We now think of classical Hodgkin lymphoma (cHL) as derived from “crippled” germinal center B cells that have frequently acquired rearranged and somatically mutated Ig genes.1,2 Despite their B-cell origin, the malignant Hodgkin and Reed-Sternberg (HRS) cells have lost most of the superficial trappings of B cells and therefore have been hidden from investigators' lenses for decades, prompting an arduous but persistent race to uncover the mystery of the HRS cell.
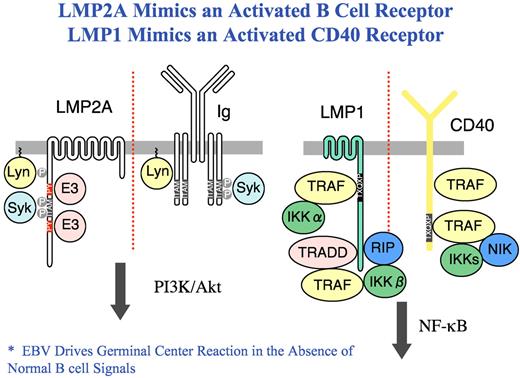
LMP1 and LMP2A mimic CD40 and Ig receptors in B cells. The C-terminal cytoplasmic domain of LMP1 contains 2 domains that associate with TNF receptor associated factors (TRAF), TNFR1-associated death domain (TRADD) and receptor interacting protein (RIP) without propagating a death signal inducing canonical and non-canonical NF-κB signaling. LMP2A activates B cell signaling by binding the Src family tyrosine kinase Lyn leading to constitutive phosphorylation of LMP2A and the recruitment of the Syk tyrosine kinase to a phosphorylated ITAM where it becomes activated resulting in the activation of the PI3K and Akt pathway. LMP2A also binds E3 ubiquitin ligases in the Nedd4 family.
LMP1 and LMP2A mimic CD40 and Ig receptors in B cells. The C-terminal cytoplasmic domain of LMP1 contains 2 domains that associate with TNF receptor associated factors (TRAF), TNFR1-associated death domain (TRADD) and receptor interacting protein (RIP) without propagating a death signal inducing canonical and non-canonical NF-κB signaling. LMP2A activates B cell signaling by binding the Src family tyrosine kinase Lyn leading to constitutive phosphorylation of LMP2A and the recruitment of the Syk tyrosine kinase to a phosphorylated ITAM where it becomes activated resulting in the activation of the PI3K and Akt pathway. LMP2A also binds E3 ubiquitin ligases in the Nedd4 family.
The mysterious origin of HRS cells has been slowly resolved3 as investigators have learned that these cells have been re-programmed by a variety of factors, including deregulated expression of B-cell molecules,4 down-regulation of B-cell transcription factors such as Notch 1 and ABF1 (reviewed in Küppers3 ), and epigenetic silencing of B-cell genes.5 Further, because Epstein-Barr virus (EBV) is found in as much as 40% of cHL, it is now clear that EBV has a pathogenic role.3 Whereas 3 EBV proteins, EBNA1, LMP1, and LMP2A, are expressed in HRS cells, LMP1 and LMP2A have attracted the most attention. LMP1 and LMP2A mimic two key signals of growth and survival of B cells. LMP1 mimics a CD40 receptor-activating NF-κB6 and other signaling pathways whereas LMP2A mimics an activated Ig receptor,7 replacing the missing Ig receptor, thereby rescuing HRS cells from inevitable apoptosis and mediating the survival of EBV-infected germinal center B cells (see figure). Thus, EBV becomes a savior to the otherwise doomed BCR-deficient HRS cell. Further, Jones et al have described circulating clonotypic B cells in the Hodgkin L428 cell line.8 These B cells expressed immunoglobulin light chain, the memory B-cell antigen CD27, and the stem cell marker aldehyde dehydrogenase (ALDH). These CD27+ ALDHhigh B cells, sharing immunoglobulin gene rearrangements with nodal HRS cells, were identified in the blood of most cHL patients, suggesting that the cHL stem cell is a CD20+ B cell, ripe for manipulation.
In this issue of Blood, Younes and colleagues and Kasamon and colleagues9,10 have taken advantage of the new biology of cHL and describe early phase 2 data adding rituximab to ABVD in patients with advanced cHL. The treatment regimens differed somewhat (rituximab was given weekly × 6 starting with C-1 day 1 ABVD by Younes et al9 and on days −7, 1, 8, 15, 22, of C-1 and days 1 of C-2, 4 and 6 of ABVD by Kasamon et al10 ), but the concept was the same, namely to add rituximab to ABVD and to frontload the regimen to achieve B-cell depletion.
The results of this approach are difficult to interpret and beg caution because both were phase 2 studies that were compared with historic controls for response and toxicity analysis. Of interest, however, the response rates (RRs), event-free survival (EFS), and overall survival (OS) were similar in the 2 trials but also similar to what one might have expected without the addition of rituximab. Kasamon et al found that of 49 evaluable patients, the 3-year EFS and OS were 83% and 98%, respectively, while Younes et al found in more mature data (median follow-up 68 months) that 5-year EFS and OS were 83% and 96%, respectively. Younes et al found toxicity that was similar to historic controls, although there was 1 case of Pneumocystis pneumonia (no PCP prophylaxis was given) and 1 patient was not able to receive all 6 cycles because of prolonged cytopenias. In the study by Kasamon et al, hospitalization occurred 27 times among 16 patients during or within 2 months after chemotherapy for a variety of causes, including febrile neutropenia (n = 7), nonneutropenic infections (n = 9), thrombosis (n = 4), pulmonary toxicity (n = 3), and other causes (n = 4), an unusually high rate of complications compared with large studies of advanced cHL or smaller retrospective analyses.
Interestingly, only about 20% to 30% of cHL cells express the B-cell antigen CD20,3 the target for rituximab in most B-cell non-Hodgkin lymphomas (NHLs). In the study by Younes et al CD20 was expressed in 20%, while in the study by Kasamon et al only in 8%. So much for targeting cancer. Yet that was not the point of these studies, as the target was not the HRS cell (not the malignant cell as we have come to expect in NHL and in other cancers), but the clonotypic stem cell (Kasamon et al) or the B cell harbored in the microenvironment (Younes et al). Therein lies the importance of these trials. The concept of “off-targeting” the HRS cell is appealing if one looks at the biology of cHL. The data from Jones et al documenting the presence of a CD20+ clonotypic B cells in cHL suggests a potential target for rituximab, but the low frequency of these cells in the circulation may limit utility.8 Frequency of clonotypic B cells was measured in the Kasamon study reported here. Of the 24 patients with baseline levels, 88% had detectable clonal CD27+ALDHhigh B cells before rituximab or rituximab-ABVD, representing 0.3% of total circulating CD19+ cells. After treatment they became undetectable in 19 patients. It would be informative to know if levels were measured after rituximab alone. Three of these 19 had re-emergence of the clone but are still in remission. The microenvironment in cHL may provide another potential “off target” for CD20-directed therapy.11 In solid tumors, data are accumulating that B-cell depletion may help to retard tumor growth. Kim et al have shown that in murine tumor models, depletion of CD20+ B cells by a mouse anti-CD20 antibody slowed the growth of new CD20− solid tumors and retarded the growth of established tumors but did not induce tumor regression.12 However, when an active immunotherapy approach using an adenovirus vaccine bearing the human papilloma virus E7 was combined with B-cell depletion against a murine lung cancer expressing the E7 virus, there was enhanced tumor regression and an immune response as measured by increased tetramer+ CD8+ T cells. Further, work from de Visser and colleagues has demonstrated that de novo carcinogenesis related to chronic inflammation depends on B-lymphocytes, and data are accumulating that suggest that B cells may inhibit Th-1 mediated anti tumor immune responses.13
Approximately 20% to 40% of cHLs harbor EBV in the HRS cells, and EBV has been shown to predict for a negative outcome in older patients. EBV can be detected in whole blood, plasma, and mononuclear cells by real-time PCR for viral DNA (reviewed in Küppers3 ). Correlates for EBV in HRS cells found high sensitivity (90%) and low specificity (65%), but interestingly, plasma EBV-DNA at diagnosis was an indicator of disease activity and correlated with expression of CD68+ macrophages,14 known to be an independent negative prognostic factor in cHL.15 In the Kasamon study, EBV copy number was measured and fell dramatically during cycle 1 in patients with EBV+ tumors.10
As we begin to unravel the underlying biology of cHL and other malignancies, it seems likely that our approach will be different and subtler. That subtlety underlies the importance of these two papers. More correlative studies looking at “off-target” effects of new agents and ultimately randomized clinical trials designed to test the biology will be needed, but these two articles provide an important statement if not proof of principle.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal