To evaluate the association between fetal hemoglobin (HbF) levels and morbidity in β-thalassemia intermedia (TI), we analyzed data from 63 untransfused patients who had also never received HbF induction therapy. Patient records were reviewed for any history of 10 predefined morbidities. Laboratory measurements for markers of ineffective erythropoiesis were also obtained. The mean age of patients was 32.1 years, 47.6% were males, and the median HbF level was 37.2%. HbF levels correlated positively with total hemoglobin, yet negatively with growth differentiation factor-15 and non–transferrin-bound iron levels. Median HbF levels were significantly lower in patients with the majority of evaluated morbidities than in those without. There was a strong negative adjusted linear correlation between the HbF level and the total number of morbidities (R2 = 0.825, P < .001). The HbF threshold of 63.7% had 95.5% sensitivity and 100% specificity for ensuring absence of morbidity. There exists a strong association between HbF levels and morbidity in the subset of untransfused patients with TI.
Introduction
Patients with β-thalassemia intermedia (TI) usually present to medical attention after 2 years of age and maintain hemoglobin values between 70 and 90 g/L without the need for a regular transfusion regimen.1 Nonetheless, the diagnosis of TI can be associated with a number of serious complications involving several organ systems.1,2 Although the mechanisms underlying the disease process have been studied extensively, our understanding of the risk factors that govern the clinical heterogeneity of the disease remains limited, and only a few genetic and environmental modifiers of disease severity have been elucidated.1 Some of the variability in the observed morbidities in this patient population has been attributed to the choice of treatment approach, which in most instances does not follow clear guidelines, in contrast to approaches used in patients with β-thalassemia major.2 Fetal hemoglobin (HbF) levels have been shown to be an important modifier of morbidity and mortality in adults with sickle cell disease.3,–5 Furthermore, it is known that patients with the same β-thalassemia mutations are more likely to have thalassemia major if they have lower production of HbF.6 HbF is clearly an important contributor to the clinical variability in β-thalassemia; however, the extent to which interindividual variation in HbF levels contributes to the clinical heterogeneity observed in TI patients has never been evaluated. Here, we examine the association between HbF levels and morbidity in untransfused patients with TI. By selecting patients who have never received transfusions or HbF induction therapy, we could ensure that the effects seen were more likely to be caused by the variation in HbF and not by other confounding variables.
Methods
This was a cross-sectional study of all TI patients treated at 2 centers in Milan, Italy, and Beirut, Lebanon (n = 260). At both centers, an age of diagnosis > 2 years and hemoglobin values between 70 and 90 g/L without the need for a regular transfusion regimen, with or without splenomegaly, were the main criteria to define the TI phenotype on presentation.1 It should be noted, however, that TI patients may have changes in the total hemoglobin level as they grow, and some patients may eventually require transfusion therapy.1 After the exclusion of all patients with any history of blood transfusion, iron chelation, or HbF induction therapy, 63 patients were available for analysis. The current study utilized a completely de-identified dataset. Data was collected as part of now completed clinical studies, and which were approved by the Institutional Review Board at both institutions. All patients had signed an informed consent form for participating in the original studies in accordance with the Declaration of Helsinki. By review of genetic records, none of the patients had coinheritance of α-thalassemia (α+ [−α3.7 and −α4.2] or α0 [−−Med and −−SEA]) or determinants associated with increased γ-globin chain production (Xmn-I +/+ genotype at position −158 of HβG2). β-Globin and α-globin genotypes of the study sample are described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). None of the patients had hemoglobin S, C, E/β-thalassemia, or δβ-thalassemia. Medical charts were reviewed to retrieve data on demographics (age and sex), splenectomy status, and presence of morbidities relevant in patients with TI1 (extramedullary hematopoiesis, pulmonary hypertension, venous thromboembolism, heart failure, leg ulcers, abnormal liver function, diabetes mellitus, hypothyroidism, hypogonadism, and osteoporosis), defined according to criteria described in the OPTIMAL CARE study.2 Patients were also assigned a morbidity score, which was the total number of morbidities experienced up to the time of study. For each patient, we also retrieved the HbF level determined at the time of diagnosis (measured at a median age of 9 years, range 5-21 years; the date of measurement preceded the dates of splenectomy, other laboratory measurements, and morbidity occurrence). Without intervention, HbF levels are thought to remain stable throughout the course of the disease. To adjust for the confounding effect of ineffective erythropoiesis on any observed association, we also retrieved data on the following relevant laboratory indices from all available laboratory records: mean total hemoglobin, nucleated red blood cells, growth differentiation factor (GDF)–15, serum ferritin, and non–transferrin-bound iron (NTBI) levels. In both centers, GDF-15 and NTBI levels were determined as described elsewhere.7,8
Statistical analysis
Data are described as mean ± SD, median (interquartile range), or percentage. Bivariate correlations to determine the association between HbF levels and study parameters were conducted with the Mann-Whitney U test for categorical variables and the Spearman correlation coefficient for continuous variables. Linear regression analysis, with adjustment for clinically relevant variables, was used to evaluate the independent correlation between HbF levels and the occurrence of morbidity. Receiver operating characteristic curve analysis was used to determine the HbF level threshold with the highest sum of sensitivity and specificity to discriminate patients who experienced morbidity from those who did not. All P values were 2 sided, with the level of significance set at < .05.
Results and discussion
The mean age of patients in the present study was 32.1 ± 16.2 years (range 8-78 years). A total of 30 patients (47.6%) were males, and 37 (58.7%) were splenectomized. The mean total hemoglobin level was 89.0 ± 16.1 g/L (range 57-131 g/L), and the median HbF level was 37.2% (interquartile range 57.8%, range 1.1%-100%). Bivariate correlations between HbF level and study parameters are summarized in Table 1. Clinical and laboratory quantification of the severity of ineffective erythropoiesis remains a challenge. Ineffective erythropoiesis is the hallmark of the disease process in TI, leading to anemia, hemolysis, and iron overload because of excessive intestinal absorption.1,9 Measurement of GDF-15, a member of the transforming growth factor-β superfamily, is thought to indicate the extent of ineffective erythropoiesis.10 NTBI levels are thought to reflect the erythropoietic rate and are increased by ineffective erythropoiesis.11,12 The negative correlation between HbF levels and both of these markers in the present report, as well as the positive correlation with total hemoglobin levels, suggests an ameliorating role of HbF level on the severity of ineffective erythropoiesis, probably through an effect on globin chain imbalance. Previous work on small cohorts of TI patients showed that high levels of circulating HbF (> 40%) could be associated with higher erythropoietin activity and expansion of erythropoiesis.13,14 The present study suggests that this increase in erythropoietic drive is also less “ineffective.” The exact mechanisms that could explain such an association, however, merit further investigation.
Bivariate correlations between fetal hemoglobin level and study parameters
| Parameter . | Value . | P . |
|---|---|---|
| Age (y) | rs −0.164 | .199 |
| Sex | ||
| Male (n = 30) | 37 (54) | .625 |
| Female (n = 33) | 42.3 (62.4) | |
| Splenectomy | ||
| No (n = 26) | 18.5 (51) | .007 |
| Yes (n = 37) | 47.3 (51.4) | |
| Total hemoglobin level (g/L) | rs 0.595 | < .001 |
| NRBC count (× 106/L) | rs −0.073 | .567 |
| GDF-15 level (pg/mL) | rs −0.522 | < .001 |
| Serum ferritin level (μg/L) | rs −0.092 | .471 |
| NTBI level (μmol/L) | rs −0.444 | < .001 |
| Morbidities | ||
| Extramedullary hematopoiesis | ||
| No (n = 50) | 50.2 (59.8) | .003 |
| Yes (n = 13) | 18.8 (31.1) | |
| Pulmonary hypertension | ||
| No (n = 44) | 58.8 (56.6) | < .001 |
| Yes (n = 19) | 8.7 (25.9) | |
| Venous thromboembolism | ||
| No (n = 51) | 47.3 (57.1) | .003 |
| Yes (n = 12) | 18.1 (17.8) | |
| Heart failure | ||
| No (n = 57) | 43.3 (56.2) | .002 |
| Yes (n = 6) | 4.4 (8.5) | |
| Leg ulcers | ||
| No (n = 52) | 45.0 (57.4) | .002 |
| Yes (n = 11) | 10.5 (24.9) | |
| Abnormal liver function | ||
| No (n = 49) | 55.5 (55.2) | < .001 |
| Yes (n = 14) | 13.9 (20.1) | |
| Diabetes mellitus | ||
| No (n = 60) | 40.6 (60.4) | .309 |
| Yes (n = 3) | 22.8 (0.5) | |
| Hypothyroidism | ||
| No (n = 53) | 47.3 (56.3) | < .001 |
| Yes (n = 10) | 5.5 (15.0) | |
| Hypogonadism | ||
| No (n = 53)* | 49.3 (49.7) | < .001 |
| Yes (n = 8) | 8.1 (8.6) | |
| Osteoporosis | ||
| No (n = 49) | 55.5 (57.0) | < .001 |
| Yes (n = 14) | 9.6 (18.5) |
| Parameter . | Value . | P . |
|---|---|---|
| Age (y) | rs −0.164 | .199 |
| Sex | ||
| Male (n = 30) | 37 (54) | .625 |
| Female (n = 33) | 42.3 (62.4) | |
| Splenectomy | ||
| No (n = 26) | 18.5 (51) | .007 |
| Yes (n = 37) | 47.3 (51.4) | |
| Total hemoglobin level (g/L) | rs 0.595 | < .001 |
| NRBC count (× 106/L) | rs −0.073 | .567 |
| GDF-15 level (pg/mL) | rs −0.522 | < .001 |
| Serum ferritin level (μg/L) | rs −0.092 | .471 |
| NTBI level (μmol/L) | rs −0.444 | < .001 |
| Morbidities | ||
| Extramedullary hematopoiesis | ||
| No (n = 50) | 50.2 (59.8) | .003 |
| Yes (n = 13) | 18.8 (31.1) | |
| Pulmonary hypertension | ||
| No (n = 44) | 58.8 (56.6) | < .001 |
| Yes (n = 19) | 8.7 (25.9) | |
| Venous thromboembolism | ||
| No (n = 51) | 47.3 (57.1) | .003 |
| Yes (n = 12) | 18.1 (17.8) | |
| Heart failure | ||
| No (n = 57) | 43.3 (56.2) | .002 |
| Yes (n = 6) | 4.4 (8.5) | |
| Leg ulcers | ||
| No (n = 52) | 45.0 (57.4) | .002 |
| Yes (n = 11) | 10.5 (24.9) | |
| Abnormal liver function | ||
| No (n = 49) | 55.5 (55.2) | < .001 |
| Yes (n = 14) | 13.9 (20.1) | |
| Diabetes mellitus | ||
| No (n = 60) | 40.6 (60.4) | .309 |
| Yes (n = 3) | 22.8 (0.5) | |
| Hypothyroidism | ||
| No (n = 53) | 47.3 (56.3) | < .001 |
| Yes (n = 10) | 5.5 (15.0) | |
| Hypogonadism | ||
| No (n = 53)* | 49.3 (49.7) | < .001 |
| Yes (n = 8) | 8.1 (8.6) | |
| Osteoporosis | ||
| No (n = 49) | 55.5 (57.0) | < .001 |
| Yes (n = 14) | 9.6 (18.5) |
Data are presented as median fetal hemoglobin in % (interquartile range) unless otherwise indicated.
NRBC indicates nucleated red blood cell; GDF, growth differentiation factor; NTBI, non–transferrin-bound iron; and rs, Spearman correlation coefficient.
Two patients were younger than the age-appropriate criteria to define hypogonadism.
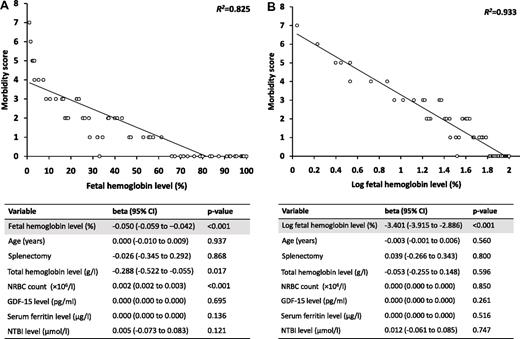
A total of 41 patients (65.1%) experienced at least 1 morbidity, and 30 patients (47.6%) experienced multiple morbidities. The median HbF level was significantly lower in patients with the majority of evaluated morbidities than in those without (Table 1). On linear regression analysis, and after adjustment for age, splenectomy, total hemoglobin, nucleated red blood cells, GDF-15, serum ferritin, and NTBI levels, there was a strong negative linear correlation between HbF level and the morbidity score (β −0.050, 95% confidence interval [CI] −0.059 to −0.042; R2 = 0.825, P < .001; Figure 1A). These results remained unchanged when the analysis was stratified for splenectomized and nonsplenectomized patients (splenectomized: beta −0.053, 95% CI −0.063 to −0.042, R2 = 0.785, P < .001; nonsplenectomized: beta −0.045, 95% CI −0.060 to −0.030, R2 = 0.852, P < .001). A regression analysis was also performed with the total HbF level in grams per liter rather than the % HbF, and the results remained essentially unchanged (beta −0.040, 95% CI −0.048 to −0.033, R2 = 0.882, P < .001). An even stronger correlation was found using the log of the % HbF (beta −3.401, 95% CI −3.915 to −2.886, R2 = 0.933, P < .001; Figure 1B). The HbF threshold 63.7% had 95.5% sensitivity and 100% specificity for ensuring absence of morbidity on receiver operating characteristic curve analysis (area under the curve 0.986, 95% CI 0.957 to 1.000).
Association between fetal hemoglobin level and the morbidity score. Figures show linear regression of (A) fetal hemoglobin level and (B) log fetal hemoglobin level against the morbidity score. Tables represent results of linear regression analysis after adjustment for indicated variables. NRBC indicates nucleated red blood cell; GDF, growth differentiation factor; NTBI, non–transferrin-bound iron; and CI, confidence interval.
Association between fetal hemoglobin level and the morbidity score. Figures show linear regression of (A) fetal hemoglobin level and (B) log fetal hemoglobin level against the morbidity score. Tables represent results of linear regression analysis after adjustment for indicated variables. NRBC indicates nucleated red blood cell; GDF, growth differentiation factor; NTBI, non–transferrin-bound iron; and CI, confidence interval.
We were able to demonstrate here the quantitative ameliorating effect of HbF levels on morbidity in TI. Although we included a select group of TI patients not receiving transfusion or HbF induction therapy in the present study, we believe that our results will be more broadly applicable in TI. Despite the observation of a statistically independent effect of HbF levels on morbidity, these increased levels could still be acting to reduce the extent of ineffective erythropoiesis and increase total hemoglobin levels in the patients with TI. The mechanisms underlying these observations should be the target of future studies.
In the absence of intervention, the heterogeneity of HbF levels in these patients may be rooted at the molecular level. Recent genome-wide association studies have identified 3 loci (at BCL11A, HBS1L-MYB, and the β-globin locus) that carry DNA polymorphisms that modulate HbF levels.15,,,–19 These polymorphisms have been associated with higher levels of HbF in patients with sickle cell disease and were shown to correlate with a milder disease course.16,18,19 They were found to occur at higher frequencies in patients with TI than in patients with β-thalassemia major6,16,20 and were associated with milder disease severity in patients with hemoglobin E/β-thalassemia.21 Whether these polymorphisms explain the spectrum of HbF levels and associated morbidity in TI patients will need to be explored in future studies.
In conclusion, the present study demonstrates that high HbF levels are associated with a milder disease course in patients with TI. The mechanisms underlying this observation should be evaluated in view of the recent advances in understanding HbF expression and could lead to potential therapeutic interventions.22 Furthermore, our demonstration that the amelioration in the extent of morbidity is quantitatively associated with HbF levels suggests that therapeutic efforts aimed at even modest induction of these levels may have a profound effect on disease course in patients with TI.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M.M., V.G.S., M.D.C., D.G.N., and A.T.T. designed the study; K.M.M., V.G.S., and L.D. prepared and analyzed the data; K.M.M., V.G.S., M.D.C., L.D., D.G.N., and A.T.T. reviewed the analysis and prepared the manuscript; and K.M.M., V.G.S., M.D.C., L.D., D.G.N., and A.T.T. approved the final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ali T. Taher, MD, FRCP, Professor of Medicine, Hematology & Oncology, Associate Chair–Research, Department of Internal Medicine, American University of Beirut Medical Center, PO Box 11-0236, Riad El-Solh 1107 2020, Beirut, Lebanon; e-mail: ataher@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal