In 1960, Duckert et al described the first clinical case of congenital FXIII deficiency.2 They investigated a young Swiss boy presenting with severe bleeding diathesis associated with slow and poor wound healing but normal routine coagulation tests. They observed an increased lability of the clot and although no proof was available, they postulated the deficiency of a plasma protein that makes the fibrin clot insoluble in urea and suggested a deficiency of fibrin stabilizing factor described by Laki and Lorand in 1948.3 After transfusing the patient with fresh frozen plasma, his bleeding symptoms improved. It is interesting to note that this very patient took part in a genetic study 45 years later and congenital FXIII A-subunit deficiency could be confirmed by sequencing, structural analysis, and cell expression.4
What do we learn from this case report published 52 years ago? Quite a lot. First of all, congenital FXIII deficiency needs prophylactic replacement therapy to avoid fatal or severely disabling bleeding complications after only minor trauma or even spontaneous intracranial haemorrhages. Secondly, the diagnosis of congenital FXIII deficiency must be based on accurate laboratory tests. As shown in 1960, the usual screening tests for coagulopathies such as prothrombin time, activated partial thromboplastin time, and thrombin time, do show normal values in case of FXIII deficiency. Therefore, if clinical symptoms indicate a bleeding disorder, a complete evaluation of the clotting system is required including a specific test that detects FXIII deficiency. Nowadays clot solubility tests like those used by Duckert et al are no longer recommended because of the high number of undiagnosed or late-diagnosed FXIII deficiencies attributable to this test.5 Correct diagnosis is as important as the correct treatment because every patient left undiagnosed will suffer from severe bleeding complications and probably death. Hence, diagnosis of congenital FXIII deficiency should not be delayed in any child with an unknown bleeding tendency especially when prolonged umbilical cord bleeding is observed after birth.
Treatment of patients with congenital FXIII deficiency normally consists of prophylactic administration of plasma-derived pasteurized FXIII concentrate every 4 to 6 weeks at a dosage ranging from 10 to 35 U/kg.6 Prophylaxis is highly efficient because of the long half-life of FXIII. When prophylactic treatment is available the prognosis is very good, although there is a lifelong risk of bleeding.
Inbal et al have now taken the treatment of such patients a big step further because plasma-derived FXIII concentrates do carry a risk for infection with blood-borne pathogens but also allergic reactions. In their multinational, open-label, single-arm, phase 3 prophylaxis trial in patients with congenital FXIII A-subunit deficiency, they investigated the efficacy and safety of a new rFXIII manufactured in Saccharomyces cerevisiae (baker's yeast).1 This rFXIII A-subunit associates in plasma with the endogenous FXIII B-subunit to form a stable FXIII heterotetramer (see figure) because FXIII circulates in plasma as a tetramer consisting of 2 catalytic A-subunits and 2 carrier B-subunits (A2B2).7 In a phase 1 clinical trial, rFXIII had a half-life similar to that of native FXIII and was found to show a good safety profile.8 Inbal et al also address the issue of the development of nonneutralizing antibodies, which is a major problem in patients with hemophilia. For their study, 41 patients were enrolled at 23 centers in 11 countries for a 52-week treatment period of monthly 35 IU/kg of rFXIII intravenously.1 Throughout the treatment period, only 5 trauma-induced bleeding episodes in 4 patients required additional treatment with FXIII-containing products. Transient, nonneutralizing, low-titer anti-rFXIII antibodies developed in only 4 patients. However, these nonneutralizing antibodies declined below detection limits in all patients despite further exposure to rFXIII.1
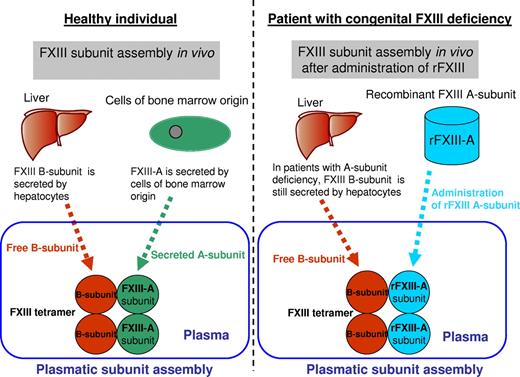
FXIII B-subunit, the carrier protein, is synthesized and secreted by the liver, whereas FXIII A-subunit is synthesized and released by cells of bone marrow origin. Both subunits are assembled in plasma to a tetrameric molecule consisting of 2 A- and 2 B-subunits (A2B2). In patients with congenital FXIII A-subunit deficiency, only free FXIII B-subunit circulates in plasma. After administration of rFXIII-A, both subunits are assembled in plasma to tetrameric FXIII in the same way as in healthy subjects.
FXIII B-subunit, the carrier protein, is synthesized and secreted by the liver, whereas FXIII A-subunit is synthesized and released by cells of bone marrow origin. Both subunits are assembled in plasma to a tetrameric molecule consisting of 2 A- and 2 B-subunits (A2B2). In patients with congenital FXIII A-subunit deficiency, only free FXIII B-subunit circulates in plasma. After administration of rFXIII-A, both subunits are assembled in plasma to tetrameric FXIII in the same way as in healthy subjects.
These findings have important implications for the treatment of patients with congenital FXIII deficiency by providing a novel and safe treatment with rFXIII instead of plasma-derived products. This work by Inbal and colleagues establishes a new treatment standard for FXIII deficient patients.
What should we expect next from rFXIII? A favorable cost-effectiveness profile needs to be established so that rFXIII becomes widely used. In this context it must be noted that, especially in some developing countries, congenital FXIII deficiency is expected to be much higher because of traditional consanguineous marriages. Inherited bleeding disorders are a major problem in such countries and the safety of plasma-derived factor concentrates as well as multidisciplinary comprehensive care is not always guaranteed.
In the future, the availability of rFXIII also has possible implications in patients with acquired FXIII deficiency. Congenital FXIII deficiency is associated with a complete lack of circulating plasma FXIII A-subunit antigen, but clinically significant reductions in FXIII levels have also been reported in a number of conditions, such as major surgery, liver cirrhosis, graft-versus-host disease, sepsis, and chronic inflammatory bowel disease. In these acquired FXIII deficiency states, FXIII A-subunit levels can drop into a range of 20% to 50%.9,10 The reduction is caused by decreased synthesis or consumption. Further studies are needed to see whether FXIII substitution therapy with rFXIII will be an option in patients with acquired FXIII deficiency.
In conclusion, Inbal et al describe a safe and novel treatment of patients with congenital FXIII. This is good news for all patients with this rare bleeding disorder. However, it is also important that all patients with this hemorrhagic diathesis are correctly investigated and diagnosed because congenital FXIII deficieny is probably the most underdiagnosed rare bleeding disorder in the world.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal