Abstract
Adoptive cell therapy with tumor-targeted T cells is a promising approach to cancer therapy. Enhanced clinical outcome using this approach requires conditioning regimens with total body irradiation, lymphodepleting chemotherapy, and/or additional cytokine support. However, the need for prior conditioning precludes optimal application of this approach to a significant number of cancer patients intolerant to these regimens. Herein, we present preclinical studies demonstrating that treatment with CD19-specific, chimeric antigen receptor (CAR)–modified T cells that are further modified to constitutively secrete IL-12 are able to safely eradicate established disease in the absence of prior conditioning. We demonstrate in a novel syngeneic tumor model that tumor elimination requires both CD4+ and CD8+ T-cell subsets, autocrine IL-12 stimulation, and subsequent IFNγ secretion by the CAR+ T cells. Importantly, IL-12–secreting, tumor-targeted T cells acquire intrinsic resistance to T regulatory cell–mediated inhibition. Based on these preclinical data, we anticipate that adoptive therapy using CAR-targeted T cells modified to secrete IL-12 will obviate or reduce the need for potentially hazardous conditioning regimens to achieve optimal antitumor responses in cancer patients.
Introduction
Adoptive transfer of tumor-targeted T cells has yielded promising results in early clinical trials.1-3 However, optimal antitumor- mediated efficacy of these adoptively transferred tumor-specific T cells is dependent on prior conditioning of the host with total body irradiation, high-dose chemotherapy, and/or postinfusion cytokine support. In fact, optimal clinical responses after adoptive T-cell therapy has been shown to correlate positively with the intensity of the prior conditioning regimen.4 Significantly, these optimized conditioning regimens with associated toxicities probably preclude application of this approach to a majority of patients who would otherwise benefit from such tumor-targeted adoptive T-cell therapies.
We and others have previously demonstrated that adoptive transfer of human T cells modified to express a human CD19 (hCD19)–targeted chimeric antigen receptor (CAR) successfully eradicate established human hCD19+ tumors in immune compromised mice.5-7 CD19 is a B-cell specific antigen, which is expressed on normal B cells as well as most B-cell malignancies including most non-Hodgkin lymphomas (NHLs), as well as chronic lymphocytic leukemias (CLLs), and B-cell acute lymphoblastic leukemias (B-ALLs). Significantly, CD19 is not expressed on bone marrow (BM) stem cells and therefore targeting of CD19 through this novel approach would be deemed relatively safe. Based on these findings we are currently investigating this approach in the clinical setting.
In our recently published clinical trial studies, we further validated the role of prior conditioning chemotherapy on the clinical outcomes of patients treated with CD19-targeted CAR-modified autologous T cells. We found that an initial cohort of 3 CLL patients treated with CAR-modified T cells in the absence of conditioning chemotherapy exhibited no objective responses and continued progressive disease.3 However, in contrast, a subsequent cohort of patients treated with lower numbers of CAR-modified T cells but after high-dose cyclophosphamide conditioning therapy demonstrated marked tumor regression, B-cell aplasias, and lengthy periods of stable disease after therapy. In addition, other published clinical studies using CAR-modified T-cell therapies in the absence of prior conditioning have similarly failed to demonstrate significant clinical benefit.8-12 Collectively, these studies highlight the necessity for conditioning pretreatment for effective adoptive therapy with CAR-modified autologous T cells.
To better asses the mechanisms of conditioning therapy on the biology of CD19-targeted CAR-modified T cells in vivo we have generated an immune-competent, conditioning-dependent tumor model using systemic hCD19 modified EL4 (EL4[hCD19]) thymoma tumors infused into C57BL6 mice with a knockout murine CD19 (mCD19−/−), knockin human CD19 (hCD19+/−) phenotype (C57BL6 [mCD19−/− hCD19+/−]).13 These mice have restricted expression of 1 functional copy of the hCD19 gene in normal B cells, resulting in a retained immune-competent phenotype.
Herein we demonstrate that the efficacy of hCD19-targeted CAR-modified T cells is dependent on prior cyclophosphamide chemotherapy to induce both tumor eradication as well as predicted B-cell aplasias. Additional serum cytokine analyses of conditioned mice demonstrated both a decrease in regulatory CD4+ T cells (Tregs) as well as significant increase in the serum levels of the IL-12 and IFNγ cytokines. Based on these findings, we demonstrate that additional modification of infused hCD19-targeted CAR+ T cells to secrete IL-12 allowed for efficient eradication of systemic EL4(hCD19) tumors, as well as induction of B-cell aplasias, in the absence of prior cyclophosphamide conditioning. This outcome was dependent on both CD4 and CD8 T-cell subsets and required continued in vivo autocrine stimulation of IL-12 as well as modified T cell–IFNγ secretion, which in turn resulted in resistance to Treg-mediated suppression. Collectively, our data support the concept of a “conditioning free” approach to adoptive T-cell therapy of cancer, which in turn may broaden the application of this promising therapeutic approach to patients otherwise intolerant of further conditioning chemotherapy or radiation therapy.
Methods
Cell lines
EL4 thymoma cell line (ATCC), was modified to express human CD19 and mouse CD80, maintained in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated FBS (Atlanta Biologicals), nonessential amino acids, sodium pyruvate, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), l-glutamine and penicillin/streptomycin and 2-mercaptoethanol (Invitrogen). Phoenix ecotropic packaging cells (ATCC) were maintained in DMEM with 10% FBS, l-glutamine, and penicillin/streptomycin, and used as previously described.6 Murine T cells were maintained in RPMI 1640 medium, supplemented as previously described, with the addition of 60 IU/mL rhIL-2 (Novartis Pharmaceuticals).
Generation of retroviral constructs
SFG-19z1 vector,5 was modified by exchange of the human CD3ζ chain cytoplasmic signaling domain with the murine CD3ζ chain, amplified from C57BL6 splenocyte derived cDNA. The human CD8 transmembrane domain was fused to the mouse CD3ζ chain and to either the CD19-specific scFv from 19z1 to generate 19mz, or to the prostate-specific membrane antigen (PSMA) scFv from the Pz1 CAR,5 to generate the control Pmz CAR. The fusion gene encoding the complete murine IL-12 with a serine-glycine repeat between the p35 and p40 chain-coding domains (mIL12f) was generously provided by Alan Houghton and Jedd Wolchok,14 and modified to include an hCD8 leader peptide and an internal ribosome entry site (IRES).
T-cell isolation and gene transfer
Murine T cells were isolated and genetically modified as previously described.15 For some experiments, 19mζ T cells were cultured in 10 ng/mL exogenous recombinant mouse IL-12 (R&D Systems). For isolation of T-cell subsets, transduced T cells were separated using a Cytomation MoFlo FACS sorter (Beckman Coulter) after staining with rat anti–mouse antibodies for CD4 or CD8. CD4+ Tregs were isolated from splenocytes using the Dynabeads Flowcomp Mouse CD4+CD25+ Treg cell isolation kit (Invitrogen) and activated with 500 IU/mL of rhIL-2.
Cytotoxicity assays
The cytolytic capacity of transduced murine T cells was determined using a standard 51Cr release assay, as previously described.16 For cytotoxicity in the presence of Tregs, effector T cells were incubated with Tregs at a 1:4 ratio for 24 hours, cells were then subsequently incubated with tumor cells at an effector:target ratio of 1:1. After 4 hours, cytotoxicity was assessed by flow cytometry to detect residual CD19+ tumor cells.17
Serum cytokine analyses
Cytokine detection was achieved using the MILLIPLEX MAP Mouse Cytokine/Chemokine, Premixed 13 Plex kit (Millipore), and the Luminex IS100 system.
FACS analyses
Flow cytometric analysis of EL4(hCD19) and transduced murine T cells was performed using a FACScan cytometer and FlowJo Version 9.2 software (TreeStar). EL4(hCD19) cells were stained with rat anti–mouse CD19 and CD80 mouse anti–human CD19 (Invitrogen). Mouse T cells were stained with rat anti–mouse CD8 (Invitrogen), CD4, CD25, CD44, and CD62L (eBioscience). Analysis of Tregs was achieved using the Mouse Regulatory T-cell staining kit (eBioscience). CARs expression was detected using Armenian hamster 12D11 antibody (Memorial Sloan-Kettering Cancer Center monoclonal antibody facility).
Mice and in vivo model
CD19 knockout C57BL6(mCD19−/− hCD19−/−) and human CD19 transgenic C57BL6(mCD19+/+ hCD19+/+) were used to generate C57BL6(mCD19−/−hCD19+/−) mice, which were used as both donor and recipients.13 Donor T cells were also isolated from IL-12Rβ2−/− and IFNγ−/− mice (B6.129S1-Il12btm1Jm/J and B6.129S7-Ifngtm1Ts/J; Jackson Laboratories). C57BL6(mCD19−/− hCD19+/−) mice were inoculated with 1 × 106 EL4(hCD19mCD80) tumor cells intravenously and then treated with 3 to 5 × 106 CAR+ T cells intravenously the following day. For conditioning studies, mice received 250 mg/kg cyclophosphamide intraperitoneally on day −3 (before tumor) or day 2 (after tumor). For B-cell depletion, mice were injected intraperitoneally on day −1 with 250 μg of anti-CD20 antibody, MB2011.18 B-cell aplasia was detected with flow cytometric analysis. All murine studies were done in the context of a Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee approved protocol (00-05-065).
Statistical analysis
All analyses were calculated using Graphpad Prism 5.0 software, survival data were assessed using a log-rank analysis and all other analyses were achieved with a Mann-Whitney test.
Results
Generation of an immune competent murine model using C57BL6(mCD19−/− hCD19+/−) mice bearing systemic EL4(hCD19) tumors
Using flow cytometry, we verified the phenotype of C57BL6(mCD19−/− hCD19+/−) peripheral B cells demonstrating loss of mCD19 expression with expression of hCD19 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Further, through retroviral gene modification and FACS we isolated the EL4(hCD19) cell line, which expressed both hCD19 and mouse CD80 (supplemental Figure 1B). Infusion of these tumor cells into C57BL6(mCD19−/− hCD19+/−) mice led to progressive disease as evidenced by ruffled fur, decreased response to stimuli, and hind limb paralysis at 3 to 5 weeks after tumor cell infusion, at which time the mice were euthanized. Tumor involvement in the BM, liver, and lymph nodes was verified by subsequent histologic analysis of tumor-bearing mice (supplemental Figure 1C).
T cells targeted to hCD19 require prior cyclophosphamide conditioning therapy to eradicate EL4(hCD19) tumors and induce B-cell aplasias
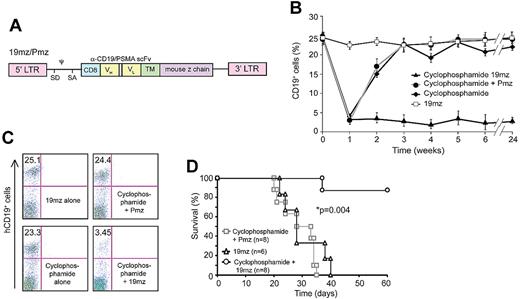
In initial experiments, we infused C57BL6(mCD19−/− hCD19+/−) mice with EL4(hCD19) followed by infusion of syngeneic CAR-modified T cells specific to either hCD19 (19mz) or as a control, T cells specific to the irrelevant PSMA-targeted Pmz CAR (Figure 1A, supplemental Figure 2A). Significantly, infusion of tumor-bearing mice with 19mz+ T cells did not enhance survival of EL4(hCD19) tumor-bearing mice compared with control mice treated with Pmz+ T cells (supplemental Figure 2B). Infusion of 19mz+ T cells into nontumor-bearing mice further failed to induce predicted aplasias of normal hCD19+ B cells (Figures 1B-C). Treatment of nontumor-bearing mice with 19mz+ T cells after conditioning cyclophosphamide chemotherapy, in contrast to untreated or Pmz+ T cell–treated mice, resulted in an aplasia of normal hCD19+ B cells, indicative of hCD19-targeted cytotoxic activity of the 19mz-modified T cells (Figures 1B-C). Cyclophosphamide conditioning of mice before and even after EL4(hCD19+) tumor-cell infusion, followed by infusion with 19mz+ T cells, but not control Pmz+ T cells, resulted in tumor eradication and a marked long-term survival (Figure 1D, supplemental Figure 3). Significantly, this antitumor efficacy was dependent on both CD4+ helper and CD8+ cytotoxic 19mz+ T cells (supplemental Figure 4).
Prior cyclophosphamide conditioning results in 19mz+ T cell–mediated B-cell aplasias and eradication of systemic EL4(hCD19) tumors. (A) Schematic of retroviral construct encoding the 19mz and Pmz CARs. CD8 indicates CD8 leader sequence; scFv, single chain variable fragment; VH and VL, variable heavy and light chains; TM, transmembrane domain; LTR, long terminal repeat; SD, SA, splice donor and acceptor; and ψ, packaging element. (B) Persistent B-cell aplasias in C57BL6(mCD19−/− hCD19+/−) mice pretreated with cyclophosphamide followed by 19mz+ but not control Pmz+ T-cell therapy. 19mz+ T-cell therapy alone or cyclophosphamide alone failed to induce persistent B-cell aplasias. (C) Representative flow cytometry demonstrating B-cell aplasias in mice conditioned with cyclophosphamide followed by 19mz+ T-cell infusion at 4 weeks after T-cell infusion. (D) Cyclophosphamide conditioned EL4(hCD19) tumor-bearing C57BL6(mCD19−/− hCD19+/−) mice treated with 19mz+ T cells have enhanced survival compared with mice treated with 19mz+ T cells alone or control Pmz+ T cell–treated mice. All results are representative of at least 2 experiments.
Prior cyclophosphamide conditioning results in 19mz+ T cell–mediated B-cell aplasias and eradication of systemic EL4(hCD19) tumors. (A) Schematic of retroviral construct encoding the 19mz and Pmz CARs. CD8 indicates CD8 leader sequence; scFv, single chain variable fragment; VH and VL, variable heavy and light chains; TM, transmembrane domain; LTR, long terminal repeat; SD, SA, splice donor and acceptor; and ψ, packaging element. (B) Persistent B-cell aplasias in C57BL6(mCD19−/− hCD19+/−) mice pretreated with cyclophosphamide followed by 19mz+ but not control Pmz+ T-cell therapy. 19mz+ T-cell therapy alone or cyclophosphamide alone failed to induce persistent B-cell aplasias. (C) Representative flow cytometry demonstrating B-cell aplasias in mice conditioned with cyclophosphamide followed by 19mz+ T-cell infusion at 4 weeks after T-cell infusion. (D) Cyclophosphamide conditioned EL4(hCD19) tumor-bearing C57BL6(mCD19−/− hCD19+/−) mice treated with 19mz+ T cells have enhanced survival compared with mice treated with 19mz+ T cells alone or control Pmz+ T cell–treated mice. All results are representative of at least 2 experiments.
Selective antibody-mediated depletion of normal hCD19+ B cells failed to recapitulate cyclophosphamide-induced enhanced antitumor efficacy of 19mz+ T cells
Because treated mice contain a substantial number of normal B cells bearing the targeted hCD19 antigen, we postulated that these normal B cells served as an “antigen sink,” which in turn resulted in either activation-induced cell death or anergy of the infused 19mz+ tumor-targeted T cells to explain the lack of resulting B-cell aplasias and tumor eradication observed in our earlier experiments.19 To address this, we pretreated mice with a murine CD20-specific antibody (MB2011) to generate an aplasia of normal B cells, thereby reducing the burden of cells targeted by the 19mz-modified T cells (Figure 2A).18 However, tumor inoculation in these B cell–depleted mice followed by infusion of 19mz+ T cells failed to enhance long-term survival of treated mice compared with untreated or control Pmz+ T cell–treated mice (Figure 2B).
Cyclophosphamide conditioning decreases Treg numbers, and induces an increase in serum IL-12 and IFNγ. (A) EL4(hCD19) tumor-bearing C57BL6(mCD19−/− hCD19+/−) mice pretreated with the B cell depleting antibody, MB2011 induced B-cell aplasias but failed to mediate tumor eradication on subsequent infusion with 19mz+ T-cell infusion (B). (C) Cyclophos-phamide conditioning mediated a significant decrease in peripheral blood Tregs as assessed by flow cytometry. (D) Serum cytokine analyses of cyclophosphamide conditioned C57BL6(mCD19−/−hCD19+/−) mice demonstrates transient increases in IL-12p70 and IFNγ as assessed by Luminex IS100 multiplex studies. All results are representative of at least 2 independent experiments.
Cyclophosphamide conditioning decreases Treg numbers, and induces an increase in serum IL-12 and IFNγ. (A) EL4(hCD19) tumor-bearing C57BL6(mCD19−/− hCD19+/−) mice pretreated with the B cell depleting antibody, MB2011 induced B-cell aplasias but failed to mediate tumor eradication on subsequent infusion with 19mz+ T-cell infusion (B). (C) Cyclophos-phamide conditioning mediated a significant decrease in peripheral blood Tregs as assessed by flow cytometry. (D) Serum cytokine analyses of cyclophosphamide conditioned C57BL6(mCD19−/−hCD19+/−) mice demonstrates transient increases in IL-12p70 and IFNγ as assessed by Luminex IS100 multiplex studies. All results are representative of at least 2 independent experiments.
Cyclophosphamide conditioning depletes endogenous Tregs while concurrently inducing marked increases of the proinflammatory cytokines IL-12 and IFNγ in the serum
To further investigate the mechanism of cyclophosphamide mediated enhanced antitumor efficacy of 19mz modified T cells, we assessed the impact of this conditioning regimen on endogenous immune-suppressive Tregs. Significantly, we found a marked depletion of Tregs after cyclophosphamide therapy in the peripheral blood of conditioned mice (Figure 2C). In addition, we assessed the impact of cyclophosphamide conditioning therapy on the serum cytokine profiles of these mice, demonstrating increased levels of the proinflammatory cytokines IL-12 and IFNγ (Figure 2D).
19mz+ T cells further genetically modified to secrete IL-12 induce B-cell aplasias and eradicate EL4(hCD19) tumors in the absence of prior cyclophosphamide conditioning
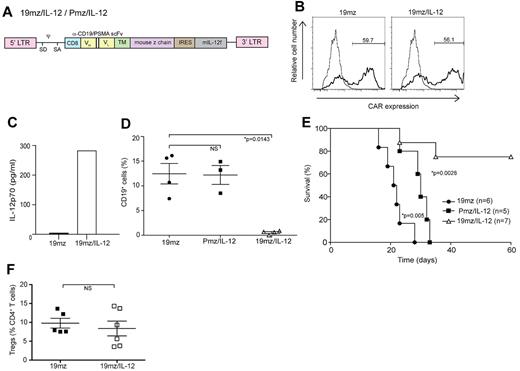
We next assessed whether the observed cyclophosphamide-mediated IL-12 cytokine surge, with subsequent presumed IL-12– induced IFNγ up-regulation, was relevant to the enhanced efficacy of 19mz-modified T-cell in vivo activity. We modified the 19mz and Pmz retroviral constructs with an IRES followed by a gene encoding a murine IL-12 α and β subunit fusion (flexi–IL-12 [mIL-12f]; Figure 3A).14 Gene transfer of the 19mz/IL-12 retroviral vector into mouse T cells was comparable to that of the parental 19mz retroviral construct (Figure 3B), with the former but not the latter T cells secreting marked levels of IL-12 (Figure 3C). Biologic activity of the secreted mIL-12f was further validated by measured IFNγ secretion from mouse splenocytes when cultured in conditioned 19mz/IL-12–transduced retroviral phoenix ecotropic viral-producing cell line supernatant compared with 19mz conditioned supernatant or unconditioned media alone (supplemental Figure 5).
T cells (19mz/IL-12+) induce B-cell aplasias and tumor eradication in the absence of prior cyclophosphamide conditioning. (A) Schematic of CAR/IL-12 retroviral constructs. IRES indicates internal ribosome entry site. (B) 19mz and 19mz/IL-12 expression after retroviral gene transfer as assessed by flow cytometry demonstrated similar gene transfer. (C) Supernatant from 19mz/IL-12+ but not 19mz+ T cells demonstrates enhanced levels of IL-12p70. (D) B-cell aplasias in EL4(hCD19) tumor-bearing C57BL6(mCD19−/−hCD19+/−) mice treated with 19mz/IL-12, but not Pmz/IL-12 or 19mz+ T cells, as assessed by flow cytometric analysis of peripheral blood samples at 2 weeks postmodified T-cell infusion. (E) 19mz/IL-12+ T cells eradicated disease and enhanced the survival of EL4(hCD19) tumor-bearing mice. In contrast, Pmz/IL-12 T cells significantly enhanced the survival of EL4(hCD19) tumor-bearing mice compared with 19mz+ T cell treated mice but similarly failed to eradicate disease. (F) Tregs in the BM of 19mz/IL-12+ and 19mz+ T cell treated mice as assessed by flow cytometry. All results are representative of at least 2 independent experiments.
T cells (19mz/IL-12+) induce B-cell aplasias and tumor eradication in the absence of prior cyclophosphamide conditioning. (A) Schematic of CAR/IL-12 retroviral constructs. IRES indicates internal ribosome entry site. (B) 19mz and 19mz/IL-12 expression after retroviral gene transfer as assessed by flow cytometry demonstrated similar gene transfer. (C) Supernatant from 19mz/IL-12+ but not 19mz+ T cells demonstrates enhanced levels of IL-12p70. (D) B-cell aplasias in EL4(hCD19) tumor-bearing C57BL6(mCD19−/−hCD19+/−) mice treated with 19mz/IL-12, but not Pmz/IL-12 or 19mz+ T cells, as assessed by flow cytometric analysis of peripheral blood samples at 2 weeks postmodified T-cell infusion. (E) 19mz/IL-12+ T cells eradicated disease and enhanced the survival of EL4(hCD19) tumor-bearing mice. In contrast, Pmz/IL-12 T cells significantly enhanced the survival of EL4(hCD19) tumor-bearing mice compared with 19mz+ T cell treated mice but similarly failed to eradicate disease. (F) Tregs in the BM of 19mz/IL-12+ and 19mz+ T cell treated mice as assessed by flow cytometry. All results are representative of at least 2 independent experiments.
Infusion of 19mz/IL-12+ but not 19mz+ or control Pmz/IL-12+ T cells induced a B-cell aplasia and long-term survival of tumor-bearing mice in the absence of prior cyclophosphamide conditioning (Figures 3D-E). Of note, IL-12–secreting Pmz-modified T cells significantly enhanced survival but failed to eradicate systemic EL4(hCD19) tumors (Figure 3E). Treatment with 19mz/IL-12+ T cells did not affect the percentage of CD4+ Tregs relative to non-Treg CD4+ T cells in the BM compared with tumor-bearing mice treated with 19mz+ T cells (Figure 3F). Collectively, these data demonstrate that IL-12–secreting hCD19-targeted T cells overcome the need for prior conditioning to eradicate targeted hCD19+ normal B cells and hCD19+ tumor cells in vivo.
19mz/IL-12+ T cells display enhanced cytotoxcity, resistance to Treg-mediated inhibition, an altered cytokine profile, and up-regulated expression of CD25 in vitro
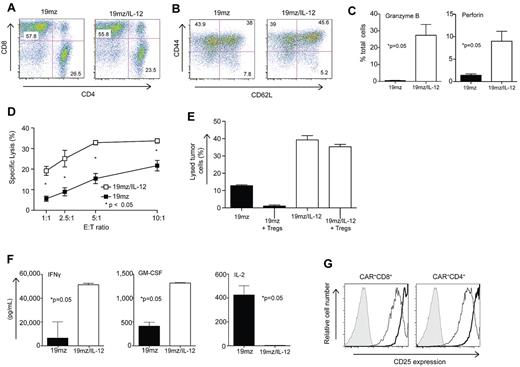
In vitro analysis comparing 19mz/IL-12+ to 19mz+ T cells revealed a preserved CD4/CD8 ratio (Figure 4A), but a trend toward an increased fraction of T cells with a Tcm phenotype in the IL-12–secreting T-cell population (46% vs 38%; Figure 4B), consistent with previously published data.20 Furthermore, IL-12–secreting 19mz+ T cells exhibited a statistically significant increase in the percentage of T cells expressing intracellular perforin and granzyme B compared with 19mz+ T cells (Figure 4C), corresponding to an enhanced in vitro cytotoxicity as assessed by a standard 51Cr release assay (Figure 4D). Significantly, in vitro, 19mz/IL-12 T cells have enhanced killing compared with 19mz+ T cells when previously cocultured with isolated Tregs (Figure 4E). In addition, in vitro cytokine analyses from tissue culture supernatants derived from activated 19mz/IL-12+ T cells demonstrated predicted increases in IL-12 (Figure 3C) and IFN-γ, as well as enhanced granulocyte macrophage colony–stimulating factor (GM-CSF), and a marked absence of IL-2 compared with supernatants from activated 19mz+ T cells (Figure 4F). IL-12–secreting T cells also demonstrated increased expression of the CD25 activation marker (Figure 4G).
In vitro, 19mz/IL-12–modified T cells, compared with 19mz+ T cells, exhibit enhanced cytotoxicity, resistance to Treg-mediated inhibition, a proinflammatory cytokine profile and enhanced CD25 expression. (A) 19mz/IL-12 and 19mz transduced T-cell populations were composed of equivalent CD4 and CD8 T cells, as detected by flow cytometry with the former exhibiting enhanced but not statistically significant increase in expression of memory T-cell markers (B). (C) 19mz/IL-12+ T cells have significantly higher expression of perforin and granzyme B compared with 19mz+ T cells. (D) As determined by standard 51Cr release assays 19mz/IL-12+ T cells have significantly increased ability to lyse EL4(hCD19) tumor cells compared with 19mz+ T cells. (E) 19mz/IL-12+ T cells, but not 19mz+ T cells retain capacity to lyse EL4(hCD19) tumor after preincubation with Tregs. (F) 19mz/IL-12+ T cells secrete increased levels of IFNγ, GM-CSF but fail to secrete IL-2 compared with 19mz+ T cells after coculture with EL4(hCD19) tumor cells at an E:T of 1:10 for 24 hours. (G) 19mz/IL-12+ T cells (thick lines) express higher levels of CD25 compared with 19mz+ T cells (thin lines), as detected by flow cytometry. Results are representative of at least 2 independent experiments.
In vitro, 19mz/IL-12–modified T cells, compared with 19mz+ T cells, exhibit enhanced cytotoxicity, resistance to Treg-mediated inhibition, a proinflammatory cytokine profile and enhanced CD25 expression. (A) 19mz/IL-12 and 19mz transduced T-cell populations were composed of equivalent CD4 and CD8 T cells, as detected by flow cytometry with the former exhibiting enhanced but not statistically significant increase in expression of memory T-cell markers (B). (C) 19mz/IL-12+ T cells have significantly higher expression of perforin and granzyme B compared with 19mz+ T cells. (D) As determined by standard 51Cr release assays 19mz/IL-12+ T cells have significantly increased ability to lyse EL4(hCD19) tumor cells compared with 19mz+ T cells. (E) 19mz/IL-12+ T cells, but not 19mz+ T cells retain capacity to lyse EL4(hCD19) tumor after preincubation with Tregs. (F) 19mz/IL-12+ T cells secrete increased levels of IFNγ, GM-CSF but fail to secrete IL-2 compared with 19mz+ T cells after coculture with EL4(hCD19) tumor cells at an E:T of 1:10 for 24 hours. (G) 19mz/IL-12+ T cells (thick lines) express higher levels of CD25 compared with 19mz+ T cells (thin lines), as detected by flow cytometry. Results are representative of at least 2 independent experiments.
hCD19-targeted efficacy of 19mz+ T cells is dependent on sustained IL-12 stimulation in vivo and requires both CD4+ and CD8+ 19mz/IL-12+ T-cell subsets
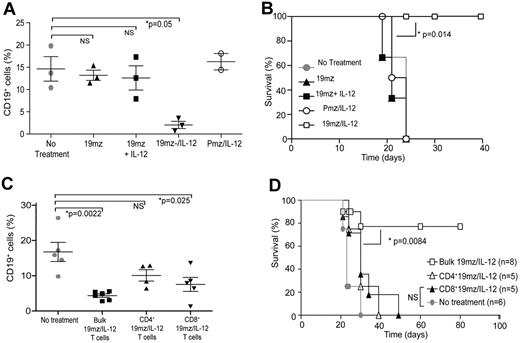
We next assessed whether ex vivo expansion of 19mz+ T cells in the context of exogenous IL-12 would be sufficient to induce a T-cell population capable of similar in vivo hCD19-targeted efficacy in the absence of prior conditioning, compared with treatment with 19mz/IL-12+ T cells. We found that treatment of mice with 19mz+ T cells “primed” with exogenous IL-12 during ex vivo gene transfer and culture failed to recapitulate the subsequent B-cell aplasias and enhanced survival observed in mice treated with IL-12–secreting T cells, despite these cells having a similar phenotype to 19mz/IL-12+ T cells at the time of infusion (Figure 5A-B), demonstrating the need for continuous IL-12 activation of tumor-targeted T cells in vivo to sustain the observed enhanced anti-hCD19 efficacy of 19mz/IL-12–modified T cells.
In vivo antitumor efficacy of 19mz+ T cells is dependent on continued IL-12 activation in vivo, and requires the presence of both CD4 and CD8 T cells subsets. (A) Similar to 19mz+ and Pmz/IL-12+ T-cell therapy, treatment of C57BL6(mCD19−/−hCD19+/−) mice bearing systemic EL4(hCD19) tumors with 19mz+ T cells cultured ex vivo with exogenous IL-12, in contrast to 19mz/IL-12+ T-cell treated mice failed to induce either B-cell aplasias or mediate tumor eradication (B). (C) Sorted CD4+ 19mz/IL-12+ T cells failed to induce B-cell aplasias in EL4(hCD19mCD80) tumor-bearing mice, in contrast to CD8+ 19mz/IL-12+ T cells, which induced a partial but significant B-cell aplasia and bulk 19mz/IL-12+ T cells, which induced significant B-cell aplasias. (D) In addition, in contrast to bulk 19mz/IL-12+ T cells, treatment with either sorted CD4+ or CD8+ 19mz/IL-12+ T cells alone failed to eradicate systemic EL4(hCD19+) tumors. Results shown are representative of 2 independent experiments with similar results.
In vivo antitumor efficacy of 19mz+ T cells is dependent on continued IL-12 activation in vivo, and requires the presence of both CD4 and CD8 T cells subsets. (A) Similar to 19mz+ and Pmz/IL-12+ T-cell therapy, treatment of C57BL6(mCD19−/−hCD19+/−) mice bearing systemic EL4(hCD19) tumors with 19mz+ T cells cultured ex vivo with exogenous IL-12, in contrast to 19mz/IL-12+ T-cell treated mice failed to induce either B-cell aplasias or mediate tumor eradication (B). (C) Sorted CD4+ 19mz/IL-12+ T cells failed to induce B-cell aplasias in EL4(hCD19mCD80) tumor-bearing mice, in contrast to CD8+ 19mz/IL-12+ T cells, which induced a partial but significant B-cell aplasia and bulk 19mz/IL-12+ T cells, which induced significant B-cell aplasias. (D) In addition, in contrast to bulk 19mz/IL-12+ T cells, treatment with either sorted CD4+ or CD8+ 19mz/IL-12+ T cells alone failed to eradicate systemic EL4(hCD19+) tumors. Results shown are representative of 2 independent experiments with similar results.
In addition, we assessed the individual and complimentary roles of CD4+ and CD8+ 19mz/IL-12+ T-cell subsets in the observed in vivo efficacy of these IL-12–secreting tumor-targeted T cells. Similar to our cyclophosphamide conditioning studies (supplemental Figure 3), we found that optimal antitumor efficacy was dependent on both CD4+ and CD8+ CAR-modified T-cell subsets because treatment of tumor-bearing mice with either 19mz/IL-12 modified CD4+ or CD8+ T cells induced only relative B-cell aplasias (Figure 5C) and failed to eradicate systemic EL4(hCD19) tumors (Figure 5D).
In vivo hCD19-targeted efficacy of 19mz/IL-12+ T cells is dependent on both autocrine IL-12 stimulation as well as IFNγ secretion by 19mz/IL-12–modified T cells
We next assessed whether observed IL-12 mediated in vivo anti-hCD19–targeted efficacy was dependent on autocrine IL-12 stimulation of 19mz/IL-12–modified T cells. We compared the in vivo efficacy of 19mz/IL-12–transduced T cells derived from IL-12Rβ2−/− transgenic mice (no autocrine IL-12 stimulation) to wild-type (WT)–transduced T cells (autocrine IL-12 stimulation). Collectively, we found that 19mz/IL-12+ IL-12Rβ2−/− T cells, in contrast to WT 19mz/IL-12+ T cells, unequivocally failed to induce either B-cell aplasias (Figure 6A) or promote survival of EL4(hCD19) tumor-bearing mice (Figure 6B). These data demonstrate a requisite role for in vivo autocrine IL-12 stimulation of CAR-modified T cells to achieve the observed enhanced hCD19-targeted efficacy of IL-12–secreting tumor targeted T cells.
In vivo antitumor efficacy of IL-12–secreting targeted T cells is dependent on autocrine IL-12 stimulation and IFNγ secretion. (A) 19mz/IL-12+ T cells derived from C57BL6 IL-12Rβ2−/− mice, compared with 19mz/IL-12+ T cells derived from syngeneic C57BL6(mCD19−/− hCD19+/−) mice failed to induce either B-cell aplasias or eradicate systemic EL4(hCD19) tumors in C57BL6(mCD19−/− hCD19+/−) mice (B). (C) Similarly, 19mz/IL-12+ T cells derived from C57BL6 IFNγ−/− mice, compared with 19mz/IL-12+ T cells derived from syngeneic C57BL6(mCD19−/− hCD19+/−) mice were unable to induce B-cell aplasias, or eradicate systemic EL4(hCD19) tumors in C57BL6(mCD19−/− hCD19+/−) mice (D). Results shown are representative of 2 independent experiments.
In vivo antitumor efficacy of IL-12–secreting targeted T cells is dependent on autocrine IL-12 stimulation and IFNγ secretion. (A) 19mz/IL-12+ T cells derived from C57BL6 IL-12Rβ2−/− mice, compared with 19mz/IL-12+ T cells derived from syngeneic C57BL6(mCD19−/− hCD19+/−) mice failed to induce either B-cell aplasias or eradicate systemic EL4(hCD19) tumors in C57BL6(mCD19−/− hCD19+/−) mice (B). (C) Similarly, 19mz/IL-12+ T cells derived from C57BL6 IFNγ−/− mice, compared with 19mz/IL-12+ T cells derived from syngeneic C57BL6(mCD19−/− hCD19+/−) mice were unable to induce B-cell aplasias, or eradicate systemic EL4(hCD19) tumors in C57BL6(mCD19−/− hCD19+/−) mice (D). Results shown are representative of 2 independent experiments.
Given that IL-12 up-regulates IFN-γ secretion from T cells, we next assessed whether secretion of this proinflammatory cytokine played a role in the observed enhanced targeted efficacy of IL-12 modified hCD19-targeted T cells. To this end, we compared mice treated with 19mz/IL-12+ T cells derived from C57BL6 INF-γ−/− mice to mice treated with WT 19mz/IL-12+ T cells. In contrast to the WT 19mz/IL-12+ T cell treated cohort, mice treated with 19mz/IL-12+ INF-γ−/− T cells failed to induce either B-cell aplasias (Figure 6C) or eradicate systemic EL4(hCD19) tumors as assessed by long-term survival of tumor-bearing mice (Figure 6D). These findings are consistent with a requisite role for IFNγ in the observed enhanced anti-hCD19–targeted efficacy of 19mz/IL-12 modified T cells.
Discussion
Adoptive therapy with tumor-specific T cells has demonstrated significant antitumor responses in the clinical setting.2-4,21 However, the observed clinical responses require prior conditioning of patients with total body irradiation and/or lymphodepleting chemotherapy. Optimal antitumor responses correlate to increased intensity of these conditioning regimens.4 Unfortunately, many patients with cancer, because of either age or prior chemotherapy and radiation therapy, are intolerant to the intense conditioning regimens requisite to achieving optimal antitumor benefit from adoptively transferred tumor-targeted T cells. Therefore, alternative approaches are needed to generate optimal antitumor efficacy in the absence of prior conditioning to make the adoptive T-cell approach universally applicable.
Although the majority of published adoptive T-cell clinical studies are focused on ex vivo expanded autologous tumor–derived T cells (tumor infiltrating lymphocytes [TILs]), recent clinical trial reports from our center and others have demonstrated very promising antitumor responses on infusion with autologous gene–modified T cells genetically targeted to tumor through a CD19- specific CAR. Although currently largely anecdotal, responses to therapy were universally achieved in the context of conditioning chemotherapy before CAR-modified T-cell infusion.1-3 These clinical data are consistent with that of patients treated with ex vivo expanded TILs, demonstrating the critical role of conditioning on subsequent antitumor efficacy. In contrast, similar studies using a GD2-specific CAR for the treatment of neuroblastoma demonstrated antitumor efficacy in the absence of preconditioning; however, the reason for this observed difference is currently unclear.22,23
The precise mechanisms whereby prior conditioning therapy enhances the antitumor efficacy of infused tumor-targeted T cells are speculative. However, based on preclinical mouse studies several mechanisms have been proposed, including depletion of endogenous lymphocytes through conditioning regimens, thereby removing these endogenous lymphocytes, which crowd lymphoid tissues and otherwise serve as “cytokine sinks” absorbing proinflammatory cytokines.24 Therefore, depletion of endogenous lymphocytes in turn may allow for “homeostatic” proliferation and enhanced access to proinflammatory cytokines by the adoptively transferred T-cell population.25 In addition, prior conditioning eradicates endogenous Tregs that may otherwise suppress infused tumor-targeted T cells.26 More recently, preclinical models demonstrate that conditioning regimens induce translocation of bacterial products from the gut, which may stimulate T cells through toll-like receptors.27 Finally, in the context of CAR-modified T cells targeted to the B-cell CD20 antigen, investigators have demonstrated enhanced antitumor activity of CD20-targeted T cells against CD20+ lymphomas in mice on prior depletion of normal B cells expressing the CD20 antigen.19 In this case, lymphodepletion removes what could be viewed as an “antigen sink” distracting modified T-cell trafficking to the tumor sites and potentially inducing modified T-cell anergy or apoptosis at sites of normal B cells disparate from the tumor.
To better understand the in vivo biology of CAR-modified T cells, we generated a more clinically relevant murine tumor model using immune competent transgenic C57BL6(mCD19−/− hCD19+/−) mice with restricted expression of functional hCD19 on normal B cells. When infused systemically with hCD19-targeted syngeneic T cells alone, this therapy failed to either eradicate systemic EL4(hCD19) tumor cells or induce predicted aplasias of normal hCD19+ B cells. However, consistent with published clinical studies,3,4 prior conditioning with cyclophosphamide chemotherapy induced profound B-cell aplasias as well as eradicated systemic EL4(hCD19) tumors. These findings validate the clinical relevance of this model.
Having established a conditioning-dependent model of CAR-modified T cell–mediated tumor eradication with an easily monitored surrogate marker of CAR-modified T-cell in vivo efficacy, namely B-cell aplasias, we used this model to investigate potential mechanisms whereby cyclophosphamide conditioning enhanced CAR T-cell therapy. In our model, the hCD19+ normal B-cell compartment does not appear to be a primary mechanism of 19mz+ T-cell failure because prior monoclonal antibody (mAb) depletion of this hCD19 “antigen sink” did not overcome the need for conditioning for 19mz+ T cells to eradicate systemic EL4(hCD19) tumors. Our results contrast with previously published findings wherein mAb-mediated B-cell depletion resulted in increased trafficking of CD20-targeted CAR-modified T cells to tumor and increased antitumor efficacy.19 Although the etiologies of these differing outcomes are unclear, it is worth noting that there are significant differences in the tumor models and chimeric receptors used that may account for these contrasting results.
We next assessed the impact of cyclophosphamide conditioning on endogenous Treg persistence and the serum cytokine milieu. We found a significant decrease in circulating Tregs, consistent with previous reports.26 In addition, we noted an altered cytokine profile after cyclophosphamide treatment, with a marked increase in the levels of the proinflammatory cytokines IL-12 and IFNγ. The former finding was expected; however, the latter was interesting. Given the proinflammatory effects of IL-12, including the induction of T-cell IFNγ secretion, we postulated that the cyclophosphamide-induced release of this cytokine may play a role in the enhanced antitumor activity of the adoptively transferred CAR+ T cells after conditioning chemotherapy.
IL-12 is a pleiotropic cytokine with several immune-stimulatory functions. IL-12 stimulation of T cells results in increased IFNγ secretion and enhanced expression of the cytolytic proteins granzyme B and perforin, leading to increased cytotoxic capacity.28 In the context of T-cell stimulation and costimulation (signals 1 and 2), IL-12 has been demonstrated to provide a signal 3, resulting in increased function and homeostatic expansion of T cells.29,30 In addition, it has been reported that IL-12 can prevent the cycling of Tregs as well as mediate resistance to these suppressive cells in an IFNγ-dependent fashion.31,32 The necessity of IFNγ for the immune-stimulatory effects of IL-12 are well documented.28,32,33 Given the favorable proinflammatory and immune-stimulatory effects of IL-12, combined with our finding of increased IL-12 levels after cyclophosphamide conditioning, we postulated that CAR+ T cells also modified to express IL-12 could serve as a surrogate to the conditioning required for effective adoptive T-cell transfer therapy.
Using this approach, we found that IL-12–secreting hCD19-targeted T cells induced both aplasias of normal B cells as well as eradication of established systemic hCD19+ tumor cells in the absence of prior cyclophosphamide conditioning. The mechanisms by which IL-12 obviates the need for prior conditioning in our model are probably multifactorial. IL-12 itself may induce or enhance the endogenous immune system to target established tumors. To this end, we noted that tumor-bearing mice treated with control Pmz/IL-12 T cells mediated a significant but modest enhanced survival but not complete tumor eradication compared with nontreated control mice (Figure 3E). This finding is consistent with previously published reports demonstrating enhanced antitumor efficacy mediated by IL-12 alone.33,34
Our data support a role for enhanced cytotoxic potential of 19mz/IL-12+ T cells as one contributing mechanism for the observed in vivo CD19-targeted activity. However, this in vitro finding is unlikely to fully explain our in vivo results given the fact that B-cell aplasias, as well as tumor eradication, were synergistically dependent on both CD8+ T cell–mediated cytotoxicity, as well as CD4+ T-cell helper function. Interestingly, this finding may also reconcile our results compared with previously published studies wherein infusion with IL-12–secreting tumor-targeted CD8+ T cells alone still required prior conditioning to achieve optimal antitumor efficacy.35 In these studies, the authors proposed that conditioning therapy retained relevance because of continued dependence on depletion of endogenous lymphocyte “cytokine sinks” and Tregs, which have been previously demonstrated to suppress the function of CAR-modified T cells.17 However, the lack of targeted CD4+ IL-12–secreting T cells in these latter studies may be an alternative explanation for these conflicting results.
We significantly observed that ex vivo expansion of 19mz T cells in the context of exogenous IL-12 induced a Tcm phenotype with up-regulated CD25 (at the time of infusion) failed to replicate our in vivo findings using 19mz/IL-12+ T cells consistent with the notion that CAR-modified T cells required continuous IL-12 stimulation in vivo. This conclusion was further verified by additional in vivo studies where treatment with 19mz/IL-12+ T cells derived from either IL-12Rβ2−/− or IFNγ−/− mice failed to either induce B-cell aplasias or eradicate established EL4(hCD19) tumors. Collectively, these data imply that a critical role of continued autocrine IL-12 stimulation with consequent IFNγ release in the observed targeted hCD19 T cell mediated efficacy in the absence of prior conditioning therapy.
Given previously published reports regarding the role of IFNγ in mediating T-cell resistance to Treg immune suppression,32 we further explored whether 19mz/IL-12+ T cells were similarly refractory to Treg-mediated inhibition. We found that 19mz/IL-12+ T cells retain cytotoxic capacity in the presence of Tregs in vitro. This finding is consistent with the dependence of 19mz/IL-12+ T cells on autocrine IL-12 stimulation to induce in vivo B-cell aplasias and tumor eradication in our tumor model. Findings further in line with this Treg-mediated resistance mechanism include decreased levels of IL-2 within in vitro 19mz/IL-12+ T-cell cultures and a concomitant increase in CD25 expression, which in turn could deprive endogenous Tregs of IL-2.20 This mechanism could also explain the observed outcomes despite the presence of Tregs in 19mz/IL-12+ T-cell treated mice (Figure 3F). In addition, the dependence of 19mz/IL-12+ T cells on secretion of IFNγ, a cytokine previously described to mediate resistance to Treg inhibition,32 is consistent with this mechanism. Therefore, although it is probable that several mechanisms contribute to the observed findings, our studies and others implicate a significant role of autocrine IL-12 and IFNγ secretion by modified T cells in overcoming Treg inhibition in vivo.31,32
Promising preclinical studies demonstrating IL-12 as a potential cytokine mediating endogenous antitumor responses have led to several clinical studies exploring systemic infusion of IL-12 in patients with a variety of advanced tumors.36-38 To date the clinical outcomes from these studies have been modest with significant treatment-related toxicities.39,40 For this reason, enthusiasm for IL-12 as an antitumor agent has been tempered. However, subsequent preclinical and clinical reports demonstrate increased safety with delivery of IL-12 directly to the tumor site.41 Given the fact that our targeted T cells ideally would deliver IL-12 directly to the tumor site, we anticipate an increased safety profile on translation to the clinical setting. Consistent with this hypothesis, in our clinically relevant tumor model, treatment with IL-12–secreting T cells was well tolerated with no toxicities noted in treated mice. This outcome is in contrast to previously published reports demonstrating dose- limiting toxicity of IL-12–secreting, tumor-targeted T cells,35 where transfer of > 500 000 IL-12–secreting T cells into sublethally irradiated mice resulted in decreased survival, attributed by the authors to the increase in systemic levels of IL-12. We failed to observe enhanced levels of IL-12 or IFNγ in the serum of treated mice (data not shown), which may potentially be related to the lack of preconditioning in our studies. In addition, previously published preclinical studies of adoptive therapy using IL-12–secreting T cells implicate important IL-12–mediated effects on macrophages and myeloid- derived suppressor cells demonstrated in subcutaneous tumor models.42,43 In contrast, we did not find significant changes in myeloid-derived suppressor cells or dendritic cells but did note increased macrophages in the BM of treated mice (H.J.P., unpublished observations) in our studies. Once again, these conflicting outcomes may be due to the differences in pretreatment conditioning, tumor targets, and T-cell targeting strategies.
In conclusion, we present a novel “conditioning-free” approach of adoptive targeted T-cell therapy of cancer using IL-12–secreting tumor-targeted T cells, which will allow for the application of CAR-modified T cells to virtually all patient populations including those otherwise intolerant to currently requisite toxic conditioning therapies. Translation of this technology to the clinical setting in the form of clinical trials using IL-12–secreting CAR-modified T cells is planned with safety mechanisms including control of IL-12 expression through an NFAT (nuclear factor of activated T cells) promoter and additional inclusion of a suicide gene in the retroviral vector.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health (grants CA95152, CA138738, CA059350, CA08748); Alliance for Cancer Gene Therapy; Damon Runyon Clinical Investigator Award; The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York; Kate's Team; Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center; and the Geoffrey Beene Cancer Foundation. T.F.T. is supported by AI56363 and AI057157. E.H. is a Howard Hughes Medical Institute award recipient.
National Institutes of Health
Authorship
Contribution: R.J.B., M.S., H.J.P., J.C.L., and E.G.H. designed the concept and experiments, and interpreted the data; H.J.P., J.C.L., E.G.H., and G.H.I. performed experiments; R.J.B. and J.C.L. developed the syngeneic model; T.F.T. contributed to the conception of the project and provided critical reagents; and R.J.B. and H.J.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renier Brentjens, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 42, New York, NY 10021; e-mail: brentjer@mskcc.org.
References
Author notes
H.J.P. and J.C.L. contributed equally to this work



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal