This phase 1 study evaluated the safety, tolerability, pharmacokinetics, and antitumor activity of obinutuzumab (GA101), a glycoengineered type II anti-CD20 monoclonal antibody administered as induction followed by 2 years of maintenance. Cohorts of 3 to 6 patients received obinutuzumab (200-2000 mg) intravenously weekly for 4 weeks. Patients with a complete or partial response (or stable disease and clinical benefit) continued to receive obinutuzumab every 3 months, for a maximum of 8 doses. Twenty-two patients with relapsed CD20-positive non-Hodgkin lymphoma or chronic lymphocytic leukemia with an indication for treatment and no therapy of higher priority were enrolled. Patients received a median of 4 prior regimens; 86% had received at least 1 rituximab-containing regimen. No dose-limiting or unexpected AEs were observed. Infusion-related reactions were most common (all grades, 73%; grade 3/4, 18%), followed by infection (32%), pyrexia (23%), neutropenia (23%), headache (18%), and nausea (18%). At end of induction, 5 (23%) patients achieved partial responses and 12 (54%) had stable disease. Eight patients received maintenance; best overall response was 32% (6 partial responses/1 complete response). Obinutuzumab induction and maintenance therapy was well tolerated with promising efficacy in this heterogeneous, highly pretreated population and warrants further investigation. This study was registered at www.clinicaltrials.gov (identifier NCT00576758).
Introduction
Monoclonal antibody therapy has markedly improved the outcome of B-cell malignancies and represents one of the most important advances in the treatment of B-cell lymphoma in the past 30 years. Rituximab, a type I chimeric IgG1 anti-CD20 antibody, given alone or in combination with standard chemotherapy regimens, is associated with increased response rates and improved progression free-survival and overall survival with an acceptable safety profile.1,,,,,,,,,,,,,–15 Maintenance therapy with rituximab has been demonstrated to significantly improve responses and progression free-survival in both previously untreated and relapsed follicular lymphoma (FL).16,–18 However, indolent lymphomas, such as FL and chronic lymphocytic leukemia (CLL) remain incurable, with patients exhibiting relapses after each treatment, highlighting the need for more effective therapies.
Obinutuzumab (GA101) is a unique, glycoengineered type II anti-CD20 monoclonal antibody designed to have improved therapeutic efficacy compared with previously developed type I agents.19,20 The mechanism of action of anti-CD20 antibodies includes activation of complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), and the induction of direct cell death, although the exact contribution of each component in vivo is unknown.21,22 Obinutuzumab recognizes a CD20 epitope overlapping with that of rituximab, but it exhibits a different elbow hinge angle and binds CD20 in a different orientation compared with type I anti-CD20 antibodies, the latter of which may form the basis for the functional differences between type I and type II antibodies.21 In addition, the Fc portion of obinutuzumab has been glycoengineered to reduce fucosylation, resulting in optimized affinity for the FcγRIIIa receptor and enhanced ADCC potency.19,21 Importantly, GA101 as a type II antibody more potently induces direct cell death and may provide an advantage when combined with chemotherapy.19 Obinutuzumab has demonstrated significantly increased ADCC compared with rituximab in in vitro models.19 In contrast to type I antibodies, type II antibodies do not stabilize CD20 in lipid rafts and thus exhibit reduced binding to C1q, resulting in lower levels of complement-dependent cytotoxicity. In vivo studies have demonstrated that obinutuzumab more effectively induces B-cell depletion in cynomolgus monkeys and can induce complete tumor remission in diffuse large B-cell lymphoma (DLBCL) xenograft models.19
Initial phase 1 studies of escalating doses of obinutuzumab administered in a 3-weekly schedule in heavily pretreated patients with relapsed/refractory CLL or CD20-positive non-Hodgkin lymphoma (NHL) have been reported recently.23,24 In doses ranging from 200 to 2000 mg, obinutuzumab was well tolerated, and no dose-limiting toxicity was observed. Reported adverse events (AEs) included infusion-related reactions, neutropenia, thrombocytopenia, and infection. Here, we report results of the first study of obinutuzumab administered as weekly × 4 induction followed by 2 years of maintenance therapy to assess the tolerability, pharmacokinetics, efficacy, and long-term safety associated with this extended dosing schedule.
Methods
Patient selection
Adult patients 18 years of age or older, with relapsed or refractory CD20-positive NHL or CLL, with a clinical indication for treatment and for which no therapy of higher priority existed were eligible for the study. Patients were required to have measurable disease (> 1.5 cm), adequate renal and hepatic function, adequate hematologic reserve (unless because of lymphoma), and an Eastern Cooperative Oncology Group performance status of 0 or 1. Histology and CD20 positivity were assessed locally before enrollment and subsequently confirmed by central pathology review. Patients could not have received rituximab within 56 days, prior radioimmunotherapy within 3 months, or an investigational monoclonal antibody within 6 months of study entry. Patients with evidence of central nervous system involvement, exposure to HIV, hepatitis B, or hepatitis C were excluded. All patients provided informed consent before participation. This study was approved by the research ethics boards of participating institutions and was conducted in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. This study was registered at www.clinicaltrials.gov (identifier NCT00576758).25
Study design and dosing
This was a multicenter, open-label, phase 1 study evaluating the safety, tolerability, pharmacokinetics, and antitumor activity of escalating doses of obinutuzumab administered as induction monotherapy followed by maintenance. Before therapy, patients underwent baseline staging investigations that included a history and physical examination; routine laboratory tests; bone marrow biopsy; and computed tomography (CT) scanning of the neck, chest, abdomen, and pelvis.
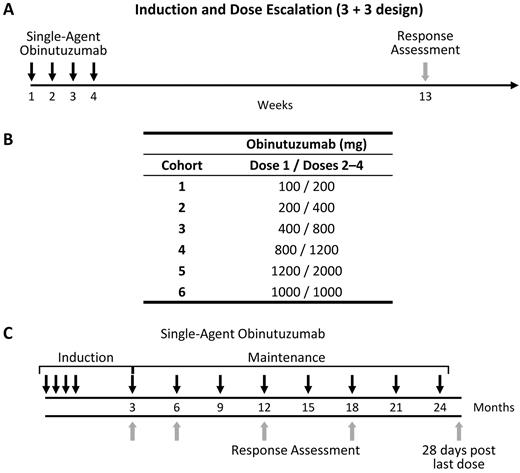
Obinutuzumab was administered as a weekly intravenous infusion for up to 4 treatments during induction therapy, with the first induction infusion given at 50% to 60% of the dose for that cohort (Figure 1). Patients were pretreated with oral acetaminophen/paracetamol (1000 mg) and an antihistamine (eg, diphenhydramine, 50-100 mg) 30 to 60 minutes before each infusion. Steroids were administered at the discretion of the investigator. The first infusion was administered at a rate of 50 mg/hour and escalated in increments of 50 mg/hour every 30 minutes to a maximum rate of 400 mg/hour. If well tolerated, subsequent infusions were administered at an initial rate of 100 mg/hour and increased by 100 mg/hour every 30 minutes to a maximum rate of 400 mg/hour. Obinutuzumab doses were escalated in a standard 3 + 3 design, in doses ranging from 200 to 2000 mg or until the maximum tolerated dose (MTD) was reached. A dose expansion cohort was added for further exploration of a proposed phase 2 dose. Dose-limiting toxicities (DLTs) were assessed during the 28-day induction period and included related grade 3 or higher nonhematologic toxicities or grade 3 or 4 neutropenia or thrombocytopenia. If grade 3 or 4 neutropenia, thrombocytopenia, or both were observed, that did not meet the definition of a DLT, the patient received subsequent treatments to complete induction, provided the neutropenia, thrombocytopenia, or both had resolved to less than grade 2. Infusion-related DLTs (first infusion only) were defined as any grade 4 infusion-related toxicity or any grade 3 infusion-related toxicity that could not be resolved by infusion rate reduction, dose interruption or discontinuation, or supportive care.
Study design. (A) Cohorts of 3 to 6 patients received intravenous (IV) infusions of obinutuzumab weekly for 4 weeks, with tumor assessment at week 13. (B) Cohort doses were escalated in a standard 3 + 3 design up to 2000 mg or until the MTD was reached. For each cohort, the first infusion was ∼ 50% to 60% of the cohort dose. (C) Patients who had a complete or partial response (or stable disease and clinical benefit) were eligible to enter a 2-year maintenance therapy regimen of single-agent obinutuzumab once every 3 months, for a maximum of 8 infusions, beginning 3 months after the last induction infusion. The first administration of maintenance therapy was initiated 3 months after the last infusion of induction obinutuzumab.
Study design. (A) Cohorts of 3 to 6 patients received intravenous (IV) infusions of obinutuzumab weekly for 4 weeks, with tumor assessment at week 13. (B) Cohort doses were escalated in a standard 3 + 3 design up to 2000 mg or until the MTD was reached. For each cohort, the first infusion was ∼ 50% to 60% of the cohort dose. (C) Patients who had a complete or partial response (or stable disease and clinical benefit) were eligible to enter a 2-year maintenance therapy regimen of single-agent obinutuzumab once every 3 months, for a maximum of 8 infusions, beginning 3 months after the last induction infusion. The first administration of maintenance therapy was initiated 3 months after the last infusion of induction obinutuzumab.
Patients with a complete response (CR) or partial response (PR) at the end of induction were eligible to receive extended therapy with obinutuzumab administered at the same dose as induction once every 3 months for a maximum of 8 infusions, or until disease progression, unacceptable toxicity, or initiation of nonprotocol antilymphoma therapy. During the study, the protocol was modified to allow patients with stable disease (SD) and clinical benefit (significant tumor shrinkage not meeting the definition for PR and associated with symptom improvement) to continue with maintenance treatment with obinutuzumab. This modification allowed patients with evidence of tumor shrinkage but who did not meet criteria for a response after the short induction period to continue receiving treatment with obinutuzumab.
Evaluation of safety and tolerability
Safety and tolerability data were collected throughout the study and consisted of routine clinical laboratory testing and toxicity assessments, including documentation of AEs and serious AEs (SAEs) graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 guidelines.26 On completion of 4 infusions of obinutuzumab, all patients attended a safety follow-up visit 28 days after their last dose of obinutuzumab. At the end of induction, patients who were not eligible for obinutuzumab maintenance therapy and did not go on to receive a new antilymphoma therapy were followed for safety for up to 6 months after B-cell recovery or for a maximum duration of 2 years from the end of induction. Patients who received maintenance therapy were followed for safety for up to 28 days after their last dose of obinutuzumab. Patients who had not progressed at the conclusion of 2 years of maintenance therapy were followed for safety for up to 6 months after B-cell recovery or for up to 2.5 years after the last patient had entered the study. Additional laboratory tests including immunoglobulin levels, analysis of white blood cell subsets, and human anti–human antibodies (HAHAs), were measured at baseline, during induction and maintenance therapy, and after the completion of therapy. Immunoglobulin levels were measured locally at each site by an enzyme-linked immunosorbent assay (ELISA). B-cell subsets were measured centrally by flow cytometry. An ELISA-based assay was developed and centrally measured to detect antidrug antibodies against obinutuzumab in human serum samples (Roche Diagnostics). The presence of HAHAs in study samples was quantitatively determined based on a threshold value designed to produce 5% false-positive results to avoid any false-negative results. Both complement (C3, C3a, C4, C5a, and Bb) and cytokine levels (IL-6, IL-8, IL-10, and TNF-α) were measured at baseline and during induction therapy by ELISA and cytometric bead array (Covance Laboratories).
Evaluation of response
Tumor response was assessed using the International Working Group Criteria for NHL and the National Cancer Institute Working Group Criteria for CLL.27,28 Response evaluations included both clinical and radiologic assessments (CT scanning) with bone marrow biopsy performed to confirm complete remission. Response to induction therapy was assessed 9 weeks after the last induction infusion of obinutuzumab (∼ 12 weeks after the start of therapy). After induction, response was assessed by CT and physical exam every 3 months in the first 6 months and then every 6 months. Patients were followed for disease progression for up to 2.5 years after the last patient was enrolled (ie, end of study).
Pharmacokinetic methods and analyses
During induction and maintenance therapy, pre- and postinfusion pharmacokinetic samples were obtained with each infusion. After maintenance therapy (or after induction therapy in those patients, not eligible to receive maintenance), single pharmacokinetic samples were taken every 3 months for up to 1 year after the last dose of obinutuzumab. An ELISA-based assay was used to measure obinutuzumab levels.
Statistical analyses
The primary end point of the study was safety. A classic phase 1 3 + 3 dose escalation design was used to determine the MTD and explore the safety margins of obinutuzumab. For this dose escalation study, no formal hypothesis testing was planned or conducted.
Results
Patient characteristics and drug exposure
From January 2008 to January 2009, 22 patients in total with CD20-positive hematologic malignancies were enrolled and treated with obinutuzumab at 5 study centers throughout Canada. Patient characteristics are listed in Table 1. The median observation time for all patients on study was 8.5 months (range, 0.8-28.3 months). Histologic subtype of the 22 patients enrolled were as follows: 10 FL, 5 CLL, 3 DLBCL, 2 small lymphocytic lymphoma, 1 mantle cell lymphoma, and 1 transformed lymphoma. The median age of patients at study entry was 60 years (range, 47-77 years) and 59% were male. Patients had received a median of 4 (range, 1-7) prior therapies. Nineteen (86%) patients received at least 1 prior rituximab-containing regimen (median 2; range 1-4). Of the 19 patients who received prior rituximab, 13 (68%) were refractory to rituximab (nonresponse or progression within 6 months of treatment). Three patients had undergone prior autologous stem cell transplantation. All 5 (100%) CLL patients had previously received and were considered refractory to fludarabine treatment. The proportion of patients with low-affinity FcγRIIIa receptor polymorphisms 158FF and 158FV was 45% (10/22) for each genotype respectively. Two patients had the high-affinity 158VV polymorphism.
Patient demographics and baseline disease characteristics
| Characteristic . | Value (range) . |
|---|---|
| All patients, n | 22 |
| Median age, y (range) | 60 (47-77) |
| Male, n (%) | 13 (59) |
| Histologic subtype, n (%) | |
| FL | 10 (45) |
| DLBCL | 3 (14) |
| Other aggressive histology (MCL + transformed MZL) | 2 (9) |
| Small lymphocytic lymphoma | 2 (9) |
| CLL | 5 (23) |
| No. of prior therapies, median (range) | 4 (1–7) |
| Received prior rituximab-containing regimen, n | 19 |
| No. of rituximab-containing regimens, median (range) | 2 (1–4) |
| No. of rituximab-refractory patients (n = 19), n (%) | 13 (68) |
| NHL patients, n | 17 |
| Received prior anthracycline, n (%) | 11 (65) |
| Received prior ASCT | 3 (18) |
| Clinical stage (Ann Arbor), n (%) | |
| I/II | 2 (12) |
| III/IV | 15 (88) |
| Follicular lymphoma prognostic index (n = 10), n (%) | |
| Low risk (0-1 risk factors) | 3 (30) |
| Intermediate risk (2 risk factors) | 2 (20) |
| High risk (3-5 risk factors) | 4 (40) |
| Unknown | 1 (10) |
| CLL patients, n | 5 |
| Refractory to fludarabine | 5 (100) |
| Rai stage, n (%) | |
| Intermediate risk (I and II) | 4 (80) |
| High risk (III and IV) | 1 (20) |
| Characteristic . | Value (range) . |
|---|---|
| All patients, n | 22 |
| Median age, y (range) | 60 (47-77) |
| Male, n (%) | 13 (59) |
| Histologic subtype, n (%) | |
| FL | 10 (45) |
| DLBCL | 3 (14) |
| Other aggressive histology (MCL + transformed MZL) | 2 (9) |
| Small lymphocytic lymphoma | 2 (9) |
| CLL | 5 (23) |
| No. of prior therapies, median (range) | 4 (1–7) |
| Received prior rituximab-containing regimen, n | 19 |
| No. of rituximab-containing regimens, median (range) | 2 (1–4) |
| No. of rituximab-refractory patients (n = 19), n (%) | 13 (68) |
| NHL patients, n | 17 |
| Received prior anthracycline, n (%) | 11 (65) |
| Received prior ASCT | 3 (18) |
| Clinical stage (Ann Arbor), n (%) | |
| I/II | 2 (12) |
| III/IV | 15 (88) |
| Follicular lymphoma prognostic index (n = 10), n (%) | |
| Low risk (0-1 risk factors) | 3 (30) |
| Intermediate risk (2 risk factors) | 2 (20) |
| High risk (3-5 risk factors) | 4 (40) |
| Unknown | 1 (10) |
| CLL patients, n | 5 |
| Refractory to fludarabine | 5 (100) |
| Rai stage, n (%) | |
| Intermediate risk (I and II) | 4 (80) |
| High risk (III and IV) | 1 (20) |
MCL indicates mantle cell lymphoma; MZL, marginal zone lymphoma; and ASCT, autologous stem cell transplantation.
In total, 5 dose escalation cohorts and a sixth dose expansion cohort were tested. All 22 patients enrolled received at least 1 infusion of obinutuzumab and were included in the safety analysis. Twenty-one of 22 patients completed all 4 planned induction infusions. Eight patients proceeded to maintenance therapy and received a range of 1 to 8 infusions.
Safety
No dose-limiting toxicities were observed during the dose escalation portion of the study at levels, ranging between 200 and 2000 mg. The most common AEs during induction were infusion-related reactions (IRRs) that occurred in 16 (73%) patients (Table 2). The majority of IRRs were grade 1 or 2, with 4 (18%) patients experiencing grade 3 or 4 IRRs; 1 grade 3 IRR was associated with tumor lysis syndrome and 1 grade 4 IRR resulted in treatment discontinuation. The grade 4 IRR occurred during the first infusion in a patient with CLL (with an initial lymphocyte count of 3.4 × 109/L) who was planned to receive the 1000-mg dose in the expansion cohort and therefore was not considered a DLT. Infusion-related reactions were primarily associated with the initial obinutuzumab infusion and resolved with slowing or interruption of the infusion, steroid administration, or both, with patients ultimately receiving the full dose. IRRs decreased in both severity and frequency with subsequent infusions. Corticosteroids were administered as treatment for IRRs in 9 patients.
Safety outcomes: most common adverse events with obinutuzumab
| Most common AEs . | No. of patients with at least 1 AE, n (%) . | |
|---|---|---|
| All grades . | Grade 3/4 . | |
| During induction, n = 22 | ||
| Infusion-related reaction | 16 (73) | 4 (18) |
| Infection | 7 (32) | |
| Pyrexia | 5 (23) | |
| Neutropenia | 5 (23) | 5 (23) |
| Headache | 4 (18) | 1 (4) |
| Nausea | 4 (18) | |
| Diarrhea | 3 (14) | |
| Fatigue | 3 (14) | |
| During maintenance, n = 8 | ||
| Infection | 5 (62) | 1 (13) |
| Infusion-related reaction | 2 (25) | |
| Cough | 2 (25) | |
| Most common AEs . | No. of patients with at least 1 AE, n (%) . | |
|---|---|---|
| All grades . | Grade 3/4 . | |
| During induction, n = 22 | ||
| Infusion-related reaction | 16 (73) | 4 (18) |
| Infection | 7 (32) | |
| Pyrexia | 5 (23) | |
| Neutropenia | 5 (23) | 5 (23) |
| Headache | 4 (18) | 1 (4) |
| Nausea | 4 (18) | |
| Diarrhea | 3 (14) | |
| Fatigue | 3 (14) | |
| During maintenance, n = 8 | ||
| Infection | 5 (62) | 1 (13) |
| Infusion-related reaction | 2 (25) | |
| Cough | 2 (25) | |
Data are as of the August 2010 cutoff.
Seven (32%) patients experienced 9 AEs in total because of infection during the induction period. AEs included abscess, candidiasis, nail bed fungal infection, sinusitis, rhinitis, upper respiratory tract infection, vaginal infection, oral herpes, and nasopharyngitis; none of these infections were grade 3 or 4.
Five (23%) patients experienced at least 1 episode of grade 3 or 4 neutropenia, and 1 patient experienced febrile neutropenia recorded as an SAE during induction. Two patients received treatment with G-CSF for neutropenia. The most commonly observed laboratory abnormalities of grade 3 or higher during induction were thrombocytopenia (n = 9), neutropenia (n = 6), leukopenia (n = 4), and anemia (n = 1).
Twenty-five AEs were reported for 6 of the 8 patients who received maintenance treatment. The most common AEs were infection, IRRs, and cough. Only 1 patient experienced a grade 3 AE that included pneumonia and sinusitis in the same patient. No grade 4 events were reported during maintenance treatment.
Eight SAEs were reported in 7 patients, including 2 IRRs. Six SAEs occurred during induction among 6 patients and 2 SAEs occurred during maintenance in 1 patient. These SAEs occurred across various obinutuzumab dose cohorts and various histologic subtypes (Table 3). Two deaths have been reported to date: 1 patient with CLL and 1 patient with DLBCL, both of whom died of disease progression after cycle 4 of induction obinutuzumab. No treatment-related deaths were reported.
Safety outcomes: serious adverse events with obinutuzumab
| Dose level, mg . | SAE* . | |
|---|---|---|
| Diagnosis . | Event . | |
| 100/200 | SLL | Tumor flare |
| FL | Pneumonia/sinusitis | |
| 400/800 | FL | Infusion-related reaction |
| MCL | Febrile neutropenia | |
| CLL | Infusion-related reaction | |
| DLBCL | Malignant hypercalcaemia | |
| 1200/2000 | CLL | Pelvic fracture |
| Dose level, mg . | SAE* . | |
|---|---|---|
| Diagnosis . | Event . | |
| 100/200 | SLL | Tumor flare |
| FL | Pneumonia/sinusitis | |
| 400/800 | FL | Infusion-related reaction |
| MCL | Febrile neutropenia | |
| CLL | Infusion-related reaction | |
| DLBCL | Malignant hypercalcaemia | |
| 1200/2000 | CLL | Pelvic fracture |
Data are as of the August 2010 cutoff.
SLL indicates small lymphocytic lymphoma; MCL, mantle cell lymphoma; CLL, chronic lymphocytic leukemia; and DLBCL, diffuse large B cell lymphomia.
All SAEs resolved.
All patients tested negative for HAHAs at baseline. None of the 14 patients tested for HAHAs during or after therapy were found to have abnormal titers. CD19+ B-cells decreased from baseline after the first infusion and remained low throughout the induction treatment period. B-cell recovery could not be assessed in the majority of patients because of development of progressive disease or initiation of new antilymphoma therapy. Immunoglobulin levels (IgG, IgA, and IgM) remained within baseline values throughout the treatment period. Elevations in IL-6 and IL-8 levels were observed during the first induction infusion and began to decrease within 4 hours after infusion. No significant changes were noted in IL-10 and TNF-α levels. In addition, no change was observed in complement activation (data not shown).
Efficacy
Twenty-one (95%) patients completed 4 infusions of induction treatment and were evaluable for response at the completion of induction (Table 4). One patient with CLL (discussed in “Safety”) discontinued induction treatment during the first infusion because of a grade 4 IRR and was not evaluable for response. One patient with DLBCL died of progressive disease before restaging radiologic assessment but was assessed as having progressive disease (PD). Based on an intention-to-treat analysis, at the end of induction, 5 (22.7%) patients demonstrated a PR, 12 (54.5%) had SD, and 4 (18.2%) had PD.
Patient response to induction obinutuzumab by dose cohort
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | All . |
|---|---|---|---|---|---|---|---|
| Dose, mg | 100/200 | 200/400 | 400/800 | 800/1200 | 1200/2000 | 1000/1000 | |
| No. of patients | 3 | 3 | 3 | 3 | 3 | 7 | 22 |
| CR | 0 | ||||||
| PR (%) | 1 | 2 | 2 | 5 (23) | |||
| SD (%) | 1 | 1 | 2 | 3 | 5 | 12 (54) | |
| PD (%) | 1 | 1 | 1 | 1 | 4 (18) | ||
| Nonevaluable (%) | 1 | 1 (4) |
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | All . |
|---|---|---|---|---|---|---|---|
| Dose, mg | 100/200 | 200/400 | 400/800 | 800/1200 | 1200/2000 | 1000/1000 | |
| No. of patients | 3 | 3 | 3 | 3 | 3 | 7 | 22 |
| CR | 0 | ||||||
| PR (%) | 1 | 2 | 2 | 5 (23) | |||
| SD (%) | 1 | 1 | 2 | 3 | 5 | 12 (54) | |
| PD (%) | 1 | 1 | 1 | 1 | 4 (18) | ||
| Nonevaluable (%) | 1 | 1 (4) |
Twenty-one patients were evaluable for response, but only 20 were radiographically measurable. One patient with DLBCL died of PD before restaging radiologic assessment but was assessed as PD. One patient with CLL discontinued therapy during infusion 1 due to a grade 4 IRR and was inevaluable for response.
After the completion of induction, 8 (36%) patients continued to receive obinutuzumab as maintenance therapy, including the 5 patients with a PR and 3 patients with SD and clinical benefit. Of these 8 patients, 4 (50%) patients completed 2 years of maintenance treatment and entered posttreatment follow-up. One patient discontinued maintenance after 7 maintenance infusions because of progressive disease and entered posttreatment follow-up. The 3 remaining patients discontinued maintenance because of PD (after 3, 1, and 1 maintenance infusions) but did not enter posttreatment follow-up. Three patients improved their response during maintenance treatment. The best overall response was 31.8% (7/22), including 1 (4.5%) CR, 6 (27.3%) PR, 10 (45.4%) SD, and 4 (18.2%) PD. The waterfall plot in Figure 2 demonstrates patient response by treatment duration, histology, obinutuzumab dose, FcγRIIIa receptor polymorphisms, and rituximab refractory status.
Best overall response to obinutuzumab in the 20 patients who were evaluable radiographically after induction alone or induction and maintenance, compared with pretreatment baseline. Responses were assessed as per the International Working Group criteria/National Cancer Institute criteria in addition to radiologic assessment. Duration of response was defined as the time from when a CR or PR was first recorded to the date of death, the date on which PD was first noted, or the last tumor assessment in patients who did not have PD. The plus (+) sign reflects patients with ongoing responses at the time of the analysis who are continuing to receive follow-up assessment. One patient with DLBCL died of PD before radiologic assessment but was assessed as PD. One patient with CLL discontinued therapy during infusion 1 because of a grade 4 IRR and was not evaluable for response. One patient had a reduction of more than 50% in the sum of product diameters but was assessed as having stable disease by the investigator. FcγRIIIa receptor polymorphisms shown at amino acid residue 158 (F, phenylalanine; V, valine). TL indicates transformed lymphoma; and SPD, sum of product diameters.
Best overall response to obinutuzumab in the 20 patients who were evaluable radiographically after induction alone or induction and maintenance, compared with pretreatment baseline. Responses were assessed as per the International Working Group criteria/National Cancer Institute criteria in addition to radiologic assessment. Duration of response was defined as the time from when a CR or PR was first recorded to the date of death, the date on which PD was first noted, or the last tumor assessment in patients who did not have PD. The plus (+) sign reflects patients with ongoing responses at the time of the analysis who are continuing to receive follow-up assessment. One patient with DLBCL died of PD before radiologic assessment but was assessed as PD. One patient with CLL discontinued therapy during infusion 1 because of a grade 4 IRR and was not evaluable for response. One patient had a reduction of more than 50% in the sum of product diameters but was assessed as having stable disease by the investigator. FcγRIIIa receptor polymorphisms shown at amino acid residue 158 (F, phenylalanine; V, valine). TL indicates transformed lymphoma; and SPD, sum of product diameters.
In the subset of patients with FL, the best overall response was 40% (4/10, including 1 CR and 3 PR). One of 3 patients with DLBCL demonstrated a partial response. One of 2 patients with small lymphocytic lymphoma achieved a response, but none of the CLL (0/5) patients enrolled on study met criteria for response. One patient with transformed marginal zone lymphoma also achieved a response. For rituximab-refractory patients, the best overall response was 15% (2/13; 1 CR, 1 PR). The time dependent analyses are immature, but the time to response in responders ranged from 2.4 to 11.0 months, and the duration of response in responding patients ranged from 3.0 to 21.1 months.
Pharmacokinetics and pharmacodynamics
Figure 3A presents the average obinutuzumab concentration during the 4 induction infusions, demonstrating higher concentrations achieved with higher doses administered. Both the Cmax and Ctrough values increased over the 4 induction infusions across the dose range tested. However, a high degree of variability was observed in the serum concentrations of obinutuzumab between individual patients. During the induction phase, similar plasma concentrations were observed for the 1000- and 800/1200-mg cohorts for the first 3 doses. Comparison of obinutuzumab serum concentrations between responding patients and nonresponders indicated consistently higher serum concentrations in responders. After administration of the fourth obinutuzumab dose, the plasma concentrations measured 14 days later demonstrated substantially higher values (∼ 514 μg/mL) for the 1000-mg dose in comparison with the lower doses (∼ 100-250 μg/mL).
Pharmacokinetics of obinutuzumab. Pharmacokinetics of obinutuzumab during induction (A) and maintenance therapy (B).
Pharmacokinetics of obinutuzumab. Pharmacokinetics of obinutuzumab during induction (A) and maintenance therapy (B).
After completion of induction, obinutuzumab concentration levels decreased rapidly in patients who did not receive maintenance. Serum samples were available for 5 of 8 patients who received maintenance obinutuzumab. During maintenance therapy, the peak serum obinutuzumab levels achieved with each 3-monthly infusion remained constant over time and were proportional to the dose administered (Figure 3B). The Ctrough serum levels of obinutuzumab observed during maintenance were within the range of 15 to 40 μg/mL for all doses tested.
Discussion
This phase 1 dose escalation study is the first trial to assess the safety and efficacy of obinutuzumab monotherapy administered weekly during induction followed by 2 years of maintenance therapy in patients with relapsed or refractory CD20-positive B-cell NHL or CLL. Overall, obinutuzumab was well tolerated, with a safety profile that seemed similar to rituximab. The most common AEs were infusion-related reactions, the majority of which were low grade and occurred during the first infusion. Although 1 patient discontinued therapy because of an infusion-related reaction, the remainder were treated with supportive therapy including steroids when necessary and completed treatment as planned.
Low-grade infections were observed in approximately one third (32%) of patients during induction and in 5 of 8 patients during maintenance and were easily managed. The only grade 3 or 4 infection reported occurred during maintenance therapy, consisting of a grade 3 respiratory infection and sinusitis that responded to antimicrobial therapy. Of the 8 patients who went on to receive maintenance treatment, there were no discontinuations for treatment-related toxicity. IgG levels remained stable during therapy, and no patients were observed to develop antidrug antibodies (HAHAs). Furthermore, the safety profile reported here is comparable to that reported in other phase 1 studies of obinutuzumab in patients with NHL and CLL.25,26 No unexpected toxicities or new safety signals were identified during induction or extended therapy.
In this heterogeneous and heavily pretreated patient population, responses were observed after induction treatment, including 2 patients known to be refractory to rituximab. Three of 8 patients receiving maintenance therapy improved their response with extended treatment, resulting in a best overall response rate of 32%. The majority of responses observed in patients with NHL were durable. The duration of response in responders ranged from 3.0 to 21.1 months, and 4 of 7 responses lasted more than a year, with 3 patients having an ongoing response. The efficacy in CLL patients differed from that reported in other phase 1 studies of obinutuzumab.25 However, in these studies patients received higher cumulative doses of obinutuzumab and were evaluated at a later time point (week 25 vs week 12).
Pharmacokinetic studies demonstrated that both peak and trough serum levels of obinutuzumab increased with each weekly infusion during induction, and levels were proportionately higher for higher doses administered. After the last dose of induction, a rapid decline of obinutuzumab serum levels was observed. During maintenance therapy, peak levels were proportional to the administered dose, but trough serum levels of obinutuzumab were similarly low for all dose cohorts. In this small and heterogeneous patient population, responses were seen across the wide range of doses studied, making selection of optimal dosing based on these outcome data alone difficult. The observed plasma concentration data across the cohorts indicated substantially higher concentrations 14 days after completion of the induction phase at doses of 1000 and 1200/2000 mg, indicating saturation of the target. Consequently, a dose of 1000 mg was chosen for further clinical studies and is being tested in the randomized phase 2 study of the current study that is comparing GA101 monotherapy with rituximab monotherapy in patients with relapsed or refractory indolent NHL.
In conclusion, obinutuzumab demonstrated a manageable safety profile and clinical activity in heavily pretreated patients with relapsed and refractory CD20-positive hematologic malignancies and prior rituximab exposure, including patients who were rituximab refractory. No MTD was observed at the doses studied and responses were seen across a wide range of doses. Based on data from this trial and other early clinical studies, obinutuzumab is currently being evaluated in several ongoing phase 2 and phase 3 trials.
There is an Inside Blood commentary on this article in this issue.
Presented in abstract form at the annual meeting of the American Society of Hematology, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Georgina Meneses-Lorente (F. Hoffmann-La Roche Ltd) for pharmacokinetic analysis, and Akiko Chai (Genentech Inc) for additional statistical analysis.
This study was sponsored by F. Hoffmann-La Roche Ltd and Genentech Inc. Support for third-party writing assistance for this manuscript furnished by Peter Flanagan was provided by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: L.H.S, S.E.A., D.A.S., J.M., and M.C. collected data; L.H.S., S.E.A., D.A.S., J.M., R.D.G., G.F., and M.C. analyzed and interpreted data; S.F.-L. analyzed and interpreted data and performed statistical analysis; and D.J.C. analyzed and interpreted data and performed pharmacokinetic analysis. All authors contributed intellectually to the development of this study.
Conflict-of-interest disclosure: S.F.-L. and D.J.C. were employees of F. Hoffmann-La Roche Ltd. at the time of the study. G.F. was an employee of Genentech at the time of the study. L.H.S. has received consultancy fees from Genentech/F. Hoffman-La Roche/Genentech. L.H.S., S.E.A., R.D.G., D.A.S., and M.C. all received honoraria from Genentech/F. Hoffmann-La Roche. J.M. declares no competing financial interests.
Correspondence: Laurie H. Sehn, British Columbia Cancer Agency, 600 West 10th Avenue, Vancouver, BC V5Z 4E6, Canada; e-mail: lsehn@bccancer.bc.ca.