Abstract
In the present study, we evaluated the efficacy and safety of rituximab in combination with standard doxorubicin, bleomycin, vinblastine, and dacarbazine (RABVD) in patients with classical Hodgkin lymphoma (cHL). In this phase 2 study, patients with chemotherapy-naive, advanced-stage cHL were treated with rituximab 375 mg/m2 weekly for 6 weeks and standard ABVD for 6 cycles. The primary outcome was event-free survival (EFS) at 5 years. Eighty-five patients were enrolled, of whom 78 were eligible. With a median follow-up duration of 68 months (range, 26-110), and based on an intent-to-treat analysis, the 5-year EFS and overall survival rates were 83% and 96%, respectively. The 5-year EFS for patients with stage III/IV cHLwas 82%. Furthermore, the 5-year EFS for patients with an International Prognostic Score of 0-2 was 88% and for those with a score of > 2, it was 73%. The most frequent treatment-related grade 3 or 4 adverse events were neutropenia (23%), fatigue (9%), and nausea (8%). Our results demonstrate that the addition of rituximab to ABVD is safe and has a promising clinical activity in patients with advanced-stage cHL. These data are currently being confirmed in a multicenter randomized trial. This trial has been completed and is registered with www.clinicaltrials.gov as NCT00504504.
Introduction
The majority of patients with newly diagnosed classical Hodgkin lymphoma (cHL) are cured with initial multiagent chemotherapy. Among multiple chemotherapy regimens, doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) remain the most widely used regimen for the treatment of patients with advanced-stage cHL.1-7 Because undesired short- and long-term treatment-related toxicity continues to be problematic for this relatively young patient population, the development of more safe and effective frontline regimens continues to be actively pursued.8-10
The anti-CD20 mAb rituximab has demonstrated a good safety profile and clinical activity in a wide variety of B-cell lymphomas, all of which express the CD20 Ag on the malignant B cells. Subsequent studies combining rituximab with frontline chemotherapy regimens resulted in improvement in the event-free survival (EFS) and, in several cases, in the overall survival (OS) of patients with different non-Hodgkin lymphoma subtypes.11,12 In Hodgkin lymphoma, the malignant cells express CD20 of the lymphocyte-predominant subtype, but only in 20%-30% of the cHL subtype.13-16 In those cases, rituximab also demonstrated single-agent activity.17 Emerging data have suggested that rituximab may also have therapeutic value in patients with cHL whose tumors do not express CD20 by either depleting reactive B lymphocytes from the microenvironment, which may enhance antitumor immunity,18 or by killing the putative CD20-expressing HL stem cells.19 With this background, we conducted a phase 2 study to evaluate the safety and efficacy of rituximab in combination with standard ABVD chemotherapy (RABVD) in patients with advanced-stage cHL.
Methods
Patients
The present study is a single-institution, open-label, nonrandomized, phase 2 study. Eligible patients were required to have histologically confirmed, chemotherapy-naive, advanced-stage cHL (stage II bulky, III, or IV disease) or relapsed cHL after radiation therapy alone. Patients were required to be > 16 years of age and to have bidimensionally measurable disease ≥ 2.0 cm, an absolute neutrophil count ≥ 1000/mm3, a platelet count ≥ 100 × 103/mm3, serum creatinine of 176.8 μM (2 mg/dL) or less, serum bilirubin of 34.3μM (1.5 mg/dL) or less, and left ventricular ejection function ≥ 50% as shown by nuclear cardiac scan or echocardiogram. Patients were excluded if they were pregnant, had HIV infection, active hepatitis B or C infection, or severe pulmonary disease. All patients provided written, informed consent in accordance with the Declaration of Helsinki. The study was approved by an institutional review board. This trial has been completed and is registered with www.clinicaltrials.gov as NCT00504504.
Treatment
Rituximab was given at 375 mg/m2 intravenously weekly for 6 weeks, with the first dose given on the same day of the first dose of ABVD (doxorubicin 25 mg/m2, bleomycin 10 units/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2). ABVD was given on days 1 and 15 of a 28-day cycle for 6 cycles. The use of growth factor support and consolidation radiation therapy was allowed at the treating physician's discretion.
Assessment of response and toxicity
The primary objective of the study was to evaluate the 5-year EFS rate. The secondary objectives were to assess toxicity and response rates, including complete response (CR), CR unconfirmed (CRu), partial response (PR), and 5-year OS. Response was determined according to International Working Group criteria reported in 1999 based on computed tomography scan and bone marrow biopsy results20 Assessments were performed during therapy after 2-3 cycles of ABVD and after completing the planned 6 cycles of ABVD. Subsequently, patients were evaluated every 3-4 months during years 1 and 2 and every 6 months during years 3-5. After 5 years, in the absence of new symptoms, patients were evaluated on a yearly basis. A positron emission tomography (PET) scan was not required because the trial was initiated before this type of scan was introduced to practice; however, at the treating physicians' discretion, a PET scan could be performed before, during, and at the end of therapy. The PET scan results were reported in real-time by MD Anderson Cancer Center clinical nuclear medicine radiologists using visual assessment based on the criteria published by Juweid et al.21 Briefly, mediastinal blood pool activity was used as the reference background activity to define PET positivity for a residual mass ≥ 2 cm in greatest transverse diameter regardless of its location. A smaller residual mass or a normal-sized lymph node (ie, ≤ 1 × 1 cm in diameter) was considered positive if its activity was above that of the surrounding background. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria Version 3.
Statistical analysis
EFS was defined as the time from the study entry to disease progression, relapse, or death from any cause. OS was calculated from the study entry to death from any cause. Kaplan-Meier plots were used to depict the PFS and OS, and differences in the survival of the 2 groups were compared with the log-rank test using Prism Version 5 software (GraphPad). Efficacy analyses were performed on an intent-to-treat basis. The success of the study was to be evaluated in terms of the 5-year EFS rate. We originally intended to compare the 5-year EFS rate in this study with that from a large previous study of 115 patients treated with ABVD (61%).1 We planned to enroll 70 patients over 4 years based on an expected accrual rate of 1.5 patients per month. With this number of patients, assuming exponentially distributed EFS times, the estimated 5-year EFS rate of 74% was to be declared significantly superior given 3 additional years of follow-up after the end of accrual (1-sided significance level of 0.05 and power of 80%). However, because the 5-year EFS rate was not a reasonable parameter to formulate an early stopping rule, we used a response rate instead. An early stopping rule was implemented to ensure that the response rate was not lower than the expected 95%.1 Therefore, at least 12 of the first 14 patients (90% confidence interval [CI] for the response rate, 65%-95%) were required to achieve CR, CRu, or PR before more patients could be enrolled.
When analyzing data after completing planned enrollment (March 2001 to March 2005, N = 70), we identified only 20 patients with poor-risk features (International Prognostic Score [IPS] > 2) who had an estimated 3-year EFS of 73%. This favorable outcome prompted us to seek institutional review board approval to enroll an additional 15 patients with IPS scores of > 2 to examine further the activity of RABVD in patients with poor-risk features. These patients were enrolled between March 2005 and August 2007.
Results
Patients
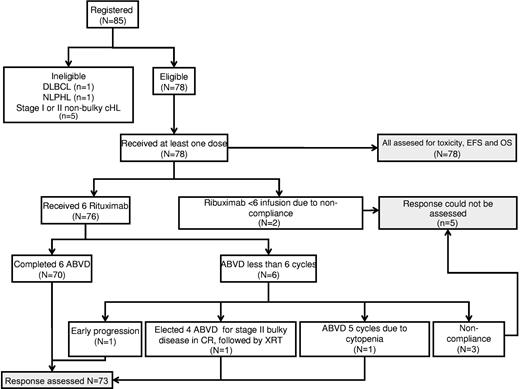
Eighty-five patients were registered in this study between March 2001 and August 2007 (Figure 1). Seven patients were found to be ineligible after registration (1 with diffuse large B-cell lymphoma, 1 with nodular lymphocyte–predominant Hodgkin lymphoma, and 5 with stage IA or stage IIA nonbulky disease). Therefore, 78 eligible patients received at least one dose of RABVD and were evaluable for treatment toxicity and efficacy. Characteristics of these 78 patients are summarized in Table 1. Five patients had poor compliance with the treatment and response assessment schedules, which precluded documentation of the end of therapy response assessment. Therefore, a total of 73 patients were evaluated for objective response. All 78 patients, including the 5 noncompliant ones, were evaluated for EFS and OS based on intent-to-treat analysis.
Characteristics of 78 eligible patients treated with RABVD
| Characteristic . | RABVD . |
|---|---|
| Total, N | 78 |
| Sex, n (%) | |
| Male | 40 (51) |
| Female | 38 (49) |
| Median age, y (range) | 32 (18-72) |
| Stage, n (%) | |
| II | 28 (36) |
| > 8 cm with B symptoms | 3 (4) |
| ≥ 10 cm or mediastinal ≥ 1/3 chest diameter | 23 (29) |
| Relapsed after previous primary radiation therapy | 2 (3) |
| III | 24 (31) |
| IV | 26 (33) |
| IPS, n (%) | |
| 0 | 6 (8) |
| 1 | 19 (24) |
| 2 | 18 (23) |
| 3 | 18 (23) |
| ≥ 4 | 17 (22) |
| CD20 status, n (%) | |
| Positive | 14 (18) |
| Negative | 56 (72) |
| Unknown | 8 (10) |
| Postchemotherapy radiation, n (%) | 26 (33) |
| Characteristic . | RABVD . |
|---|---|
| Total, N | 78 |
| Sex, n (%) | |
| Male | 40 (51) |
| Female | 38 (49) |
| Median age, y (range) | 32 (18-72) |
| Stage, n (%) | |
| II | 28 (36) |
| > 8 cm with B symptoms | 3 (4) |
| ≥ 10 cm or mediastinal ≥ 1/3 chest diameter | 23 (29) |
| Relapsed after previous primary radiation therapy | 2 (3) |
| III | 24 (31) |
| IV | 26 (33) |
| IPS, n (%) | |
| 0 | 6 (8) |
| 1 | 19 (24) |
| 2 | 18 (23) |
| 3 | 18 (23) |
| ≥ 4 | 17 (22) |
| CD20 status, n (%) | |
| Positive | 14 (18) |
| Negative | 56 (72) |
| Unknown | 8 (10) |
| Postchemotherapy radiation, n (%) | 26 (33) |
Response to therapy
Because the trial was initiated before the PET scan was introduced to practice, the response evaluation in this trial is based on computed tomography scan according to International Working Group criteria reported in 1999.20 CR and CRu were achieved in 49 and 19 patients (93%), respectively, and 4 achieved PR. One patient experienced progressive disease after 3 cycles of RABVD and received salvage chemotherapy.
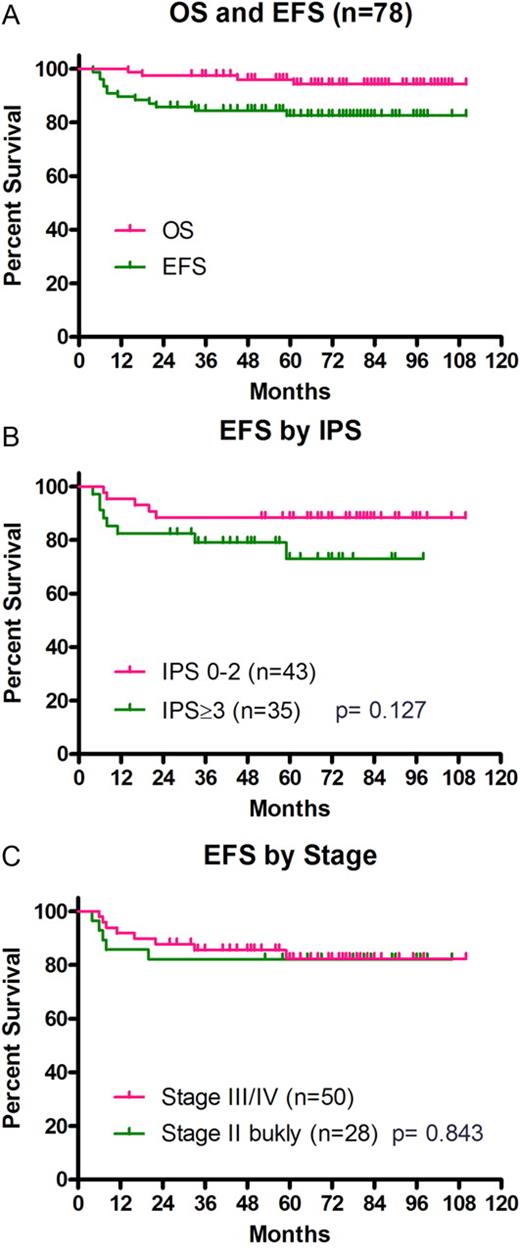
The median follow-up duration of surviving patients was 68 months (range, 26-110 months). Based on an intent-to-treat analysis, the 5-year EFS and OS rates were 83% (95% CI, 72%-90%) and 96% (95% CI, 88%-99%), respectively (Figure 2A). EFS was then analyzed by Ann Arbor stage, IPS, and the expression of CD20 on Hodgkin and Reed-Sternberg (HRS) cells. The 5-year EFS in patients with stage II and stage III/IV disease were 82% and 82%, respectively (Figure 2B). The 5-year EFS rates in patients with IPS of 0-2 (n = 43) and those with IPS of > 2 (n = 35) were 88% (95% CI, 74%-95%) and 73% (95% CI, 50%-86%), respectively (P = .127, Figure 2C).
Kaplan-Meier curves showing the survival of patients. (A) EFS and OS in all patients. (B) EFS by Ann Arbor stage. (C) EFS by IPS grouping.
Kaplan-Meier curves showing the survival of patients. (A) EFS and OS in all patients. (B) EFS by Ann Arbor stage. (C) EFS by IPS grouping.
CD20 expression on HRS cells was evaluated in 70 patients; 14 (20%) expressed CD20 and 56 (80%) did not. The 5-year EFS in patients with CD20+ tumors was 93%, compared with 77% for those with CD20− tumors (P = .230, Figure 3A).
Kaplan-Meier curves showing the survival of patients. (A) EFS by CD20 status on malignant cells. (B) EFS by interim PET result.
Kaplan-Meier curves showing the survival of patients. (A) EFS by CD20 status on malignant cells. (B) EFS by interim PET result.
Interim PET scan results have been shown to predict long-term outcome of ABVD therapy in patients with cHL.22,23 Therefore, in the present study, we investigated whether interim PET status maintains its prognostic value in patients treated with RABVD. For this analysis, we used real-time prospective interim PET results, which were performed after 2 or 3 cycles of RABVD and interpreted by MD Anderson Cancer Center clinical diagnostic nuclear medicine radiologists. An interim PET scan was performed in 65 patients. The 5-year EFS rates in patients with interim PET-negative tests (n = 52) and those with interim PET-positive tests (n = 13) were 91% and 77%, respectively (P = .076, Figure 3B).
Toxicity
All 78 eligible patients received at least 1 dose of RABVD and were evaluated for toxicity. All patients received pretreatment anti-emetics and were allowed to receive growth factor prophylaxis. Seventy-six patients received 6 weekly doses of rituximab as scheduled and 2 did not because of poor compliance. One patient experienced prolonged cytopenia after 5 cycles of RABVD and did not receive the sixth cycle. One patient developed Pneumocystis pneumonia after the third cycle that resolved with proper therapy. The most frequent treatment-related grade 3 and 4 adverse events were neutropenia in 18 patients (23%), fatigue in 7 patients (9%), and nausea in 6 patients (8%). Table 2 shows the most common toxicities observed after RABVD.
Toxicity after RABVD (N = 78)
| Toxicity . | Grade 1, % . | Grade 2, % . | Grade 3, % . | Grade 4, % . | Total, % . |
|---|---|---|---|---|---|
| General | |||||
| Fatigue | 31 | 9 | 5 | 4 | 49 |
| Pain | 10 | 23 | 6 | 0 | 40 |
| Alopecia | 23 | 19 | 0 | 0 | 42 |
| Gastrointestinal | |||||
| Nausea | 37 | 19 | 8 | 0 | 64 |
| Vomiting | 22 | 15 | 1 | 0 | 38 |
| Diarrhea | 13 | 9 | 0 | 0 | 22 |
| Constipation | 13 | 12 | 0 | 0 | 24 |
| Pulmonary | |||||
| Cough | 10 | 9 | 0 | 0 | 19 |
| Dyspnea | 10 | 9 | 1 | 0 | 21 |
| Neurologic (neuropathy) | 24 | 6 | 1 | 0 | 32 |
| Infection | 6 | 8 | 3 | 0 | 17 |
| Hematologic (granulocytopenia) | 3 | 8 | 15 | 8 | 33 |
| Toxicity . | Grade 1, % . | Grade 2, % . | Grade 3, % . | Grade 4, % . | Total, % . |
|---|---|---|---|---|---|
| General | |||||
| Fatigue | 31 | 9 | 5 | 4 | 49 |
| Pain | 10 | 23 | 6 | 0 | 40 |
| Alopecia | 23 | 19 | 0 | 0 | 42 |
| Gastrointestinal | |||||
| Nausea | 37 | 19 | 8 | 0 | 64 |
| Vomiting | 22 | 15 | 1 | 0 | 38 |
| Diarrhea | 13 | 9 | 0 | 0 | 22 |
| Constipation | 13 | 12 | 0 | 0 | 24 |
| Pulmonary | |||||
| Cough | 10 | 9 | 0 | 0 | 19 |
| Dyspnea | 10 | 9 | 1 | 0 | 21 |
| Neurologic (neuropathy) | 24 | 6 | 1 | 0 | 32 |
| Infection | 6 | 8 | 3 | 0 | 17 |
| Hematologic (granulocytopenia) | 3 | 8 | 15 | 8 | 33 |
Discussion
Over the past decade, the addition of the anti-CD20 naked mAb rituximab to standard frontline chemotherapy regimens have improved treatment outcome, including survival, of patients with B-cell non-Hodgkin lymphoma. Because HRS cells rarely express CD20 and uniformly express CD30, previous efforts focused on the development of mAbs targeting CD30. Unfortunately, several naked anti-CD30 mAb failed to produce meaningful clinical responses, precluding further clinical development. In the present study, we demonstrate that targeting CD20 in combination with ABVD is safe and has promising clinical activity for the treatment of patients with advanced-stage cHL. Although this was a single-arm, open-label study, our results compare favorably to those recently reported with ABVD alone in a similar patient population, especially those who were treated around the same period of time at our institution and elsewhere (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).4,6,24 More recently, Kasamon et al conducted a phase 2 multicenter study of RABVD in a similar patient population and independently confirmed our results, reporting a 3-year EFS rate of 83% in 49 patients.25 Given these encouraging results, we have initiated a multicenter randomized study comparing standard ABVD with RABVD in patients with advanced-stage cHL with IPS > 2 (www.clinicaltrials.gov as NCT00654732).
In Hodgkin lymphoma, CD20 expression on malignant cells is detected in all nodular lymphocyte–predominant patients and in up to 30% of cHL patients. In these patients, single-agent rituximab produced high response rates.17,26 In a pilot study, rituximab demonstrated efficacy in patients with relapsed cHL regardless of CD20 expression by HRS cells.27 Twenty-two patients with relapsed cHL were treated with 6 weekly doses of rituximab. Five (22%) patients achieved PR or CR, and 8 additional patients had stable disease. Clinical remissions were observed in patients regardless of CD20 expression by HRS cells, but responses were limited to patients whose disease was confined to the lymph nodes.27 In the present study, patients with CD20+ HRS cells tended to have a better EFS compared with those whose HRS cells did not express CD20 (93% vs 77%, respectively), although this difference was not statistically significant. The benefit of rituximab in patients with CD20− tumors might be explained by eliminating reactive B cells in the microenvironment that may support the growth and survival of HRS cells.28 Eliminating reactive B cells may also enhance the antilymphoma immune response.18 A third potential mechanism is targeting the putative HRS stem cells that are reported to express CD20.19
Patients with poor-risk features are considered candidates for investigational therapy. The 2 most widely used prognostic factors are the IPS score and interim PET scan results.23,29 In our study, patients with IPS > 2 had a 5-year EFS of 73%, which is better than expected when ABVD alone was used. Patients with negative interim PET imaging results had a better 5-year EFS compared with those with positive interim PET results (91% vs 77%). Therefore, our data confirm the prognostic value of interim PET in patients receiving RABVD. However, patients with positive interim PET had a far better outcome compared with what has been reported previously with ABVD alone.23 The different outcome between these 2 studies may be related to the retrospective nature of the ABVD study, but may also suggest that the addition of rituximab to ABVD may improve treatment outcome, especially in patients with positive interim PET results. These encouraging results provided a rationale for an ongoing multicenter, randomized clinical trial comparing RABVD with standard ABVD in patients with high-risk advanced-stage cHL (www.clinicaltrials.gov as NCT00654732). In a different strategy, the German Hodgkin Lymphoma Study Group is currently investigating the role of rituximab in combination with BEACOPP in patients with positive interim PET scan (www.clinicaltrials.gov as NCT00515554).
Since the initiation of our study, the mAb drug conjugate brentuximab vedotin has demonstrated remarkable single-agent activity in patients with relapsed cHL, resulting in its approval by the US Food and Drug Administration.30 Emerging data from a recently reported phase 1 study have indicated that combining ABVD with brentuximab vedotin is associated with a 40% incidence of pulmonary toxicity.31 In contrast, no pulmonary toxicity was observed when brentuximab vedotin was combined with AVD. A randomized study will be required to determine the contribution of brentuximab vedotin–based combination programs in the treatment of newly diagnosed patients with advanced-stage cHL. However, RABVD and brentuximab vedotin-AVD represent different strategies that may complement each other, but it remains to be seen whether combining rituximab with brentuximab vedotin plus AVD will be necessary. This question can only be answered in randomized studies.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Yu Cao for her help in collecting the data and preparing this manuscript.
Authorship
Contribution: A.Y. designed the study, provided administrative support and patient care, collected and analyzed the data, and wrote the manuscript; Y.O. provided patient care, collected and analyzed the data, and wrote the manuscript; A.R.C. provided patient care and collected and analyzed the data; P.M., A.G., B.P., H.H.C., H.A.M., F.H., J.R., F.S., M.A.F., B.S.D., M.A.R., N.D., L.W.K., S.S.N., and L.E.F. provided patient care; L.F. and Y.Y. analyzed the data; and all authors approved the final manuscript.
Conflict-of-interest disclosure: A.Y. has received research funding from Genentech, Novartis, SBIO, Seattle Genetics, Syndax, and Sanofi-Aventis and honoraria from Novartis, Seattle Genetics, and Sanofi-Aventis. P.M. is on the advisory board for Genentech. A.G. has received research funding from Genentech and Roche. M.F. has received research funding from Genentech. M.R. has received research funding from Pfizer and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Anas Younes, MD, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: ayounes@mdanderson.org.