Abstract
Autophagy is the process by which superfluous or damaged macromolecules or organelles are degraded by the lysosome. Pharmacologic and genetic evidence indicates that autophagy plays pleiotropic functions in cellular homeostasis, development, survival, and differentiation. The differentiation of human blood monocytes into macrophages is a caspase-dependent process when triggered ex vivo by colony stimulating factor-1. We show here, using pharmacologic inhibitors, siRNA approaches, and Atg7−/− mice, that autophagy initiated by ULK1 is required for proper colony stimulating factor-1–driven differentiation of human and murine monocytes. We also unravel a role for autophagy in macrophage acquisition of phagocytic functions. Collectively, these findings highlight an unexpected and essential role of autophagy during monocyte differentiation and acquisition of macrophage functions.
Introduction
Autophagy (macroautophagy) is a general mechanism for degradation and recycling of macromolecules that is characterized by the formation of double-membrane vesicles called phagosomes that derive from phagophores.1 Autophagy plays a crucial role in cellular homeostasis, development, differentiation, cell death and survival, and ageing.2-4 Although autophagy functions mainly as a safeguard mechanism during nutrient starvation, excessive autophagy leads to cell death.5
Monocytes have the unique property to migrate into tissues in response to inflammation where they are subjected to differentiation into morphologically and functionally heterogeneous cells, such as macrophages, myeloid dendritic cells, and osteoclasts, depending on the stimulus.6 The differentiation of human peripheral blood monocytes into macrophages can be reproduced ex vivo by exposure to colony stimulating factor-1 (CSF-1; also known as M-CSF), a process that requires limited activation of caspase-8 and caspase-3.7 Binding of CSF-1 to its receptor CSF-1R triggers successive waves of AKT activation, leading to the formation of a caspase-8 activating platform and differentiation of monocytes into macrophages.7 In the present study, we investigated the implication of autophagy in monocyte differentiation and acquisition of macrophage functions.
Methods
Human monocyte culture and differentiation
Human peripheral blood monocytes were obtained from healthy donors with informed consent following the Declaration of Helsinki according to recommendations of an independent scientific review board. Experiments on mice were performed with the approval of the ethics committee of the University of Nice. Human and mouse monocytes were enriched with autoMACS Separator (Miltenyi Biotec). Macrophage differentiation was visualized using standard optics (Carl Zeiss), electronic microscopy, flow cytometry, and phagocytic assay. A detailed description is in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunoblot assays
Western blot analysis has been described previously.7 Cytoplasmic and microsomal fractions were carried out according to the manufacturer's instructions (proteoExtract Subcellular Proteome Extraction KIT; Calbiochem) and analyzed by immunoblotting.
Cathepsin activity measurement
After stimulation, cells were lysed (50mM Tris, pH 7.4, 150mM NaCl, 1mM phenylmethylsulfonyl fluoride, 1% Triton X-100) and cellular extracts were incubated in 96-well plates with z-RR-AMC (cathepsin B) or z-FR-AMC (cathepsin B/L) as substrates for various times at 37°C. Cathepsin activity was measured by following emission at 460 nm (excitation at 390 nm). Each experiment was performed in quadruplicates and repeated at least 4 times.
siRNA knockdown
siRNA were introduced into monocytes by nucleoporation (Amaxa) as described previously.8 We used siRNAs targeting ULK1, Beclin-1 (BEC#1 and BEC#2), Atg7 (ATG7#1 and ATG7#2), Atg5 (ATG5#1 and ATG5#2), and luciferase as a negative control. Sequences are accessible on request (Invitrogen).
Results and discussion
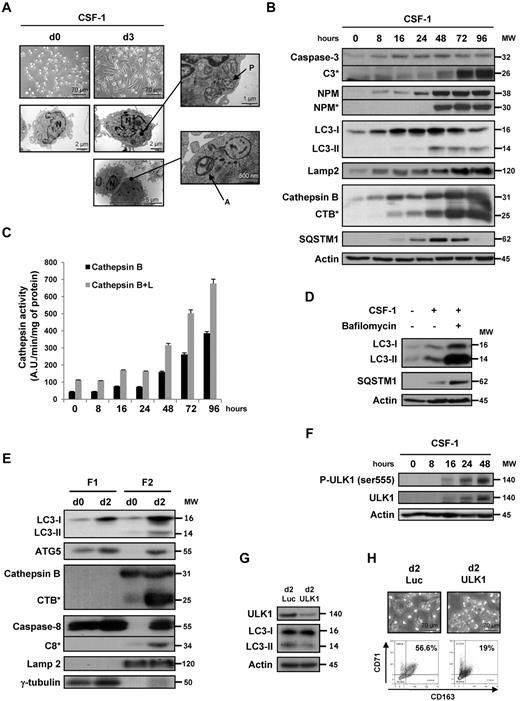
One of the features of CSF-1–induced ex vivo monocyte differentiation is rapid adherence to the culture flask with a characteristic fibroblast-like shape, as judged by phase-contrast microscopy at day 3 (Figure 1A top panels). Electronic microscopy images of these cells showed typical autophagic structures, including double membranes surrounding cytoplasmic material and/or mitochondria, which correspond to autophagosomes (Figure 1A right panels). In accordance with our previous observations,7 we detected caspase-3 proteolysis and nucleophosmin (NPM) cleavage in monocytes that have been exposed for 48 hours to CSF-1 (Figure 1B). The accumulation of LC3-II, which is a hallmark of autophagy, was detected in CSF-1–treated monocytes as soon as 16 hours after the addition of CSF-1, which was earlier than caspase activation. Concomitantly, cathepsin B (CTSB) was cleaved into its active form, which is a feature of lysosomal activation, and the expression of p62/SQSTM1 (sequestosome) and LAMP2, a lysosomal marker, increased. Accumulation of p62 during CSF-1–induced differentiation is the balance between increased expression of the protein and its degradation by lysosomal proteases.5 This is probably the reason why p62 increased in the first 2 days after CSF-1 addition and next decreased, reflecting its specific degradation in autophagosomes. Cathepsin activities increased gradually as a function of time during macrophagic differentiation (Figure 1C). The occurrence of autophagy reflected increased lysosomal flux because LC3-II and p62 accumulations raised in the presence of bafilomycin A1, an inhibitor of the lysosomal vacuolar H+-ATPase (Figure 1D). Autophagy was not observed in human monocytes that differentiate into dendritic cells on exposure to GM-CSF plus IL-4, as judged by the lack of accumulation of LC3-II, CTSB activation, and caspase-3 and NPM proteolysis (supplemental Figure 1A). Finally, autophagy induction was also detected during differentiation of monocytes induced by GM-CSF or CSF-1 plus RANK-ligand but not on IL-6 treatment (supplemental Figure 1B).
Autophagy is induced during macrophagic differentiation of human monocytes. Human peripheral blood monocytes from healthy donors were exposed for the indicated times to 100 ng/mL CSF-1. (A) Electron microscopy images showing ultrastructural features of a representative monocyte (d0) and morphologic features of autophagy in monocytes treated for 3 days (d3) with CSF-1. P indicates phagophore; A, autophagosome; and N, nuclei. (B) Immunoblot analysis of caspase-3, NPM, LC3, Lamp2, CTSB, and SQSTM1 in monocytes exposed for the indicated times to CSF-1. Actin is used as a loading control. *Cleavage fragments. Molecular weights (MW) are in kDa. (C) Measurement of CTSB and B + L activities using Z-RR-AMC or Z-FR-AMC as substrates, respectively, in monocytes treated with CSF-1. Results, expressed as arbitrary units (A.U.) per minute and per milligram of protein, are the mean ± SD of 4 independent experiments performed in quadruplicate. (D) Monocytes were exposed for 3 days to 100 ng/mL alone or in association with bafilomycin A1 (10nM) added 48 hours after CSF-1 treatment, and protein expression was analyzed by immunoblot. Actin is used as a loading control. (E) Monocytes were exposed for 2 days to CSF-1 before collecting cytoplasmic (F1) and microsomal (F2) extracts that were analyzed by immunoblot. Lamp2 and γ-tubulin are used as a control for microsomal and cytoplasmic fractions, respectively. (F) Immunoblot analysis of phospho-ULK1 (Ser555) and ULK1 in monocytes exposed for the indicated times to CSF-1. (G) Monocytes were transfected with siRNA targeting Luciferase (Luc) or ULK1 and exposed 2 days to CSF-1. The expression of ULK1 in transfected cells was analyzed by immunoblot. (F-G) Actin is used as a loading control. (H) Monocytes were transfected with siRNA targeting Luciferase (Luc) or ULK1 and exposed 2 days to CSF-1. Macrophage differentiation was examined morphologically (fibroblastic shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163.
Autophagy is induced during macrophagic differentiation of human monocytes. Human peripheral blood monocytes from healthy donors were exposed for the indicated times to 100 ng/mL CSF-1. (A) Electron microscopy images showing ultrastructural features of a representative monocyte (d0) and morphologic features of autophagy in monocytes treated for 3 days (d3) with CSF-1. P indicates phagophore; A, autophagosome; and N, nuclei. (B) Immunoblot analysis of caspase-3, NPM, LC3, Lamp2, CTSB, and SQSTM1 in monocytes exposed for the indicated times to CSF-1. Actin is used as a loading control. *Cleavage fragments. Molecular weights (MW) are in kDa. (C) Measurement of CTSB and B + L activities using Z-RR-AMC or Z-FR-AMC as substrates, respectively, in monocytes treated with CSF-1. Results, expressed as arbitrary units (A.U.) per minute and per milligram of protein, are the mean ± SD of 4 independent experiments performed in quadruplicate. (D) Monocytes were exposed for 3 days to 100 ng/mL alone or in association with bafilomycin A1 (10nM) added 48 hours after CSF-1 treatment, and protein expression was analyzed by immunoblot. Actin is used as a loading control. (E) Monocytes were exposed for 2 days to CSF-1 before collecting cytoplasmic (F1) and microsomal (F2) extracts that were analyzed by immunoblot. Lamp2 and γ-tubulin are used as a control for microsomal and cytoplasmic fractions, respectively. (F) Immunoblot analysis of phospho-ULK1 (Ser555) and ULK1 in monocytes exposed for the indicated times to CSF-1. (G) Monocytes were transfected with siRNA targeting Luciferase (Luc) or ULK1 and exposed 2 days to CSF-1. The expression of ULK1 in transfected cells was analyzed by immunoblot. (F-G) Actin is used as a loading control. (H) Monocytes were transfected with siRNA targeting Luciferase (Luc) or ULK1 and exposed 2 days to CSF-1. Macrophage differentiation was examined morphologically (fibroblastic shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163.
Cellular fractionation demonstrated the accumulation of LC3-II in the microsomal fraction, in accordance with its well-admitted lipidation that targets it to the membrane of phagophores (Figure 1E). Cleaved caspase-8 and active CTSB were also detected in this fraction, with very high amounts of both active proteases at day 2 of differentiation. In addition, ATG5 was found in this fraction, implying that ATG proteins and caspase-8 coexist in the same compartment (Figure 1E). Altogether, autophagy is induced during the CSF-1–induced differentiation of monocytes into macrophages. To get insights into the mechanism of autophagy induction by CSF-1, we investigated the implication of ULK1, a kinase that initiates autophagy, in macrophagic differentiation. CSF-1 was shown to increase ULK1 protein level and phosphorylation on Ser555 (Figure 1F). Importantly, knockdown of ULK1 inhibited both LC3-II accumulation and macrophagic differentiation (Figure 1G-H).
To further determine the role of autophagy in the process of macrophage differentiation, we investigated the consequence of inhibiting autophagy by both pharmacologic and other siRNA approaches. Both 3-methyladenine (3-MA), a Vps34 inhibitor, and CA-074-Me (CA), a CTSB and L inhibitor, prevented CSF-1–induced differentiation of human monocytes, as indicated by the lack of characteristic morphologic changes and CD71 and CD163 expression increase (supplemental Figure 1C), without increasing the rate of cell death as judged by annexin-V/4,6-diamidino-2-phenylindole staining and caspase-3 and -8 activities (supplemental Figure 2A-C). In addition, autophagy inhibition was totally reversible when pharmacologic inhibitors were withdrawn for the culture medium at day 2 and cells restimulated with CSF-1 for 3 days (supplemental Figure 3A-B), indicating that this way of inhibiting autophagy was not deleterious for monocytes. Importantly, 3-MA and CA also abolished LC3-II accumulation and NPM cleavage at day 2 (supplemental Figure 1D). As expected, CA abrogated, whereas 3-MA only partially affected, CTSB and L activity (supplemental Figure 1E). Finally, autophagy was required for production of functional macrophages because 3-MA and CA treatment abolished their capacity to phagocyte bacteria (supplemental Figure 1F). To substantiate further the implication of autophagy in monocyte differentiation, we knocked down ATG6 (Beclin-1), ATG7, and ATG5 using 2 specific siRNAs (Figure 2A; supplemental Figure 1G). All siRNAs were found to dampen concomitantly the expression of their respective targets (Figure 2B) and to inhibit monocyte differentiation, as assessed by flow cytometry (Figure 2A), but none was observed to increase the rate of cell death as judged by annexin-V/4,6-diamidino-2-phenylindole staining and caspase 3 activity (supplemental Figure 2A-B). Finally, Beclin-1, ATG5, or ATG7 knockdown also impaired the ability of differentiated monocytes to phagocyte bacteria (Figure 2C).
Autophagy involvement in monocyte differentiation into macrophages. (A) Monocytes were transfected with siRNA targeting Luciferase (Luc), Beclin-1 (BEC), ATG7, or ATG5 and exposed 2 days to CSF-1. Macrophage differentiation was examined morphologically (fibroblastic shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163. (B) Monocytes were transfected with siRNA targeting Luciferase (Luc), Beclin-1 (BEC), ATG7, or ATG5 and exposed 2 days to CSF-1. The expression of Beclin-1, ATG7, ATG5, and LC3 in cells transfected with the indicated siRNA and treated for 2 days with CSF-1 was analyzed by immunoblot. Actin is used as a loading control. Molecular weights (MW) are in kDa. (C) Functional assay of monocytes transfected with Luciferase, Beclin-1, ATG7, or ATG5 siRNA and treated for 2 days with CSF-1. Results are expressed as fold induction compared with untreated monocyte and represent the mean ± SD of 4 independent experiments performed in triplicate. ***P < .001 (vs d2 LUC) according to a student paired t test. (D) Enriched bone marrow mouse monocytes were exposed for the indicated times to 100 ng/mL CSF-1. Differentiation was studied by morphologic examination (fibroblastic shape) and by 2-color flow cytometric analysis at indicated day. Percentages indicate cells that express both high CD11b and F4/80 staining. One representative of 5 independent experiments is shown. (E) Immunoblot analysis of NPM and LC3 in mouse monocytes exposed for the indicated times to CSF-1. Actin is used as a loading control. *Cleavage fragments. (F) Immunoblot analysis of ATG7 in monocytes obtained from WT or vav-Atg7−/− mice (n = 2). Actin is used as a loading control. (G) Monocytes obtained from WT or vav-Atg7−/− mice were exposed for the indicated times to 100 ng/mL CSF-1. Differentiation was studied by morphologic examination (fibroblastic shape) and by 2-color flow cytometric analysis at day 4. Percentages indicate cells that express both high CD11b and F4/80 staining.
Autophagy involvement in monocyte differentiation into macrophages. (A) Monocytes were transfected with siRNA targeting Luciferase (Luc), Beclin-1 (BEC), ATG7, or ATG5 and exposed 2 days to CSF-1. Macrophage differentiation was examined morphologically (fibroblastic shape) and by 2-color flow cytometric analysis. Percentages indicate cells that express both CD71 and CD163. (B) Monocytes were transfected with siRNA targeting Luciferase (Luc), Beclin-1 (BEC), ATG7, or ATG5 and exposed 2 days to CSF-1. The expression of Beclin-1, ATG7, ATG5, and LC3 in cells transfected with the indicated siRNA and treated for 2 days with CSF-1 was analyzed by immunoblot. Actin is used as a loading control. Molecular weights (MW) are in kDa. (C) Functional assay of monocytes transfected with Luciferase, Beclin-1, ATG7, or ATG5 siRNA and treated for 2 days with CSF-1. Results are expressed as fold induction compared with untreated monocyte and represent the mean ± SD of 4 independent experiments performed in triplicate. ***P < .001 (vs d2 LUC) according to a student paired t test. (D) Enriched bone marrow mouse monocytes were exposed for the indicated times to 100 ng/mL CSF-1. Differentiation was studied by morphologic examination (fibroblastic shape) and by 2-color flow cytometric analysis at indicated day. Percentages indicate cells that express both high CD11b and F4/80 staining. One representative of 5 independent experiments is shown. (E) Immunoblot analysis of NPM and LC3 in mouse monocytes exposed for the indicated times to CSF-1. Actin is used as a loading control. *Cleavage fragments. (F) Immunoblot analysis of ATG7 in monocytes obtained from WT or vav-Atg7−/− mice (n = 2). Actin is used as a loading control. (G) Monocytes obtained from WT or vav-Atg7−/− mice were exposed for the indicated times to 100 ng/mL CSF-1. Differentiation was studied by morphologic examination (fibroblastic shape) and by 2-color flow cytometric analysis at day 4. Percentages indicate cells that express both high CD11b and F4/80 staining.
As in human, differentiation of murine monocytes into macrophages was accompanied by NPM caspase-dependent cleavage and induction of LC3-II accumulation (Figure 2D-E). The ex vivo differentiation of monocytes from vav-Atg7−/− deficient mice (Figure 2F) into macrophages was drastically inhibited compared with monocytes from control littermate (Figure 2G). Collectively, our data demonstrate an essential role of autophagy in CSF-1–induced differentiation of monocytes into macrophages and the generation of functional phagocytes.
There is compelling evidence that autophagy can drive the rapid cellular changes necessary for proper differentiation.8 For instance, autophagy is involved in mitochondrial clearance during terminal erythroid differentiation9 as well as in megakaryocyte,10 lymphocyte,11,12 and adipocyte differentiation.13 Of note, most of these differentiation processes are also known to require caspase activation.14 We establish that autophagy plays a crucial role in monocyte differentiation into macrophages when this differentiation is reproduced ex vivo by CSF-1 stimulation. Because active caspase-8 and ATG proteins were found together in the microsomal fraction, initiation of caspase-8 activation and autophagy could take place in the same complex, as an autophagy-dependent apical activation of caspase-8 that is independent of death ligands was recently described.15 During the process of revision of the current manuscript, it was reported that GM-CSF–dependent differentiation of monocytes into macrophages was also an autophagic process.16
Human peripheral blood monocytes attracted by the chemokine CCL2 that shifted toward M2 phenotype by a local microenvironment are a main source of tumor-associated macrophages.17 These cells promote tumorigenesis via immunosuppressive effects, scavenging, and angiogenesis and are associated with poor prognosis in various tumors.18 CSF-1–mediated differentiation of human monocytes cultured ex vivo reproduces this macrophage polarization, and inhibition of the CSF-1 receptor depletes tumor-associated macrophages in vivo.19,20 Our results suggest that targeting autophagy mechanisms could be an alternative approach to prevent the deleterious effect of macrophages in tumor promotion through blockade of CSF-1–mediated differentiation of monocytes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr A. K. Simon and Dr A. J. Stranks (Oxford, United Kingdom) for providing us with bone marrow of wild-type and vav-Atg7−/− C57BL6 mice as well as the Etablissement Français du Sang for providing us with human blood from healthy donors.
This work was supported by the Ligue Nationale Contre le Cancer (Equipe Labellisée, grant 2011-2013) and INCA (grant PL2011-249). A.J. and L.B. were supported by fellowships from the Ligue Nationale Contre le Cancer. S.O. was supported by a fellowship from Inserm-Region PACAC. G.R. was supported by fellowship from the Fondation de France.
Authorship
Contribution: A.J. designed the study and performed the experimental work; S.O., L.B., M.D., G.R., and F.L. contributed to some experiments; P.G. performed electron microscopy experiments; E.L., F.L., and E.S. participated with helpful discussion; and P.A. directed the work and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrick Auberger, Inserm U1065, Bâtiment ARCHIMED, 151, route de Saint-Antoine de Ginestière, BP2 3194, 06204 Nice Cedex 03, France; e-mail: auberger@unice.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal