient use of resources while maintaining optimal clinical outcomes.
In conclusion, our study confirms the findings of prior smaller and single-institution studies demonstrating the acceptability of using unrelated donors for patients in need of HCT for AML when an HLA-identical sibling donor is not available.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement 5U01HL069294 from the NHLBI and NCI; contract HHSH234200637015C with the Health Resources and Services Administration (HRSA/DHHS); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; grants from Allos, Amgen, and Angioblast; an anonymous donation to the Medical College of Wisconsin; Ariad; the Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene; CellGenix; Children's Leukemia Research Association; Fresenius-Biotech North America; Gamida Cell Teva Joint Venture; Genentech; Genzyme; GlaxoSmithKline; Kiadis Pharma; the Leukemia & Lymphoma Society; the Medical College of Wisconsin; Millennium Pharmaceuticals; Milliman USA; Miltenyi Biotec; the National Marrow Donor Program; Optum Healthcare Solutions; Otsuka America Pharmaceutical; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix; Swedish Orphan Biovitrum; THERAKOS; and Wellpoint.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
National Institutes of Health
Authorship
Contribution: W.S., S.O., and J.S. conceived the idea for the project, designed the study, and wrote the manuscript; J.D.R. revised and approved the manuscript; W.S. and M.-J.Z. performed the statistical analyses; and M.M.H. designed the study and revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wael Saber, MD, MS, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: wsaber@mcw.edu.