The monoclonal antibody targeting CD52, alemtuzumab, has a more potent antileukemic activity compared with the antibody targeting CD20, rituximab. CLL cells express more CD52 than CD20.3 Finally, the combination of fludarabine with alemtuzumab (FA) was shown to be effective and safe.4,5 Hence, it seemed a logical step to combine alemtuzumab with FC chemotherapy, the most efficient backbone of current chemoimmunotherapies for CLL.6 Therefore, the French cooperative group on CLL and Waldenstrom Macroglobulinemia (FCGCLL/MW) and Groupe Ouest Est des Leucémies et Autres Maladies du Sang (GOELAMS) conducted a joint multicenter, phase 3 trial comparing FCA with FCR. In January 2009, the Data and Safety Monitoring Board (DSMB) decided that the recruitment had to be stopped because of an excess mortality observed in the FA arm. Overall 8 patients died in the FCA arm due to infectious complications and secondary lymphoma. No toxic death was observed in the FCR arm. Moreover, FCA induced a significantly lower rate of complete responses than FCR.
The importance of a careful, systematic, sequential, clinical development for new drug combinations cannot be over-stressed. The current trial would have benefitted from a carefully conducted phase 2 trial performed in pretreated patients prior to embarking on a phase 3 trial in untreated patients. As a matter of fact, recent data from a phase 2 trial suggested that FCA had more toxicity with inferior therapeutic efficacy (response rate and time to progression) compared with FCR or FA.7 More importantly, FCA induced an unacceptably high toxicity including fatal complications that were clearly related to the treatment in 5 of 57 patients (treatment-related mortality rate of nearly 9%).7 It is possible that knowledge of the results of this phase 2 trial would have prevented the French group from starting this trial.
Modifications of the route of administration of alemtuzumab may have contributed to these unfavorable results. In most trials using fludarabine and alemtuzumab, the antibody was given intravenously, resulting in plasma levels of > 1 μg/mL supposed to induce apoptosis of CLL cells.8 Lower levels (> 0.1 μg/mL) are sufficient to deplete normal T cells, at least in an allograft setting.9 The subcutaneous route of application seems to achieve therapeutic plasma levels only when using higher cumulative doses and only after more than 3 weeks of thrice-weekly applications of alemtuzumab.10 Therefore, it is possible that the intermittent, subcutaneous dosing regimen of alemtuzumab as applied in the French trial, every 4 weeks for 3 days, produced alemtuzumab plasma concentrations that were sufficient to deplete T cells but not high enough to exert sufficient tumor killing. Unfortunately, alemtuzumab plasma levels were not determined in the trial.
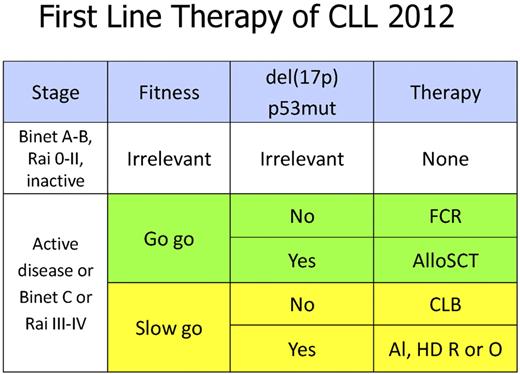
The trial confirms the current treatment algorithm that recommends FCR as standard first-line therapy for CLL patients with a good performance status (see figure). As in previous publications, FCR showed a higher rate of complete responses and remissions that were minimal residual disease (MRD) negative.2,11
Current recommendations on first line therapy of CLL. FCR indicates fludarabine, cyclophosphamide, rituximab; AlloSCT, allogeneic stem cell transplantation; CLB, chlorambucil; Al, alemtuzumab; HD, high dose; R, rituximab; O, ofatumumab; P53mut, mutation of the p53 gene; and del(17p), deletion of the short arm of chromosome 17.
Current recommendations on first line therapy of CLL. FCR indicates fludarabine, cyclophosphamide, rituximab; AlloSCT, allogeneic stem cell transplantation; CLB, chlorambucil; Al, alemtuzumab; HD, high dose; R, rituximab; O, ofatumumab; P53mut, mutation of the p53 gene; and del(17p), deletion of the short arm of chromosome 17.
In light of the results of the French study, one can only agree with the recommendation of Lepretre et al that FCA should not be used for the treatment of CLL outside of clinical trials. The question of whether alemtuzumab should no longer be used in any chemoimmunotherapy is an entirely different issue. It should be emphasized that a recent randomized trial showed that the second-line use of fludarabine plus intravenous alemtuzumab, FA, led to a significant improvement of the overall survival rate without excess toxicity, in particular in CLL patients with advanced Rai stages.5 Therefore, it is likely that alemtuzumab will have a defined, valuable role in the management of CLL, for example, for high-risk patients or at relapse.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal