Abstract
Dasatinib is a tyrosine kinase inhibitor used to treat imatinib-resistant chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia. At present, little is known about how dasatinib influences nonmalignant cells. In the present study, we tested the effect of dasatinib on functional responses of normal mature human neutrophils. Dasatinib completely blocked integrin- and Fc-receptor–mediated neutrophil functions, with the lowest IC50 values below 10nM under serum-free conditions. Dasatinib caused a partial inhibition of neutrophil responses triggered by G-protein–coupled receptors and had a moderate effect on neutrophil responses triggered by microbial compounds. Whereas dasatinib inhibited neutrophil chemotaxis under static conditions in 2 dimensions, it did not affect migration under flow conditions or in 3-dimensional environments. Dasatinib did not have any major effect on phagocytosis or killing of bacteria by neutrophils. Adhesion of human neutrophils in the presence of whole serum was significantly inhibited by 50-100nM dasatinib, which corresponds to the reported serum concentrations in dasatinib-treated patients. Finally, ex vivo adhesion of mouse peripheral blood neutrophils was strongly reduced after oral administration of 5 mg/kg of dasatinib. Those results suggest that dasatinib treatment may affect the proinflammatory functions of mature neutrophils and raise the possibility that dasatinib-related compounds may provide clinical benefit in neutrophil-mediated inflammatory diseases.
Introduction
Dasatinib (BMS-354825) is a second-generation oral tyrosine kinase inhibitor primarily used as a second-line treatment in imatinib-resistant chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia.1 Similar to imatinib, dasatinib also inhibits the Abl kinase and the Bcr-Abl fusion protein, although with a different molecular mechanism of action.2,3 In addition to Abl and Bcr-Abl, dasatinib also inhibits several additional kinases, including Src and Btk family members, c-Kit, PDGFR, and Eph receptors.3,4
In addition to its effect on malignant cells, dasatinib also inhibits certain functions of normal cells of various hematopoietic lineages, including T lymphocytes,5 natural killer cells,6 basophils,7 platelets,8,9 and osteoclasts.10 However, no information is available on the effect of dasatinib on neutrophils, the most abundant circulating leukocytes.
Neutrophils are short-lived, terminally differentiated phagocytic cells that provide the first line of defense against bacterial and fungal pathogens, but also contribute to the development of various acute and chronic inflammatory diseases.11,12 Neutrophil activation occurs through several cell-surface receptors (integrins, Fc receptors, G-protein–coupled receptors, and cytokine and innate immune receptors) that activate complex intracellular signal transduction events leading to cellular responses such as adhesion, migration, respiratory burst, granule release, phagocytosis, and bacterial killing.
Several genetic and pharmacologic studies have indicated that protein tyrosine kinases play critical roles in neutrophil activation by various cell-surface receptors.13 Src-family kinases are involved in neutrophil functions triggered through integrins14-16 or formyl-peptide receptors.15,17 Pharmacologic studies have suggested a role for Abl in integrin-mediated activation,18 L-selectin shedding,19 and respiratory burst20 of neutrophils. We and others have identified critical roles for Syk in various neutrophil-activation pathways.16,21-24
The lack of information on the effect of dasatinib on neutrophils, the putative role of Src-family kinases and c-Abl in neutrophil activation, and the inhibition of certain neutrophil functions by dasatinib in a kinase inhibitor screening study (K.F., T.V., G. Kéri, and A.M., unpublished observations, December 2010) prompted us to perform a detailed analysis of the effect of dasatinib on human neutrophil functions. Our results indicate that dasatinib exerts a robust inhibitory effect on various inflammation-related functions of mature human neutrophils.
Methods
Neutrophil isolation and inhibitor treatment
Human neutrophils were isolated from venous blood of healthy volunteers by Ficoll or Percoll gradient centrifugation, followed by hypotonic lysis of RBCs.17,25 Cells were resuspended in Ca2+- and Mg2+-free HBSS supplemented with 20mM HEPES, pH 7.4, and kept at room temperature until use.
Dasatinib (> 99% pure) was obtained from Selleck Chemicals and its purity and stability was confirmed by HPLC-MS analysis (Vichem). Dasatinib was dissolved in DMSO. The final dasatinib-treated samples contained ≤ 0.01% DMSO and inhibitor-free controls contained 0.01% DMSO.
Isolated neutrophils were diluted in the assay medium, supplemented with 0.5mM CaCl2, and then pretreated with the indicated concentrations of dasatinib or vehicle (DMSO) at 37°C for 30 minutes before activation. Unless otherwise stated, 1mM MgCl2 was added immediately before cell activation. Neutrophil assays were performed at 37°C with dasatinib being present throughout the assays. Cell viability and the basal rate of apoptosis for up to 6 hours was not affected by up to 1μM dasatinib (data not shown).
All experiments on human samples were approved by the institutional review board of Semmelweis University or Ludwig-Maximilians University.
Neutrophil activation
Plate-based activation of neutrophils was performed in Nunc Maxisorp or tissue culture–treated BD Biosciences plates. For adherent activation of neutrophils, the plates were precoated with 150 μg/mL of human fibrinogen (Calbiochem) or 10% FCS (Invitrogen), and stimulated with 20 ng/mL of human TNF-α (PeproTech), 50 ng/mL of human C5a (R&D Systems), 1 μg/mL of Pam3CSK4 (EMC Microcollections), 1 μg/mL of ultrapurified lipopolysaccharide (LPS; InVivoGen) or 100nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) as described previously.15,21,26,27 Neutrophil activation by plate-bound anti-integrin Abs (20 μg/mL of anti-CD18 clone IB4) or the polyvalent integrin ligand poly-RGD (20 μg/mL; Sigma-Aldrich) was performed as described previously.21,26,28 Immobilized IgG immune complexes were prepared using human lactoferrin (Lfr; 20 μg/mL) and anti-Lfr (1:400 dilution) or, in the case of measuring lactoferrin release, human serum albumin (20 μg/mL) and anti–human serum albumin (1:400 dilution; all reagents from Sigma-Aldrich) and used to stimulate neutrophils as described previously.29
Neutrophil activation in suspension was performed in polypropylene tubes or in FCS-coated wells in the absence of MgCl2 by stimulating the cells with 20 ng/mL of TNF, 1μM fMLP (Sigma-Aldrich), 50 ng/mL of C5a, 100 ng/mL of human IL-8 (Peprotech), 50 ng/mL of LTB4 (Santa Cruz Biotechnology), 1 μg/mL of Pam3CSK4, 1 μg/mL of ultrapurified LPS, or 10 mg/mL of zymosan (Sigma-Aldrich) opsonized or not with normal or heat-inactivated human serum. Where indicated, cells were pretreated with 10μM cytochalasin B (Sigma-Aldrich).
Functional assays and biochemical studies
For respiratory burst assays, cells were supplemented with 100nM ferricytochrome c (Sigma-Aldrich), plated at 105/well on 96-well plates, and superoxide release monitored spectrophotometrically, as described previously.21,22,26,27 Alternatively, the samples were supplemented with 50 μg/mL of lucigenin (Sigma-Aldrich) and their luminescence was followed using a Thermo Labsystems Fluoroskan Ascent FL luminometer. Cell spreading was assessed after 30 minutes of stimulation by phase-contrast microscopy of formalin-fixed cells using a Leica DMI 6000B inverted microscope (Leica Microsystems) with a 20× phase contrast objective, connected to a Leica DFC480 CCD camera, analysed by Leica Application Suite Version 3.8 software. Cellular adhesion during a 30-minute incubation was determined after several washes using an acid phosphatase assay, as described previously.21 Lactoferrin release was tested using a double-sandwich ELISA17 ; gelatinase release was followed by in-gel gelatinase zymography16,29 after 10 (fMLP) or 30 minutes (all other stimuli) of activation. Up-regulation and activation of CD11b during a 30-minute incubation was assessed using biotinylated anti–human CD11b (M1/70), followed by streptavidin-FITC (both from BD Biosciences) or FITC-labeled Abs against an activation-specific human CD11b epitope (clone CRBM1/5; eBiosciences). Samples were analyzed using a BD Biosciences FACSCalibur flow cytometer and CellQuest Pro 5.2.1 software.
Phosphorylation of intracellular proteins was tested on cell lysates prepared using a Triton X-100–based lysis buffer supplemented with protease and phosphatase inhibitors26 after incubation of the cells for 3 minutes (fMLP), 5 minutes (IL-8, C5a, LTB4), 10 minutes (TNF, Pam3CSK4, and zymosan), or 20 minutes (ultrapurified LPS) in suspension or for 10 minutes (immune complex) or 15 minutes (TNF on fibrinogen and poly-RGD) on a solid surface. Where indicated, Syk was immunoprecipitated using the 4D10 mAb (Santa Cruz Biotechnology) and Protein A/G-Sepharose beads (Invitrogen) as described previously.21,26 Total cell lysates or Syk immunoprecipitates were immunoblotted using anti-phosphotyrosine Ab (clone 4G10; Millipore), phosphospecific Abs against Syk (2701; Cell Signaling Technology), or phosphospecific (Cell Signaling Technology) or nonphosphospecific (Santa Cruz Biotechnology) Abs against the ERK and p38 MAPKs, as described previously.21,22,26
Neutrophil migration
The migration of individual neutrophils toward a gradient of 10μM fMLP or 1 μg/mL of IL-8 during a 10-minute period at 37°C on immobilized fibrinogen (250 μg/mL) under steady-state conditions was tested using a Zigmond chamber assay, as described previously.27,30
Mechanotactic crawling of human neutrophils was analyzed using IBIDI μ-slides VI 0.4 flow chambers coated with 250 μg/mL of human fibrinogen or 12.5 μg/mL of human ICAM1 (Peprotech). Cells were treated with 1μM fMLP for 10 minutes inside the chamber before application of 1 dyne/cm2 shear stress for 10 minutes using a high-precision syringe pump (KD Scientific). Time-lapse video microscopic images recorded using a Zeiss Axiovert 200 microscope were analyzed offline using ImageJ Launcher 1.38x software with a manual tracking plugin (Fabrice Cordeliès, Institute Curie, Orsay, France). Single-cell migration tracks were analyzed using IBIDI chemotaxis and migration tools.
Transwell migration assays were performed essentially as described previously16,21 using polycarbonate filters with a 3-μm pore size (Corning) precoated with human fibrinogen. Neutrophil migration toward 100nM fMLP or 10 ng/mL of IL-8 in 60 minutes was quantified using an acid phosphatase assay.21 To assess migration through an extracellular matrix, neutrophils were allowed to migrate through Transwell inserts filled with 100 μL of 8-fold–diluted Matrigel (BD Biosciences) for 3 hours.
Bacterial killing and phagocytosis
Killing of Staphylococcus aureus or Escherichia coli opsonized with pooled human serum and incubated with neutrophils for 30 minutes at a neutrophil to bacteria ratio of 1:10 was tested by a plate-based assay, as described previously.27,31 For phagocytosis assays, green fluorescent protein (GFP)–expressing S aureus bacteria32 were opsonized and incubated with neutrophils for 0, 10, or 20 minutes, washed, and resuspended in BD Biosciences FACS Lysis buffer, followed by determination of neutrophil-associated fluorescence by flow cytometry. Where indicated, neutrophils were preincubated with 10μM cytochalasin D (Sigma-Aldrich).
Adhesion of unfractionated human and mouse leukocytes
To determine the adhesion of unmanipulated leukocytes in the presence of whole serum, RBCs were sedimented from heparinized human blood by 0.4% dextran 500 (Sigma-Aldrich) and the leukocyte-rich supernatant was treated with dasatinib and incubated on an FCS-coated surface in the presence of 20 ng/mL of human TNF, 100 ng/mL of human C5a, 1 μg/mL of Pam3CSK4, or 1 μg/mL of ultrapurified LPS. After 30 minutes at 37°C, the plates were washed and leukocyte adhesion was quantified using an acid phosphatase assay.21
To determine the effect of oral administration of dasatinib, adult C57BL/6 mice were treated with the indicated doses of dasatinib by oral gavage. Two hours later, the mice were treated with heparin, killed, and leukocyte-rich plasma was obtained by dextran sedimentation of peripheral blood as described in the previous paragraph. Ex vivo adhesion of leukocytes to FCS-coated plates in the presence of 50 ng/mL of murine TNF was determined as described in the previous paragraph.
Animal experiments were authorized by the Semmelweis University Animal Experimentation Review Board.
Presentation of data and statistical analysis
All experiments were performed 3 or more times with comparable results. Respiratory burst assays were performed in triplicate. Zero time points and unstimulated control values were subtracted to simplify presentation of kinetic curves. Other quantitative assays were performed in duplicate or triplicate. In the representative graphs, error bars represent SD from a single experiment. Densitometry was performed using ImageJ Launcher 1.38x software.
For generation of the percent response values and dose-response curves, unstimulated control values were subtracted, the responses expressed in percent of vehicle-treated samples, and the data averaged across the indicated number of experiments, with error bars representing SEM. In the kinetic experiments, dose-response curves were generated using the last time point or, in the case of the luminometric respiratory burst assay, the integrated area under the curve. Maximum inhibition and IC50 values were calculated using a 4-parameter logistic algorithm by IDBS XLFit with the inhibition in the absence of dasatinib set to 0% and maximal inhibition limited to 100%.
Where indicated, statistical analysis was performed using the Student paired 2-population t test. P < .05 was considered statistically significant.
Results
Dasatinib blocks adhesion-mediated respiratory burst
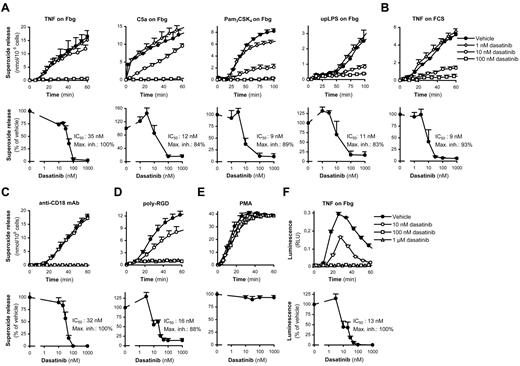
Neutrophil activation at the site of inflammation occurs through various proinflammatory agonists while the cells are adherent to the inflamed endothelium or the extracellular matrix. This can be mimicked by stimulating neutrophils by soluble agonists in the presence of an adhesive surface33 (so-called adherent activation), which requires β2-integrins.21,26,34 Proinflammatory agonists, including TNF, C5a, and TLR2 (Pam3CSK4) or TLR4 (ultrapurified LPS) ligands, potently triggered superoxide release from human neutrophils adherent to a fibrinogen- or FCS-coated surface (Figure 1A-B). Low nanomolar concentrations of dasatinib exerted a robust inhibition of those responses, with IC50 values below 10nM in the most sensitive assay conditions tested (Figure 1A-B).
Dasatinib abrogates adhesion-dependent respiratory burst of human neutrophils. Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 20 ng/mL of human TNF, 50 ng/mL of human C5a, 1 μg/mL of Pam3CSK4 or 1 μg/mL of ultrapurified LPS (upLPS) while adherent to a fibrinogen (Fbg)–coated (A,F) or FCS-coated (B,E) surface; by plate-bound Abs against human CD18 (C), by plating them on a poly-RGD–coated surface (D); or by 100nM PMA (E); followed by spectrophotometric measurement of superoxide release (A-E) or luminometric measurement of reactive oxygen production (F). Kinetic curves show mean and SD of representative experiments and dose-response curves show mean and SEM of the percent response from 3-11 independent experiments. RLU indicates relative luminescence unit.
Dasatinib abrogates adhesion-dependent respiratory burst of human neutrophils. Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 20 ng/mL of human TNF, 50 ng/mL of human C5a, 1 μg/mL of Pam3CSK4 or 1 μg/mL of ultrapurified LPS (upLPS) while adherent to a fibrinogen (Fbg)–coated (A,F) or FCS-coated (B,E) surface; by plate-bound Abs against human CD18 (C), by plating them on a poly-RGD–coated surface (D); or by 100nM PMA (E); followed by spectrophotometric measurement of superoxide release (A-E) or luminometric measurement of reactive oxygen production (F). Kinetic curves show mean and SD of representative experiments and dose-response curves show mean and SEM of the percent response from 3-11 independent experiments. RLU indicates relative luminescence unit.
Neutrophils can also be potently activated on coligation of integrins and Fc-receptors by immobilized anti-integrin Abs.28,35 As shown in Figure 1C, that response was also completely blocked by dasatinib.
In addition to these costimulatory approaches, neutrophils can also be activated by plating them on a surface coated with a polyvalent integrin ligand (poly-RGD) in the absence of another proinflammatory stimulus.21 As shown in Figure 1D, such activation was also dramatically inhibited by dasatinib.
These findings raise the possibility that dasatinib blocks the assembly of the NADPH oxidase or otherwise interferes with the superoxide release assay. However, respiratory burst triggered by the nonphysiologic activating agent PMA was not affected by dasatinib (Figure 1E) and a luminometric respiratory burst assay confirmed complete inhibition of the TNF-induced respiratory burst of fibrinogen-adherent neutrophils (Figure 1F).
Dasatinib inhibits other adhesion-mediated neutrophil functions
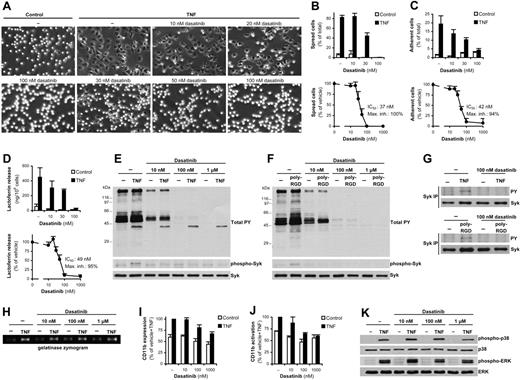
Adherent activation of neutrophils also triggers other functional responses, including spreading and adhesion of the cells and exocytosis of secondary granules. Dasatinib potently inhibited the spreading (Figure 2A-B) and adhesion (Figure 2C) response and the release of the secondary granule marker lactoferrin (Figure 2D) after stimulation of fibrinogen-adherent neutrophils with TNF, with IC50 values in the range of 35-50nM depending on the assay readout used. In contrast, dasatinib did not affect the spreading of PMA-stimulated human neutrophils (not shown), indicating that the final cytoskeletal spreading machinery remained intact after dasatinib treatment.
Dasatinib inhibits other adhesion-dependent responses but does not block TNF signaling. (A-G) Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 20 ng/mL of human TNF while adherent to a fibrinogen (Fbg)–coated surface (A-E and top part of G) or plated on a poly-RGD–coated surface (F and bottom part of G), followed by microscopic observation (A) and quantification (B) of cell spreading, measurement of cell adhesion (C), assessment of lactoferrin release (D), direct analysis of total cellular tyrosine phosphorylation and phosphorylation of Syk Tyr352 by immunoblotting (E-F), or analysis of Syk phosphorylation by immunoprecipitation (IP), followed by immunoblotting for phosphotyrosine (PY) residues (G). (H-K) Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with TNF in suspension, followed by analysis of gelatinase release by in-gel zymography (H), flow cytometric analysis of expression (I) and activation (J) of CD11b, or phosphorylation of ERK and the p38 MAPK by immunoblotting (K). Bar graphs in panels B through D show mean and SD of representative experiments, and dose-response curves in panels B through D and bar graphs in panels I and J show mean and SEM of percent response. Mean fluorescence intensity values of isotype control–stained samples were subtracted in panels I and J, and the resulting fluorescence was expressed as a percentage of that in the indicated samples. Each set of data was obtained from 3-9 independent experiments. The mean and SEM of phosphorylation of the various MAPKs at 10nM, 100nM, and 1μM dasatinib after subtraction of unstimulated control values corresponded to 101% ± 18%, 94% ± 17%, and 56% ± 8% (p38 MAPK) and 89% ± 31%, 85% ± 26%, and 89% ± 20% (ERK) of that in the absence of dasatinib, respectively.
Dasatinib inhibits other adhesion-dependent responses but does not block TNF signaling. (A-G) Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 20 ng/mL of human TNF while adherent to a fibrinogen (Fbg)–coated surface (A-E and top part of G) or plated on a poly-RGD–coated surface (F and bottom part of G), followed by microscopic observation (A) and quantification (B) of cell spreading, measurement of cell adhesion (C), assessment of lactoferrin release (D), direct analysis of total cellular tyrosine phosphorylation and phosphorylation of Syk Tyr352 by immunoblotting (E-F), or analysis of Syk phosphorylation by immunoprecipitation (IP), followed by immunoblotting for phosphotyrosine (PY) residues (G). (H-K) Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with TNF in suspension, followed by analysis of gelatinase release by in-gel zymography (H), flow cytometric analysis of expression (I) and activation (J) of CD11b, or phosphorylation of ERK and the p38 MAPK by immunoblotting (K). Bar graphs in panels B through D show mean and SD of representative experiments, and dose-response curves in panels B through D and bar graphs in panels I and J show mean and SEM of percent response. Mean fluorescence intensity values of isotype control–stained samples were subtracted in panels I and J, and the resulting fluorescence was expressed as a percentage of that in the indicated samples. Each set of data was obtained from 3-9 independent experiments. The mean and SEM of phosphorylation of the various MAPKs at 10nM, 100nM, and 1μM dasatinib after subtraction of unstimulated control values corresponded to 101% ± 18%, 94% ± 17%, and 56% ± 8% (p38 MAPK) and 89% ± 31%, 85% ± 26%, and 89% ± 20% (ERK) of that in the absence of dasatinib, respectively.
Dasatinib blocks activation of the Syk tyrosine kinase
Adherent activation of neutrophils triggers various tyrosine phosphorylation pathways.13 Tyrosine phosphorylation of cellular proteins in response to TNF stimulation of fibrinogen-adherent neutrophils (Figure 2E) or after plating the cells on poly-RGD–coated surfaces (Figure 2F) was inhibited by 10nM and completely blocked by 100nM dasatinib. The only exception was an approximately 40-kDa protein shown in Figure 2E, which likely corresponds to p38 MAPK activated by TNF in an adhesion-independent manner (see next section).
We have described previously a critical role for Syk in adherent activation of neutrophils.21 Both TNF-mediated activation of fibrinogen-adherent neutrophils (Figure 2E) and plating the cells on a poly-RGD–coated surface (Figure 2F) triggered phosphorylation of Tyr352 of Syk, and this phosphorylation was strongly reduced by as little as 10nM dasatinib. Complete inhibition of adhesion-induced Syk phosphorylation by 100nM dasatinib could also be observed on immunoprecipitation followed by immunoblotting with anti-phosphotyrosine Abs (Figure 2G). Given the essential role of Syk in adherent activation of neutrophils,21 these results indicate that the effect of dasatinib is likely mediated, at least in part, by blocking the activation of the Syk tyrosine kinase.
Dasatinib does not block TNF signal transduction
Our results thus far indicated that dasatinib blocks adhesion-induced signal transduction. To determine whether dasatinib also interferes with TNF-receptor signaling, neutrophils were stimulated by TNF under nonadherent conditions. As shown in Figure 2H, dasatinib did not affect the TNF-induced exocytosis of gelatinase granules (which, in contrast to lactoferrin release, does not require cellular adhesion). Dasatinib also did not affect TNF-induced up-regulation of CD11b substantially at the indicated 30-minute time point (Figure 2I). Dasatinib significantly reduced the TNF-induced expression of an activation-specific CD11b neoepitope during a 30-minute incubation period at 1μM but not at 10-100nM concentrations of the drug (Figure 2J). As shown in Figure 2K, dasatinib did not significantly affect TNF-induced phosphorylation of the ERK or the p38 MAPKs except for a partial inhibition of the phosphorylation of p38 MAPK at the highest concentration (1μM) tested. These results suggest that mid-nanomolar concentrations of dasatinib do not block TNF-receptor signal transduction.
Low- to mid-nanomolar dasatinib concentrations inhibit various adhesion-dependent functional responses of human neutrophils (Figure 1 and Figure 2A-D), but do not significantly affect TNF signal transduction. The most likely explanation for this is that dasatinib inhibits integrin signal transduction, at least in part by preventing the activation of the Syk tyrosine kinase.
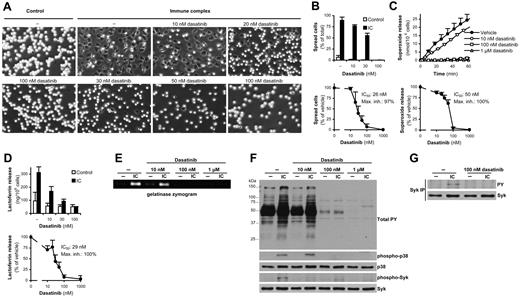
Dasatinib blocks immune complex–induced neutrophil activation
Immune complex formation is a major cause of neutrophil activation in autoimmune diseases.12 As shown in Figure 3, dasatinib abrogated immune complex–induced spreading (Figure 3A-B), superoxide production (Figure 3C), and exocytosis of specific and gelatinase granules (Figure 3D-E), with IC50 values in the range of 25-50nM. Dasatinib also inhibited basal and immune complex–induced tyrosine phosphorylation and activation of the p38 MAPK (Figure 3F). Similar to integrin-mediated activation, Syk is also thought to be required for immune complex–induced neutrophil activation.36,37 Immune complex–induced phosphorylation of the Tyr352 residue of Syk was inhibited by 10nM and completely blocked by 100nM dasatinib (Figure 3F) and Syk phosphorylation, as determined by immunoprecipitation and phosphotyrosine immunoblotting, was also completely blocked by 100nM dasatinib (Figure 3G). We conclude that mid-nanomolar concentrations of dasatinib abrogate immune complex–induced neutrophil activation, at least in part by blocking the activation of the Syk tyrosine kinase.
Dasatinib inhibits neutrophil functions triggered by immobilized immune complexes. Human neutrophils pretreated with the indicated concentrations of dasatinib were plated on a surface coated with immobilized IgG immune complexes (IC), followed by microscopic observation (A) and quantification (B) of cell spreading, measurement of respiratory burst (C), assessment of lactoferrin (D) and gelatinase (E) release, analysis of phosphorylation of total cellular proteins and the phosphorylation of the p38 MAPK and Syk (Tyr352) by immunoblotting (F), or analysis of Syk phosphorylation by immunoprecipitation (IP), followed by immunoblotting for phosphotyrosine (PY) residues (G). Panels A, E, and F show representative data from 3-4 independent experiments. The kinetic/bar graphs in panels B through D show mean and SD of representative experiments, and the dose-response curves in these panels show mean and SEM of the percent response from 3-4 independent experiments.
Dasatinib inhibits neutrophil functions triggered by immobilized immune complexes. Human neutrophils pretreated with the indicated concentrations of dasatinib were plated on a surface coated with immobilized IgG immune complexes (IC), followed by microscopic observation (A) and quantification (B) of cell spreading, measurement of respiratory burst (C), assessment of lactoferrin (D) and gelatinase (E) release, analysis of phosphorylation of total cellular proteins and the phosphorylation of the p38 MAPK and Syk (Tyr352) by immunoblotting (F), or analysis of Syk phosphorylation by immunoprecipitation (IP), followed by immunoblotting for phosphotyrosine (PY) residues (G). Panels A, E, and F show representative data from 3-4 independent experiments. The kinetic/bar graphs in panels B through D show mean and SD of representative experiments, and the dose-response curves in these panels show mean and SEM of the percent response from 3-4 independent experiments.
Partial inhibition of G-protein–coupled receptor signaling
We next investigated the effect of dasatinib on signaling by G-protein–coupled receptors that sense bacterial invasion, activation of the inflammation machinery, and tissue damage (eg, extracellular release of mitochondrial formyl peptides).
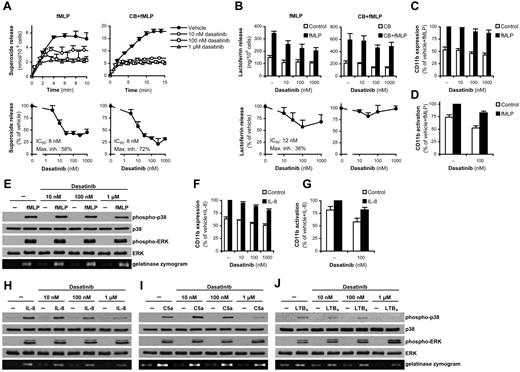
As shown in Figure 4A, dasatinib caused a considerable but not complete inhibition of superoxide production triggered by the formyl-peptide fMLP, with IC50 values below 10nM both in the presence and absence of cytochalasin B (a cytoskeleton-disrupting agent that augments certain neutrophil functions). Dasatinib also partially inhibited lactoferrin release in the absence, but not in the presence, of cytochalasin B (Figure 4B), whereas it did not significantly affect up-regulation (Figure 4C) or activation (Figure 4D) of CD11b at the indicated time point, exocytosis of gelatinase granules (Figure 4E), or phosphorylation of the ERK or p38 MAPKs (Figure 4E).
Dasatinib partially inhibits neutrophil functions triggered by G-protein–coupled receptors. Human neutrophils pretreated with the indicated concentrations of dasatinib with or without 10μM cytochalasin B (CB) were stimulated with 1μM fMLP (A-E), 100 ng/mL of human IL-8 (F-H), 50 ng/mL of C5a (I), or 50 ng/mL of LTB4 (J), followed by measurement of the respiratory burst (A), lactoferrin release (B), flow cytometric analysis of the expression (C,F) and activation (D,G) of CD11b, or assessment of phosphorylation of the ERK or p38 MAPKs by immunoblot and analysis of gelatinase release by in-gel zymography (E,H-J). The kinetic/bar graphs in panels A and B show mean and SD of representative experiments, and the dose-response curves in these panels and bar graphs in panels C and D and F and G show means and SEM of the percent response. Mean fluorescence intensity values of isotype control–stained samples were subtracted in panels C and D and F and G, and the resulting fluorescence was expressed as a percentage of that in the indicated samples. Each set of data were obtained from 3-5 independent experiments. Panels E and H through J are representative of 3 independent experiments. The mean and SEM of phosphorylation of the various MAPKs in the stimulated samples after subtraction of unstimulated controls in the presence of 10nM, 100nM, and 1μM dasatinib was, respectively: 103% ± 10%, 99% ± 14%, and 77% ± 17% (E; p38 MAPK); 99% ± 9%, 94% ± 6%, and 100% ± 2% (E; ERK); 90% ± 17%, 53% ± 5%, and 16% ± 5% (H; p38 MAPK); 123% ± 39%, 112% ± 11%, and 169% ± 42% (H; ERK); 102% ± 52%, 75% ± 27%, and 24% ± 12% (I; p38 MAPK); 98% ± 5%, 98% ± 12%, and 195% ± 55% (I; ERK); 113% ± 53%, 79% ± 29%, and 62% ± 60% (J; p38 MAPK); and 89% ± 14%, 173% ± 32%, and 236% ± 49% (J; ERK) of that in the absence of dasatinib.
Dasatinib partially inhibits neutrophil functions triggered by G-protein–coupled receptors. Human neutrophils pretreated with the indicated concentrations of dasatinib with or without 10μM cytochalasin B (CB) were stimulated with 1μM fMLP (A-E), 100 ng/mL of human IL-8 (F-H), 50 ng/mL of C5a (I), or 50 ng/mL of LTB4 (J), followed by measurement of the respiratory burst (A), lactoferrin release (B), flow cytometric analysis of the expression (C,F) and activation (D,G) of CD11b, or assessment of phosphorylation of the ERK or p38 MAPKs by immunoblot and analysis of gelatinase release by in-gel zymography (E,H-J). The kinetic/bar graphs in panels A and B show mean and SD of representative experiments, and the dose-response curves in these panels and bar graphs in panels C and D and F and G show means and SEM of the percent response. Mean fluorescence intensity values of isotype control–stained samples were subtracted in panels C and D and F and G, and the resulting fluorescence was expressed as a percentage of that in the indicated samples. Each set of data were obtained from 3-5 independent experiments. Panels E and H through J are representative of 3 independent experiments. The mean and SEM of phosphorylation of the various MAPKs in the stimulated samples after subtraction of unstimulated controls in the presence of 10nM, 100nM, and 1μM dasatinib was, respectively: 103% ± 10%, 99% ± 14%, and 77% ± 17% (E; p38 MAPK); 99% ± 9%, 94% ± 6%, and 100% ± 2% (E; ERK); 90% ± 17%, 53% ± 5%, and 16% ± 5% (H; p38 MAPK); 123% ± 39%, 112% ± 11%, and 169% ± 42% (H; ERK); 102% ± 52%, 75% ± 27%, and 24% ± 12% (I; p38 MAPK); 98% ± 5%, 98% ± 12%, and 195% ± 55% (I; ERK); 113% ± 53%, 79% ± 29%, and 62% ± 60% (J; p38 MAPK); and 89% ± 14%, 173% ± 32%, and 236% ± 49% (J; ERK) of that in the absence of dasatinib.
We next investigated neutrophil responses triggered by IL-8, C5a, and LTB4, G-protein–coupled receptor agonists that do not induce robust respiratory burst. Dasatinib did not affect the up-regulation (Figure 4F) or activation (Figure 4G) of CD11b triggered by IL-8. Lower concentrations of dasatinib did not affect, whereas the highest concentration slightly reduced, gelatinase release triggered by IL-8, C5a, and LTB4 (Figure 4H-J). Dasatinib also partially inhibited the phosphorylation of p38 MAPK in response to IL-8, C5a, and LTB4, although that inhibition was primarily seen only at the highest concentration tested (Figure 4H-J). Interestingly, whereas 10-100nM dasatinib did not affect ERK phosphorylation triggered by those agonists, the 1μM dose tended to slightly augment that response (Figure 4H-J).
We conclude that, whereas low nanomolar concentrations of dasatinib exert a partial inhibition of certain fMLP-induced responses, other G-protein–coupled receptor-mediated neutrophil functions are either not affected or are only affected at very high concentrations of dasatinib.
Effect of dasatinib on neutrophil migration
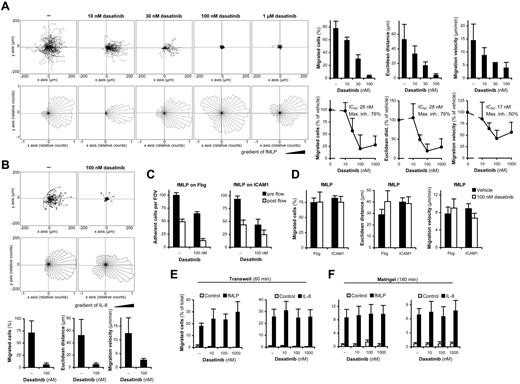
Neutrophils are able to accumulate at the site of inflammation by highly organized chemotactic migration. To determine the effect of dasatinib on that process, we first performed video microscopic assessment of neutrophil chemotaxis in a CD18-dependent23 Zigmond chamber assay under static conditions. As shown in Figure 5A, migration of human neutrophils toward fMLP on a fibrinogen-coated substrate was strongly inhibited by dasatinib, with IC50 values in the range of 15-30nM. The migration of neutrophils toward IL-8 under similar conditions was also strongly inhibited by 100nM dasatinib (Figure 5B).
The effect of dasatinib on neutrophil migration. (A-B) Human neutrophils pretreated with the indicated concentrations of dasatinib were allowed to migrate toward 10μM fMLP (A) or 1 μg/mL of human IL-8 (B) on a fibrinogen-coated coverslip in a Zigmond chamber assay. Representative single-cell tracks, rose diagrams of direction of movement, and the results of quantitative analysis are shown. Bar graphs show mean and SD of representative experiments, and the dose-response curves show mean and SEM of the percent response from 263-476 cells from 3-7 independent experiments. (C-D) Adhesion (C) and migration (D) of neutrophils in the presence of 1μM fMLP in a flow chamber assay (all samples were treated with fMLP). Panel C shows the number of adherent cells per field of view (FOV). Panel D shows migration characteristics of cells that remained adherent through the entire 10-minute flow period. Data in panels C and D show mean and SEM from 5 independent experiments. (E-F) Cells were allowed to migrate toward 100nM fMLP or 10 ng/mL of IL-8 through FCS-coated Transwell filters (E) or a Matrigel matrix (F). Data in panels E and F show mean and SEM from 4-5 independent experiments.
The effect of dasatinib on neutrophil migration. (A-B) Human neutrophils pretreated with the indicated concentrations of dasatinib were allowed to migrate toward 10μM fMLP (A) or 1 μg/mL of human IL-8 (B) on a fibrinogen-coated coverslip in a Zigmond chamber assay. Representative single-cell tracks, rose diagrams of direction of movement, and the results of quantitative analysis are shown. Bar graphs show mean and SD of representative experiments, and the dose-response curves show mean and SEM of the percent response from 263-476 cells from 3-7 independent experiments. (C-D) Adhesion (C) and migration (D) of neutrophils in the presence of 1μM fMLP in a flow chamber assay (all samples were treated with fMLP). Panel C shows the number of adherent cells per field of view (FOV). Panel D shows migration characteristics of cells that remained adherent through the entire 10-minute flow period. Data in panels C and D show mean and SEM from 5 independent experiments. (E-F) Cells were allowed to migrate toward 100nM fMLP or 10 ng/mL of IL-8 through FCS-coated Transwell filters (E) or a Matrigel matrix (F). Data in panels E and F show mean and SEM from 4-5 independent experiments.
We next investigated whether dasatinib also affected 2-dimensional mechanotactic migration of neutrophils on integrin ligand surfaces under flow conditions in the presence of a homogenous concentration of fMLP. Because of the use of integrin ligands in the absence of selectin ligands, this assay models the process of intraluminal crawling, whereas no leukocyte rolling was possible under the conditions used.24,38 As shown in Figure 5C, initiation of flow caused approximately 50% of the adherent cells to detach from both fibrinogen and ICAM1 even in the absence of dasatinib. As expected, 100nM dasatinib reduced both the pre-flow adhesion of neutrophils and, particularly in case of fibrinogen, also the remaining adhesion at the end of the 10-minute flow period. Interestingly, the migration of cells that remained adherent through the entire 10-minute flow period was not affected by 100nM dasatinib (Figure 5D), suggesting that dasatinib inhibits neutrophil adhesion but not mechanotactic migration.
We also investigated neutrophil migration through FCS-coated polycarbonate membranes in β2 integrin-dependent21 Transwell assays. As shown in Figure 5E, dasatinib did not inhibit the migration of neutrophils toward fMLP or IL-8 under those conditions.
The apparent contradiction between the results presented in Figure 5A and B and Figure 5D and E prompted us to investigate neutrophil migration in a more physiologic environment: through a complex extracellular matrix (Matrigel) preparation. Placing Matrigel in Transwell inserts significantly reduced and delayed the migration of neutrophils compared with Matrigel-free inserts (compare Figure 5E-F), indicating that Matrigel did indeed form a migration barrier. Dasatinib did not affect neutrophil migration toward fMLP or IL-8 under these conditions (Figure 5F).
We conclude that, although dasatinib inhibited 2-dimensional neutrophil migration under static conditions and reduced neutrophil adhesion under static and flow conditions, it did not affect the migration of the cells under flow or in 3-dimensional environments such as Transwell filters or Matrigel matrices.
Recognition of innate immune ligands
We next investigated whether dasatinib affected neutrophil responses triggered by innate immune ligands of microbial origin (other than bacterial formyl peptides). Zymosan, an extract of fungal walls, triggers various signal transduction pathways, including TLR and C-type lectin pathways and, when opsonized by serum proteins, may also engage complement and Fc receptors. Dasatinib inhibited the respiratory burst (Figure 6A) and the phosphorylation of ERK and the p38 MAPK (Figure 6B) triggered by unopsonized zymosan. The effect of dasatinib on normal serum-opsonized zymosan was slightly less pronounced (Figures 6A-B), especially in the case of ERK phosphorylation, which was not sensitive to dasatinib. Neutrophil responses triggered by zymosan opsonized with heat-inactivated serum were similar to those triggered by unopsonized zymosan, suggesting that complement-mediated opsonization (and possibly the concomitant release of complement fragments such as C5a) slightly reduces the sensitivity toward dasatinib.
The effect of dasatinib on recognition of innate immune ligands and antimicrobial activity. Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 10 mg/mL of zymosan (Zym) that was opsonized with normal or heat-inactivated (HI) human serum or left unopsonized (A-B), 1 μg/mL of Pam3CSK4 (Pam3; C-D), or with 1 μg/mL of ultrapurified LPS (upLPS; D), followed by assessment of respiratory burst (A), ERK and p38 MAPK phosphorylation (B,D), or gelatinase release (C). (E-F) Killing of S aureus or E coli (E) and phagocytosis of GFP-expressing S aureus (F) by human neutrophils pretreated with the indicated concentrations of dasatinib. Kinetic curves in panels A and E show mean and SD of representative experiments, and the dose-response curves in these panels show mean and SEM of the percent response from 3-6 independent experiments. Panels B through D show representative results from 3 independent experiments. Panel F shows mean and SEM of mean fluorescence intensity (MFI) values from 3-4 independent experiments after subtraction of samples without bacteria at the 0 time point and expressed as a percentage of the indicated sample. The mean and SEM of phosphorylation of the various MAPKs in the stimulated samples after subtraction of unstimulated control values in the presence of 10nM, 100nM, and 1μM dasatinib was, respectively: 62% ± 27%, 5% ± 2%, and 5% ± 3% (B; unopsonized Zym; p38 MAPK); 81% ± 23%, 43% ± 9%, and 52% ± 26% (B; unopsonized Zym; ERK); 42% ± 19%, 7% ± 7%, and 5% ± 5% (B; HI serum-opsonized Zym; p38 MAPK); 56% ± 24%, 37% ± 16%, and 72% ± 38% (B; HI serum-opsonized Zym; ERK); 73% ± 12%, 9% ± 4%, and 4% ± 4% (B; normal serum-opsonized Zym; p38 MAPK); 84% ± 1%, 69% ± 2%, and 89% ± 11% (B; normal serum-opsonized Zym; ERK); 160% ± 38%, 138% ± 57%, and 55% ± 24% (D; Pam3); and 154% ± 31%, 72% ± 24%, and 15% ± 14% (D; upLPS) of that in the absence of dasatinib.
The effect of dasatinib on recognition of innate immune ligands and antimicrobial activity. Human neutrophils pretreated with the indicated concentrations of dasatinib were stimulated with 10 mg/mL of zymosan (Zym) that was opsonized with normal or heat-inactivated (HI) human serum or left unopsonized (A-B), 1 μg/mL of Pam3CSK4 (Pam3; C-D), or with 1 μg/mL of ultrapurified LPS (upLPS; D), followed by assessment of respiratory burst (A), ERK and p38 MAPK phosphorylation (B,D), or gelatinase release (C). (E-F) Killing of S aureus or E coli (E) and phagocytosis of GFP-expressing S aureus (F) by human neutrophils pretreated with the indicated concentrations of dasatinib. Kinetic curves in panels A and E show mean and SD of representative experiments, and the dose-response curves in these panels show mean and SEM of the percent response from 3-6 independent experiments. Panels B through D show representative results from 3 independent experiments. Panel F shows mean and SEM of mean fluorescence intensity (MFI) values from 3-4 independent experiments after subtraction of samples without bacteria at the 0 time point and expressed as a percentage of the indicated sample. The mean and SEM of phosphorylation of the various MAPKs in the stimulated samples after subtraction of unstimulated control values in the presence of 10nM, 100nM, and 1μM dasatinib was, respectively: 62% ± 27%, 5% ± 2%, and 5% ± 3% (B; unopsonized Zym; p38 MAPK); 81% ± 23%, 43% ± 9%, and 52% ± 26% (B; unopsonized Zym; ERK); 42% ± 19%, 7% ± 7%, and 5% ± 5% (B; HI serum-opsonized Zym; p38 MAPK); 56% ± 24%, 37% ± 16%, and 72% ± 38% (B; HI serum-opsonized Zym; ERK); 73% ± 12%, 9% ± 4%, and 4% ± 4% (B; normal serum-opsonized Zym; p38 MAPK); 84% ± 1%, 69% ± 2%, and 89% ± 11% (B; normal serum-opsonized Zym; ERK); 160% ± 38%, 138% ± 57%, and 55% ± 24% (D; Pam3); and 154% ± 31%, 72% ± 24%, and 15% ± 14% (D; upLPS) of that in the absence of dasatinib.
We also investigated the effect of dasatinib on neutrophil responses triggered by TLR agonists. As shown in Figure 6C, dasatinib reduced gelatinase release triggered by the TLR2 agonist Pam3CSK4. Higher concentrations of dasatinib also partially reduced the activation of the p38 MAPK by Pam3CSK4, and the highest concentration of the drug caused significant inhibition of p38 MAPK activation triggered by ultrapurified LPS (Figure 6D).
We conclude that dasatinib inhibited the responses of neutrophils to unopsonized zymosan, but that this effect was slightly attenuated by complement-mediated opsonization. Dasatinib also partially inhibited neutrophil functions triggered by TLR ligands, but that inhibition was primarily seen at very high concentrations of the drug.
Effect on bacterial killing and phagocytosis
We also investigated whether dasatinib affected antimicrobial activities of neutrophils. As shown in Figure 6E, dasatinib caused a modest reduction of neutrophil-mediated killing of serum-opsonized S aureus or E coli with IC50 values above 100nM (Figure 6E). Dasatinib did not affect phagocytosis of serum-opsonized GFP-expressing S aureus bacteria (Figure 6F), whereas the same response was strongly inhibited by 10μM cytochalasin D (data not shown), indicating that we indeed tested an active phagocytosis process. We conclude that dasatinib does not have a major effect on the direct antimicrobial activities of neutrophils.
Dasatinib inhibits leukocyte adhesion in whole serum
All of the experiments discussed thus far were performed on neutrophils separated from their natural environment in the absence of any serum proteins. We next investigated whether dasatinib also inhibits neutrophils under more physiologic conditions in the presence of whole serum. RBCs were sedimented from freshly drawn human blood and the leukocyte-rich plasma supernatant was used in adhesion assays without any additional manipulation to preserve near-physiologic conditions. The majority of adherent cells in this assay showed a typical neutrophil-like morphology (not shown).
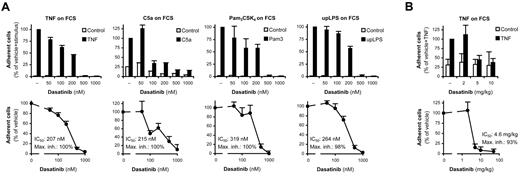
As shown in Figure 7A, adding TNF, C5a, Pam3CSK4, or ultrapurified LPS induced robust increase in leukocyte (neutrophil) adhesion to an FCS-coated surface, and all of these responses were almost completely blocked by dasatinib. In the case of TNF stimulation, dasatinib caused a modest but statistically significant (P = .013, n = 9) inhibition at as little as a 50nM concentration, whereas in case of C5a stimulation, a robust and highly significant (P = .00074; n = 6) inhibition was achieved by 100nM dasatinib. In both cases, half-maximal inhibition was attained slightly above 200nM dasatinib. We conclude that 50-100nM dasatinib significantly inhibits neutrophil adhesion in the most sensitive assay systems, and that half-maximal inhibition is reached at approximately the 200nM concentration in the presence of whole serum.
Dasatinib inhibits adhesion of human and mouse leukocytes in whole serum. (A) Leukocyte-rich human plasma pretreated with the indicated concentrations of dasatinib was incubated with 20 ng/mL of human TNF, 100 ng/mL of human C5a, 1 μg/mL of Pam3CSK4, or 1 μg/mL of ultrapurified LPS (upLPS) on an FCS-coated surface for 30 minutes, followed by determination of cell adhesion. (B) Leukocyte-rich mouse plasma collected 2 hours after oral administration of the indicated doses of dasatinib was stimulated with 50 ng/mL of murine TNF on an FCS-coated surface for 30 minutes, followed by determination of cell adhesion. Bar graphs show mean and SD of representative experiments, and the dose-response curves show mean and SEM of the percent response from 3-9 independent experiments.
Dasatinib inhibits adhesion of human and mouse leukocytes in whole serum. (A) Leukocyte-rich human plasma pretreated with the indicated concentrations of dasatinib was incubated with 20 ng/mL of human TNF, 100 ng/mL of human C5a, 1 μg/mL of Pam3CSK4, or 1 μg/mL of ultrapurified LPS (upLPS) on an FCS-coated surface for 30 minutes, followed by determination of cell adhesion. (B) Leukocyte-rich mouse plasma collected 2 hours after oral administration of the indicated doses of dasatinib was stimulated with 50 ng/mL of murine TNF on an FCS-coated surface for 30 minutes, followed by determination of cell adhesion. Bar graphs show mean and SD of representative experiments, and the dose-response curves show mean and SEM of the percent response from 3-9 independent experiments.
Oral administration of dasatinib inhibits ex vivo adhesion of murine leukocytes
Our final aim was to determine whether oral administration of dasatinib to experimental mice also affected neutrophil functions. We collected peripheral blood 2 hours after oral dasatinib treatment, which was followed by the ex vivo analysis of leukocyte adhesion from leukocyte-rich plasma as described in the previous section. As shown in Figure 7B, ex vivo administration of TNF triggered robust leukocyte adhesion to an FCS-coated surface. That response was nearly completely blocked by prior oral administration of as little as 5 mg/kg of dasatinib with a calculated IC50 value of 4.6 mg/kg. These results indicate that oral administration of dasatinib inhibits leukocyte (likely primarily neutrophil) adhesiveness in experimental mice.
Discussion
Over the past several years, tyrosine kinases have emerged as major therapeutic targets in various malignant diseases.39 Therapy of chronic myelogenous leukemia with the Abl kinase inhibitor imatinib became the first example of a tyrosine kinase inhibitor used in human therapy.40 The later emergence of imatinib-resistant leukemia41 prompted the development of second-generation agents such as dasatinib, which is able to inhibit imatinib-resistant Bcr-Abl mutants.2
Whereas dasatinib is able to suppress the proliferation of malignant hematopoietic cells, its effect on nonmalignant cells is poorly understood. This is particularly true for neutrophils. The results of the present study indicate that dasatinib exerts robust inhibitory effects on neutrophil responses triggered by cellular adhesion (Figures 1 and 2A-G) or immobilized IgG immune complexes (Figure 3), with IC50 values in the low-nanomolar range (often below 10nM) in the most sensitive assay systems under serum-free conditions. Conversely, neutrophil responses triggered by cytokines (Figure 2H-K), G-protein–coupled receptor agonists (Figure 4), innate immune ligands (Figure 6A-D), and direct antimicrobial functions of neutrophils (Figure 6E-F) were either reduced modestly or only at a very high (1μM) dasatinib concentration.
We also investigated the effect of dasatinib on the directed migration of neutrophils (Figure 5). Dasatinib blocked neutrophil migration in a 2-dimensional Zigmond chamber assay and, as expected, reduced neutrophil adhesion under both static and flow conditions. However, dasatinib did not affect the mechanotactic migration of cells that remained adherent under flow or the transmigration of neutrophils in 3-dimensional settings such as Transwell filters or extracellular matrices. In our preliminary in vivo studies, dasatinib also did not inhibit neutrophil accumulation during a thioglycollate-induced sterile peritonitis (K.F. and A.M., unpublished observations, January 2012). Although the reason for that discrepancy is unclear, these results argue against a major effect of the drug on in vivo neutrophil migration. Although it would be tempting to speculate that the discrepancy is because of the requirement for integrins during 2-dimensional but not 3-dimensional migration of neutrophils,42 the fact that the Transwell migration21 (and likely the mechanotactic migration under flow43 ) is dependent on β2-integrins argues against that possibility. It should also be mentioned that the discrepancy between defective adherent activation (Figure 1-2) and normal migration (Figure 5D-F) is in agreement with our prior conclusion that the 2 processes use different signal transduction pathways.16,21,26
Because approximately 96% of dasatinib binds to serum proteins in vivo,44 we also investigated the effect of the drug on leukocyte adhesion in whole serum. Dasatinib completely blocked leukocyte (primarily neutrophil) adhesion under these conditions with significant inhibition at 50-100nM and IC50 values of approximately 200nM in the most sensitive assay systems used (Figure 7A). We also administered dasatinib orally to experimental mice, followed by ex vivo analysis of the adhesion of their leukocytes in the presence of autologous serum. Oral administration of 5 mg/kg of dasatinib almost completely blocked leukocyte adhesion under these conditions. That dose is near or below doses used previously to treat various leukemia models in experimental mice.2,45,46
The recommended dosage of dasatinib in human patients ranges from 70 mg once or twice daily to 100-140 mg once daily.47 The maximum dasatinib serum concentration after the most thoroughly tested 70-mg dose was reported to be in the range of 50-100 ng/mL (approximately 100-200nM).44,48,49 Even higher serum concentrations are expected after administration of more than 70 mg of dasatinib.44,49,50 There is significant variation in dasatinib plasma concentrations between individual patients44,48-50 and different ethnic groups.48 A recent study in pediatric patients reported dasatinib plasma concentrations in the range of 100-200 ng/mL (approximately 200-400nM) and even 2 cases of > 240 ng/mL (approximately > 480nM) without dose-limiting toxicity.50 The results of these studies suggest that plasma dasatinib concentrations significantly above 100-200nM likely occur in certain dasatinib-treated patients, indicating that at least some of the effects on mature neutrophils likely occur during dasatinib treatment in human patients.
Dasatinib is a dual inhibitor of Abl and Src-family tyrosine kinases, but it also inhibits additional tyrosine kinases such as c-Kit and the Eph family,1 and many of these kinases may be involved in neutrophil signaling.14,15,17-19 We can nevertheless speculate about how dasatinib inhibits neutrophils. Whereas Abl-deficient mouse neutrophils showed normal integrin and Fc-receptor signaling (K.F. and A.M., unpublished observations, April 2010), Src-family kinases were clearly indispensable for neutrophil activation by both integrins14,15 and Fc receptors (Z. Jakus, M. Kovács, and A.M., unpublished observations, November 2006). The inhibitory effect of dasatinib on cellular tyrosine phosphorylation (Figure 2E-F) is very similar to that seen in Src-family–deficient neutrophils.21 Src-family kinases activate Syk downstream of integrins16,21 and Fc receptors (Z. Jakus and A.M., unpublished observations, November 2006) in neutrophils, whereas Syk is required for signaling by both integrins21 and Fc receptors (Kiefer et al36 and Z. Jakus and A.M., unpublished observations, November 2006) in these cells. Because dasatinib strongly inhibited Syk activation after integrin (Figure 2E-G) and Fc-receptor (Figure 3F-G) ligation, a feasible explanation is that dasatinib inhibits Src-family–mediated activation of Syk, thereby blocking integrin and Fc-receptor signal transduction in neutrophils.
Drugs affecting immune cells (including neutrophils) may also attenuate antimicrobial immunity. Indeed, one of the side effects of dasatinib is the emergence of mild to moderate infections.44 The neutrophil compartment may be important during these side effects either because dasatinib may trigger myelosuppression and neutropenia or because of the direct effect of dasatinib on the antimicrobial functions of mature neutrophils. Whereas our results presented in Figure 6 make it unlikely that therapeutic concentrations of dasatinib reduce the antimicrobial effect of neutrophils directly, the strong inhibition of adherent activation in the presence of antimicrobial compounds (Figure 1A) raises the possibility that dasatinib may reduce the inflammatory response required for antimicrobial immunity.
The dramatic effect of dasatinib on adhesion-mediated and immune complex–induced neutrophil functions also raises the possibility that dasatinib-related molecules may be effective in autoimmune or inflammatory diseases characterized by excessive neutrophil activation. That possibility is further emphasized by the significant inhibition of neutrophil-mediated in vivo models of inflammation (such as K/B × N serum-transfer arthritis or the Arthus reaction) by oral administration of dasatinib (K.F. and A.M., unpublished observations, March 2011). Therefore, in the future, kinase inhibitors sharing structural similarities or overlapping target profiles with dasatinib may also provide clinical benefit in autoimmune or inflammatory diseases.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Edina Simon and Anna Tóth for expert technical assistance; Csaba Szántai-Kis, Eszter Illyés, and István Varga for the HPLC-MS analysis; Csaba Tímár for help with bacterial killing assays; Erzsébet Ligeti and József Mandl for access to equipment; and William Nauseef for the GFP-expressing S aureus strain.
This work was supported by the Wellcome Trust (International Senior Research Fellowship number 087782 to A.M.), the Hungarian Office for Research and Technology (Anyos Jedlik award number NKFP-A1-0069/2006 to A.M. and T.V.), the European Research Council (Starting Independent Investigator Award number 206283 to A.M.), the Deutsche Forschungsgemeinschaft (grant SFB914/A2 to B.W.), and the European Union FP7 Cooperation Program TARKINAID project (to A.M. and B.W.).
Wellcome Trust
Authorship
Contribution: K.F., T.N., T.V., and A.M. designed the study; K.F. and T.N. performed the majority of the experiments and analyzed the data; K.F., T.N., and A.M. interpreted the data and wrote the manuscript; R.P. and B.W. designed, performed, analyzed, and interpreted the Zigmond chamber and flow chamber migration assays and wrote the relevant parts of the manuscript; and A.M. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Attila Mócsai, MD, PhD, Department of Physiology, Semmelweis University School of Medicine, PO Box 259, 1444 Budapest, Hungary; e-mail: mocsai@eok.sote.hu.