Heparin-induced thrombocytopenia (HIT) is a potentially devastating form of drug-induced thrombocytopenia that occurs in patients receiving heparin for prevention or treatment of thrombosis. Patients with HIT develop autoantibodies to the platelet factor 4 (PF4)/heparin complex, which is termed the HIT Ab complex. Despite a decrease in the platelet count, the most feared complication of HIT is thrombosis. The mechanism of thrombosis in HIT remains poorly understood. We investigated the effects of the HIT Ab complex on tissue factor (TF) expression and release of TF-positive microparticles in peripheral blood mononuclear cells and monocytes. To model these effects ex vivo, we used a murine mAb specific for the PF4/heparin complex (KKO), as well as plasma from patients with HIT. We found that the HIT Ab complex induced TF expression in monocytes and the release of TF-positive microparticles. Further, we found that induction of TF is mediated via engagement of the FcγRI receptor and activation of the MEK1-ERK1/2 signaling pathway. Our data suggest that monocyte TF may contribute to the development of thrombosis in patients with HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is a well-recognized complication of heparin therapy and is one of the most common and potentially devastating causes of drug-induced thrombocytopenia.1 Millions of Americans are exposed to heparin each year, resulting in thousands of cases of HIT annually.2 Platelet factor 4 (PF4), a strongly cationic chemokine expressed by megakaryocytes and packaged into the α granules of platelets, is released on platelet activation.3 PF4 binds with high affinity to highly anionic molecules, such as heparin and endogenous heparin-like glycosaminoglycans (GAGs).4 The precise physiologic role of PF4 in the regulation of coagulation is not known.5 A subset of patients exposed to heparin develop autoantibodies (so called HIT Abs) against PF4/heparin complexes.6,7 These Abs bind to specific epitopes on PF4, leading to the formation of trimeric complexes of PF4/heparin-IgG, which are referred to as HIT Ab complexes.8,9 These complexes bind to platelets, resulting in platelet activation and the development of thrombocytopenia.10
Immune complexes exert their effects on cells via engagement of specific Fc receptors. IgG Abs bind to Fcγ receptors. In humans, 3 different classes of FcγRs have been identified: FcγRs I, II, and III. FcγRI is a high-affinity receptor and as such can also bind monomeric IgG as opposed to the low-affinity FcγRs, FcγRII and FcγRIII, that only bind aggregated immunoglobulins or Ag-Ab complexes.11 FcγRI is expressed on monocytes/macrophages, neutrophils, eosinophils, and dendritic cells. FcγRII is further subclassified into FcγRIIa and FcγRIIb. FcγRIIa is an activating receptor and is expressed on monocytes/macrophages, neutrophils, eosinophils, platelets, and Langerhans cells, whereas FcγRIIb is expressed on B lymphocytes and mast cells. In terms of function, FcγRIIb is the only inhibitory FcγR, and all the other FcγRs result in activation of cells.12 FcγRIII is subdivided into FcγRIIIa, which is expressed on natural killer cells and macrophages, and FcγRIIIb, which is expressed on eosinophils, neutrophils, macrophages, mast cells, and follicular dendritic cells. Binding of immune complexes to the activating FcγRs leads to phosphorylation of the cytoplasmic immunoreceptor tyrosine-based activation motif by src-kinases, leading to activation of downstream signaling.12 It has been found that platelet activation by HIT Ab complexes is mediated by engagement of the FcγRIIa receptor.10
Most thrombocytopenias are associated with an increased risk of bleeding. However, the most-feared complication in HIT is the development of arterial and venous thrombosis.13,14 The precise mechanism leading to thrombosis in HIT is unknown. It has been proposed that platelet activation and the release of platelet-derived microparticles (PMPs) may be responsible for thrombosis.15,16 Microparticles (MPs) are sub-micron–sized membrane vesicles released on cell activation or apoptosis.17 PMPs generally express phosphatidylserine and are therefore considered to be procoagulant.17 Increased numbers of circulating PMPs are also seen in other autoimmune thrombocytopenias, such as idiopathic thrombocytopenic purpura,18 which is a disorder that is generally associated with an increased risk of bleeding. We posit that platelet activation and release of PMPs alone is unlikely to account for the increased thrombotic risk in patients with HIT. PF4/heparin-specific Abs have been shown to activate both monocytes and endothelial cells, thereby implicating these cells in the development of thrombosis in HIT.19,–21
Tissue factor (TF) is a transmembrane glycoprotein that binds plasma factor VII/VIIa (FVII/VIIa). The TF:FVIIa complex functions as the primary initiator of coagulation in vivo. The TF:VIIa complex leads to activation of both coagulation factors X and IX, ultimately resulting in thrombin generation and the formation of fibrin.22 Activated monocytes express TF in a variety of disease states, such as endotoxemia and sepsis,22,23 sickle cell disease, and antiphospholipid Ab syndrome.24,–26 Two brief reports have described increased TF expression by monocytes after exposure to the HIT Ab complex.19,21 In healthy persons very low levels of TF+ MPs are present in the circulation.17,27 Importantly, however, activated monocytes release MPs,28 and elevated levels of TF+ MPs have been shown in a variety of diseases, including cardiovascular disease, diabetes, cancer, sickle cell disease, and endotoxemia.17,29,,,,,–35 It has been proposed that, given their smaller size (compared with leukocytes), MPs may play a main role in thrombosis by virtue of their ability to bind to a growing thrombus and thereby deliver TF.36
In this study, we evaluated the effect of the HIT Ab complex on monocyte TF expression and the release of TF+ MPs. Given the importance of the FcγRIIa receptor in platelet activation in HIT,10 we also evaluated the role of the different Fc receptors in HIT Ab complex signaling in monocytes. We used an in vitro model in which PBMCs or monocytes were exposed to PF4, heparin, and Ab. We used a murine anti–human monoclonal Ab (KKO) that binds PF4/heparin complexes or a control Ab,37 as well as plasma from patients with HIT to model the HIT Ab complex. We found that the HIT Ab complex induced monocyte TF and the release of TF+ MPs. This effect depended on the concentrations of both PF4 and heparin. We also found that inhibition of FcγRI receptor, but not FcγRII and FcγRIII receptors, blocked HIT Ab complex–mediated induction of monocyte TF. Further, TF induction by the HIT Ab complex required the activation of the MEK1-ERK1/2 signaling pathway. These findings suggest that monocyte TF expression and monocyte-derived TF+ MPs may contribute to thrombosis in HIT.
Methods
Reagents
The murine anti–human monoclonal Ab (KKO) and the control Ab (RTO) have been described.37 PF4 was obtained from Hematologic Technologies Inc. Heparin was obtained from Abraxis Pharmaceutical Products. Normal cell-free plasma was obtained from Fisher Scientific. Human FVIIa and FX were obtained from Enzyme Research Laboratories. Lipopolysaccharide (LPS; Escherichia coli 0111:B4 A23187) was obtained from Sigma Chemicals. The MEK1 inhibitor (PD98059) was obtained from Cell Signaling Technologies. The CD14-allopphycocyanin (APC)–conjugated Ab was obtained from R&D Systems. A FITC-conjugated anti-TF Ab was obtained from American Diagnostica. A Monocyte Isolation Kit II was obtained from Miltenyi Biotec. The inhibitory anti–human TF monoclonal Ab, HTF-1, was kindly provided by Dr Ronald Bach (University of Minnesota). Anti-FcγRI (anti-CD64) and RIII Abs (anti-CD16) were obtained from BD PharMingen. The Ab against FcγRII (IV.3) was derived from an IV.3 hybridoma cell line (ATCC). RPMI 1640 tissue culture media was obtained from Invitrogen. Fetal calf serum was obtained from Omega Scientific. HEPES buffer, penicillin, streptomycin, and D+ glucose were obtained from Cellgro.
Human samples
Blood was obtained from healthy volunteers (n = 5) for isolation of PBMCs and monocytes after informed consent in accordance with the Declaration of Helsinki and with the approval of the University of North Carolina Institutional Review Board. Blood from patients with HIT (n = 3; clinical diagnosis confirmed with the 14C-serotonin release assay and by measuring Abs to PF4/heparin by ELISA) was obtained as previously described.37
PBMC and PB monocyte isolation
Blood was collected by venipuncture into citrate (3.2%) and immediately centrifuged at 250g for 10 minutes at room temperature. The buffy coat was collected, diluted 1:1 with HBSS supplemented with 5mM EDTA (HBSS + EDTA), layered over Ficoll–sodium diatrizoate (Ficoll–Paque Premium; GE Healthcare), and centrifuged at 400g for 30 minutes at room temperature. The PBMC layer was collected, and the cells were washed twice in HBSS with EDTA. PBMCs were then resuspended in tissue culture media. Monocytes were isolated by resuspending PBMCs in MACS buffer (Miltenyi Biotec). Monocyte isolation was performed via negative selection as per the manufacturer's recommendations. Briefly, nonmonocytic cells were removed by binding to specific biotinylated Abs bound to magnetic beads. Monocytes (95% purity) were then washed once in MACS buffer and resuspended in tissue culture media.
Endotoxin detection
All lots of PF4, heparin, and KKO were tested for endotoxin with the use of the Chromogenic LAL Assay (Genescript). The limit of detection of this assay is 0.005 endotoxin unit/mL. No endotoxin contamination was detected in any of the KKO, PF4, and heparin lots used for this study.
In vitro model to study the effect of HIT Ab complexes on PBMCs and monocytes
Two million cells in 1 mL of culture media (PBMCs or monocytes) were incubated with the various reagents at 37°C for either 2 hours (mRNA experiments) or 6 hours (all other experiments). KKO was added at 100 μg/mL, PF4 at 10 μg/mL, and heparin at 1 U/mL. Various concentrations of both PF4 and heparin were used in experiments in which the goal was to evaluate the effects of the concentration of PF4 and heparin on TF expression. In experiments evaluating the role of the different Fc receptors, samples were preincubated with blocking Abs for 30 minutes at 37°C. In all experiments, cells were incubated with LPS (1 μg/mL) as a positive control. Heat-inactivated plasma from healthy volunteers and patients with HIT were added to the cells in tissue culture media at a final volume of 1:25. After incubation, cells were pelleted by centrifugation at 500g for 10 minutes at room temperature. The cell pellet was then washed and frozen at −80°C. To prepare MPs the culture supernatant fluid was centrifuged at 1500g for 15 minutes to remove cell fragments and other debris, and the MP-rich supernatant fluid was collected and stored at −80°C.
TF assays
Cellular TF activity.
Frozen cell pellets (PBMCs or monocytes) were resuspended in 1 mL of HBSA (137mM NaCl, 5.38mM KCl, 5.55mM glucose, 10mM HEPES, 0.1% BSA, pH 7.5). TF activity in cells was determined with the use of a 1-stage clotting assay as previously described,38 using the STart4 analyzer (Diagnostica Stago).
MP TF activity assay.
MPs were isolated from 200 μL of cell supernatant fluid by centrifugation at 20 000g for 15 minutes at 4°C, washed twice with HBSA, and resuspended in 200 μL of HBSA. MP TF activity was determined as described previously.39
Quantitative real-time PCR
Total RNA was isolated from PBMCs with the use of the RNeasy Plus kit (QIAGEN). mRNA was reverse transcribed with the First Strand cDNA Synthesis kit with Oligo-dT primers (Fermentas). Exon-spanning primers for human TF and hypoxanthine-guanine phosphoribosyl transferase (HPRT) were synthesized by Integrated DNA Technologies (Table 1). Quantitative real-time PCR was performed on a Mastercycler Gradient (Eppendorf) with the use of the Maxima SYBR Green qPCR Master Mix (Fermentas). Relative TF mRNA levels were quantified with the ΔΔCt method40 and normalized to HPRT.
Real-time PCR primers
| Primer . | Sequence . | Exons . |
|---|---|---|
| Human TF fwd | 5′-TGACCTCACCGACGAGATTGTGAA-3′ | 3 |
| Human TF rev | 5′-TCTGAATTGTTGGCTGTCCGAGGT-3′ | 4 |
| Human HPRT fwd | 5′-ATGGACAGGACTGAACGTCTTGCT-3′ | 2/3 Junction |
| Human HPRT rev | 5′-TTGAGCACACAGAGGGCTACAATG-3′ | 3 |
| Primer . | Sequence . | Exons . |
|---|---|---|
| Human TF fwd | 5′-TGACCTCACCGACGAGATTGTGAA-3′ | 3 |
| Human TF rev | 5′-TCTGAATTGTTGGCTGTCCGAGGT-3′ | 4 |
| Human HPRT fwd | 5′-ATGGACAGGACTGAACGTCTTGCT-3′ | 2/3 Junction |
| Human HPRT rev | 5′-TTGAGCACACAGAGGGCTACAATG-3′ | 3 |
Fwd indicates forward; and rev, reverse.
Flow cytometry
Cellular TF expression (PBMCs or monocytes) was measured by flow cytometry with the use of a Beckman Coulter Cyan flow cytometer. One million fresh cells were resuspended in 100 μL of PBS (Sigma-Aldrich) for Ab staining. Fluorescently labeled Abs were added to cells and then incubated in the dark at room temperature for 30 minutes. Cells were then washed with PBS and stained with fluorescently labeled Abs against CD14 (APC, 1 μg/mL) and TF (FITC, 1 μg/mL). TF expression in monocytes was measured according to the detection of CD14+ and TF+ double-positive events, and the results were expressed as mean fluorescence intensity [MFI; arbitrary units (AU)]. MPs were measured by flow cytometry according to the methods recommended by the protocol on standardization of enumeration of MPs by the International Society on Thrombosis and Haemostasis Scientific and Standardization Subcommittees.41 Pelleted MPs (20 000g for 15 minutes at 4°C) were washed twice with HBSA and resuspended in HBSA for staining. MPs were incubated for 30 minutes in the dark at room temperature with Abs, washed once with HBSA, and then resuspended in HBSA. MPs were then stained as before with fluorescently labeled Abs against CD14 (APC) and TF (FITC). The number of CD14+ and TF+ double-positive events was then measured, and the results were expressed as MFIs. TF expression in MPs was analyzed with a BD LSRII flow cytometer (BD Biosciences) because the LSRII instrument provided better resolution compared with the Cyan for detection of MPs.42 Data analysis was performed with FlowJo software (TreeStar).
Activation of ERK1/2
Monocytes were preincubated for 30 minutes with anti-FcγRI Ab (10 μg/mL) or control Ab (10 μg/mL) before the addition of KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). Total ERK and phospho-ERK1/2 were measured with commercial ELISA kits (R&D Systems).
Statistics
All statistical analyses were performed with GraphPad Prism 5 for Windows (GraphPad Software). All data are presented as means ± SDs. Two group comparisons were performed using a 2-tailed Student t test. P ≤ .05 was considered statistically significant.
Results
HIT Ab complex induces TF expression in both PBMCs and monocytes
We found that PBMCs and monocytes exhibited similar induction of TF in response to the HIT Ab complex (TF activity in control PBMCs, 7.70 ± 3.02, and in PBMCs after 6-hour incubation with HIT Ab complex, 88.86 ± 13.89; TF activity in control monocytes, 6.22 ± 1.06, and in monocytes after 6-hour incubation with HIT Ab complex, 72.08 ± 18.47; n = 3. Data from 1 million PBMCs and 250 000 monocytes. TF activity is expressed as pg/106 cells). Therefore, most experiments were performed with PBMCs.
Increased TF expression in PBMCs exposed to the HIT Ab complex
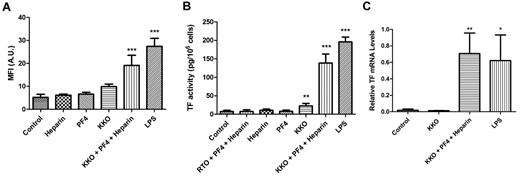
PBMCs were incubated with culture media (control), KKO (100 μg/mL), heparin (1 U/mL), and PF4 (10 μg/mL) both alone and together (HIT Ab complex), or LPS (1 μg/mL) for 6 hours. An additional control for the experiments measuring TF activity was incubation of cells with heparin (1 U/mL), PF4 (10 μg/mL), and RTO, a murine anti-PF4 mAb (100 μg/mL). The HIT Ab complex increased TF protein expression and activity on the cells, whereas heparin, PF4, or KKO alone had no effect (Figure 1A-B). A small increase in cellular TF activity was observed when KKO alone was used, but no significant increase in TF activity was observed with heparin or PF4 alone, as well as when the control Ab RTO was used with heparin and PF4 (Figure 1B). LPS strongly induced TF protein expression and activity (Figure 1A-B). To assess whether aggregation of KKO could explain the small increase in TF expression observed with Ab alone, KKO was ultracentrifuged (100 000g for 60 minutes) to remove Ab aggregates. Ultracentrifugation did not decrease the small induction of TF expression by KKO alone (TF activity in KKO, 100.7 ± 31.67 pg/106 cells; TF activity with ultracentrifuged KKO, 113.7 ± 33.57 pg/106 cells; P = .65; n = 3). This suggests that Ab aggregation was not the reason for the small amount of TF induction observed with KKO alone.
The HIT Ab complex induces TF expression in PBMCs and monocytes. (A) PBMCs were incubated for 6 hours with the different reagents, and the number of TF+/CD14+ double-positive events was measured with flow cytometry. Results are expressed as MFI (of the anti-TF Ab on CD14+ cells). (B) Measurement of cellular TF activity. An additional control consisted of RTO (isotype control Ab for KKO, 100 μg/mL) + heparin + PF4. TF activity was measured with a 1-stage clotting assay. (C) Monocytes were incubated in media alone (control), KKO (100 μg/mL), KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL), and LPS (1 μg/mL) for 2 hours. Total RNA was isolated from the cells, and TF mRNA levels were determined by real-time PCR. Results are presented as relative TF mRNA levels compared with HPRT. Results are from 5 independent experiments. ***P < .001, **P < .01, and *P < .05 compared with control.
The HIT Ab complex induces TF expression in PBMCs and monocytes. (A) PBMCs were incubated for 6 hours with the different reagents, and the number of TF+/CD14+ double-positive events was measured with flow cytometry. Results are expressed as MFI (of the anti-TF Ab on CD14+ cells). (B) Measurement of cellular TF activity. An additional control consisted of RTO (isotype control Ab for KKO, 100 μg/mL) + heparin + PF4. TF activity was measured with a 1-stage clotting assay. (C) Monocytes were incubated in media alone (control), KKO (100 μg/mL), KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL), and LPS (1 μg/mL) for 2 hours. Total RNA was isolated from the cells, and TF mRNA levels were determined by real-time PCR. Results are presented as relative TF mRNA levels compared with HPRT. Results are from 5 independent experiments. ***P < .001, **P < .01, and *P < .05 compared with control.
The effect of preformed HIT Ab complexes on TF induction in PBMCs was also evaluated. PF4, heparin, and KKO (at the same concentrations as in the previous paragraph) were incubated at 37°C for 30 minutes to allow formation of HIT Ab complexes. These complexes were then incubated with PBMCs at 37°C for 6 hours, but no induction of TF activity was observed (data not shown). Therefore, all experiments were conducted with sequential addition of PF4, heparin, and KKO to PBMCs and/or monocytes.
Induction of TF mRNA in monocytes exposed to the HIT Ab complex
Monocytes (2 × 106/mL) were incubated in culture media (control), KKO (100 μg/mL) alone, heparin (1 U/mL) + PF4 (10 μg/mL) + KKO (100 μg/mL; HIT Ab complex), or LPS (1 μg/mL) for 2 hours at 37°C, and TF mRNA levels were measured by quantitative PCR. Incubation of monocytes with the HIT Ab complex resulted in a 37-fold increase in TF mRNA levels compared with control cells (0.70 ± 0.14 AU vs 0.02 ± .01 AU; P ≤ .005; Figure 1C). Incubation with KKO alone did not have any effect. As expected, LPS increased TF mRNA levels (Figure 1C).
HIT Ab complex induces the release of TF+ MPs from PBMCs
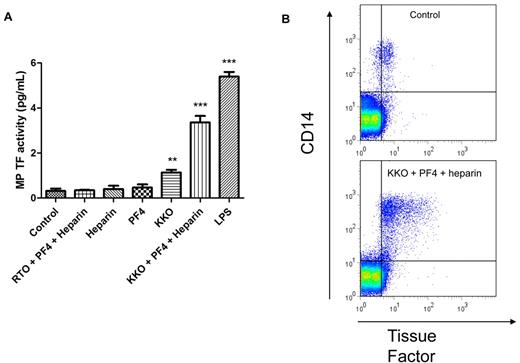
PBMCs were incubated for 6 hours under the same experimental conditions described earlier. MPs were isolated from the cell supernatant fluid, and levels of TF activity and TF Ag expression were determined. Treatment of PBMCs with the HIT Ab complex but not heparin and PF4 alone or together with the control Ab RTO induced the release of TF+ MPs into the culture supernatant fluid (Figure 2A). As observed in the cellular fraction, treatment with KKO alone also resulted in a small increase in MP TF activity. LPS also increased the number of TF+/CD14+ MPs (data not shown) and MP TF activity (Figure 2A). The number of CD14+ and TF+ (double-positive) events in the MP fraction was measured with flow cytometry. Incubation with the HIT Ab complex led to a significant increase in the number of TF+/CD14+ MPs compared with the control (Figure 2B).
The HIT Ab complex induces release of TF+ MPs from PBMCs. PBMCs were incubated for 6 hours at 37°C under the same experimental conditions as outlined under Figure 1B. (A) MPs were isolated from the cell supernatant fluid, and MP TF activity was measured with a 2-stage chromogenic assay. (B) The number of TF+ MPs was determined with flow cytometry–based detection of TF+/CD14+ events. The top plot shows the number of MPs from PBMCs incubated with media alone (control), and the bottom plot shows the number of MPs from PBMCs incubated with the HIT Ab complex. Increases were seen in the number of TF+/CD14+ MPs and the MFI of TF (later not shown). Results are from 5 independent experiments. ***P < .001 and **P < .01 compared with control.
The HIT Ab complex induces release of TF+ MPs from PBMCs. PBMCs were incubated for 6 hours at 37°C under the same experimental conditions as outlined under Figure 1B. (A) MPs were isolated from the cell supernatant fluid, and MP TF activity was measured with a 2-stage chromogenic assay. (B) The number of TF+ MPs was determined with flow cytometry–based detection of TF+/CD14+ events. The top plot shows the number of MPs from PBMCs incubated with media alone (control), and the bottom plot shows the number of MPs from PBMCs incubated with the HIT Ab complex. Increases were seen in the number of TF+/CD14+ MPs and the MFI of TF (later not shown). Results are from 5 independent experiments. ***P < .001 and **P < .01 compared with control.
Effect of PF4 and heparin concentrations on the induction of TF expression in PBMCs
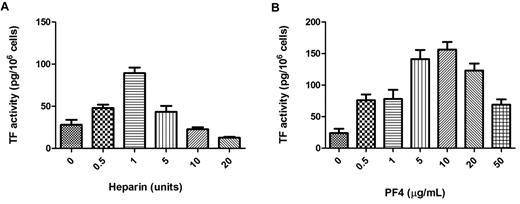
PBMCs were incubated for 6 hours with PF4 (10 μg/mL) and KKO (100 μg/mL) as before, but the amount of heparin in the experiment was varied (0.5-20 U/mL). Titration of heparin resulted in a bell-shaped curve for TF activity with maximal levels observed at a concentration of heparin ∼ 1 U/mL (Figure 3A). A similar experiment was performed when PBMCs were incubated with heparin (1 U/mL) and KKO (100 μg/mL), but the amount of PF4 was varied (0-50 μg/mL). TF induction was maximal at 10 μg/mL of PF4 and, again, was significantly decreased at both higher and lower concentrations (Figure 3B). These results indicate that HIT Ab complex–mediated TF induction in PBMCs critically depends on the concentrations of PF4 and heparin.
Effect of varying the concentrations of PF4 and heparin on the induction of TF expression in PBMCs. (A) Increasing amounts of heparin were added to PBMCs containing 100 μg/mL KKO and 10 μg of PF4. Samples were incubated for 6 hours at 37°C, and TF activity was measured with a one-stage clotting assay. (B) Increasing amounts of PF4 were added to PBMCs containing 100 μg/mL KKO and 1 U/mL heparin. TF activity was measured with a one-stage clotting assay. Results are from 3 independent experiments.
Effect of varying the concentrations of PF4 and heparin on the induction of TF expression in PBMCs. (A) Increasing amounts of heparin were added to PBMCs containing 100 μg/mL KKO and 10 μg of PF4. Samples were incubated for 6 hours at 37°C, and TF activity was measured with a one-stage clotting assay. (B) Increasing amounts of PF4 were added to PBMCs containing 100 μg/mL KKO and 1 U/mL heparin. TF activity was measured with a one-stage clotting assay. Results are from 3 independent experiments.
HIT patient plasma induces the release of TF+ MPs from PBMCs
To evaluate the ability of Abs from patients with HIT to induce PBMC TF, heat-inactivated plasma from patients with HIT and healthy volunteers were used. PBMCs were incubated for 6 hours at 37°C in the presence of heparin (1 U/mL), PF4 (10 μg/mL), and either heat-inactivated plasma from patients with HIT (HIT plasma) or heat-inactivated control plasma. PBMCs were also incubated with culture media alone, HIT plasma alone, or LPS. Incubation with heparin, PF4, and HIT plasma resulted in increased TF activity (Figure 4A). Incubation of PBMCs with HIT patient plasma alone also resulted in slightly increased TF activity compared with control (Figure 4A). MPs were isolated from the culture supernatant fluid from the above experimental conditions, and MP TF activity was measured. PBMCs incubated with heparin, PF4, and HIT plasma released MP TF activity in the culture supernatant fluid compared with those incubated with media (Figure 4B). We observed a slight increase in MP TF activity when cells were incubated with HIT patient plasma alone, whereas no increase was observed with control plasma, PF4, and heparin. As expected, LPS significantly increased cellular TF activity in and the release of MP TF activity (Figure 4A-B).
Plasma from patients with HIT induces TF in PBMCs. Plasma from healthy volunteers (n = 3) and from patients with HIT (n = 3) was heat-inactivated and used in the place of RTO and KKO, respectively. (A) TF activity in PBMCs. (B) MP TF activity in MPs isolated from the cell supernatant fluid. ***P < .001, **P < .01, and *P < .05 compared with control.
Plasma from patients with HIT induces TF in PBMCs. Plasma from healthy volunteers (n = 3) and from patients with HIT (n = 3) was heat-inactivated and used in the place of RTO and KKO, respectively. (A) TF activity in PBMCs. (B) MP TF activity in MPs isolated from the cell supernatant fluid. ***P < .001, **P < .01, and *P < .05 compared with control.
Induction of PBMC TF expression by the HIT Ab complex is inhibited by Abs against FcγRI
PBMCs were preincubated with Abs against FcγRI, FcγRII, or FcγRIII for 30 minutes at 37°C, followed by incubation with the HIT Ab complex for 6 hours. Cells that were preincubated with the anti-FcγRI Ab expressed significantly less cellular TF activity and MP TF activity compared with controls (Figure 5A-B). In contrast, Abs against FcγRII or FcγRIII did not affect induction of TF expression (Figure 5A-B). In these experiments PBMCs incubated with the HIT Ab complex or culture media alone were used as positive and negative controls, respectively. These results indicate that the HIT Ab complex induces TF induction in PBMCs by activation of the FcγRI receptor.
Role of different Fc receptors in the induction of TF expression in PBMCs by the HIT Ab complex. PBMCs were preincubated for 30 minutes with either anti-FcγRI Ab (10 μg/mL), anti-FcγRII Ab (10 μg/mL), or anti-FcγRIII Ab (10 μg/mL) before the addition of control Ab or KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). (A) TF activity in PBMCs. (B) MP TF activity in MPs isolated from the cell supernatant fluid. The results are from 3 independent experiments. Complex refers to addition of KKO + PF4 + heparin. ***P < .001.
Role of different Fc receptors in the induction of TF expression in PBMCs by the HIT Ab complex. PBMCs were preincubated for 30 minutes with either anti-FcγRI Ab (10 μg/mL), anti-FcγRII Ab (10 μg/mL), or anti-FcγRIII Ab (10 μg/mL) before the addition of control Ab or KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). (A) TF activity in PBMCs. (B) MP TF activity in MPs isolated from the cell supernatant fluid. The results are from 3 independent experiments. Complex refers to addition of KKO + PF4 + heparin. ***P < .001.
Induction of monocyte TF expression by the HIT Ab complex is mediated by activation of the MEK-ERK1/2 signaling pathway
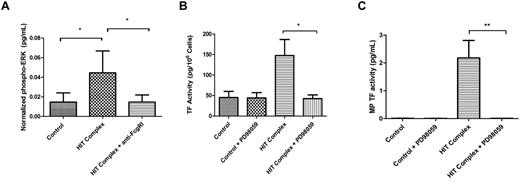
We analyzed the role of the MEK-ERK1/2 pathway in induction of TF expression by the HIT Ab complex. Monocytes were preincubated with an Ab against FcγRI or control Ab for 30 minutes at 37°C, followed by incubation with the HIT Ab complex for 30 minutes. Total ERK1/2 and phospho-ERK1/2 were measured by ELISA. The HIT Ab complex induced a significant increase in phospho-ERK1/2, and this phosphorylation was inhibited by the addition of the anti-FcγRI Ab (Figure 6A). Next, monocytes were incubated with the MEK-1 inhibitor PD98059 for 30 minutes at 37°C, followed by incubation with the HIT Ab complex for 6 hours. A significant decrease was observed in both cellular TF activity and MP TF activity in the cells that were preincubated with PD98059 compared with cells incubated with the HIT Ab complex with vehicle (Figure 6B-C). No change was observed in samples left untreated (control) or in samples preincubated with PD98059 without the HIT complex. These results indicate that activation of the FcγRI receptor by the HIT Ab complex and the subsequent induction of TF in monocytes required activation of the ERK1/2 pathway.
Role of ERK-1/2 in the induction of TF expression in monocytes by the HIT Ab complex. (A) Monocytes were preincubated for 30 minutes with anti-FcγRI Ab (10 μg/mL) or control Ab before the addition of KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). Total ERK1 and phospho-ERK1/2 were measured by ELISA. (B-C) Monocytes were preincubated for 1 hour with MEK1 inhibitor PD98059 (50 μg/mL) or control Ab before the addition of KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). Cellular TF (B) and MP TF activity in MPs isolated from the cell supernatant fluid (C) were measured. The results are from 3 independent experiments. The HIT complex refers to addition of KKO + PF4 + heparin. **P < .01 and *P < .05.
Role of ERK-1/2 in the induction of TF expression in monocytes by the HIT Ab complex. (A) Monocytes were preincubated for 30 minutes with anti-FcγRI Ab (10 μg/mL) or control Ab before the addition of KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). Total ERK1 and phospho-ERK1/2 were measured by ELISA. (B-C) Monocytes were preincubated for 1 hour with MEK1 inhibitor PD98059 (50 μg/mL) or control Ab before the addition of KKO (100 μg/mL) + heparin (1 U/mL) + PF4 (10 μg/mL). Cellular TF (B) and MP TF activity in MPs isolated from the cell supernatant fluid (C) were measured. The results are from 3 independent experiments. The HIT complex refers to addition of KKO + PF4 + heparin. **P < .01 and *P < .05.
Discussion
HIT is associated with a significant risk of thrombosis despite the thrombocytopenia observed in affected persons. The mechanism of thrombosis in HIT remains poorly understood. We found that the HIT Ab complex induces TF expression and the release of TF+ MPs from monocytes. Two groups have previously reported that the HIT Ab complex induces TF expression in monocytes in vitro.19,21 One of these reports used PBMCs from healthy volunteers and either KKO or plasma from patients with HIT as the Ab source and found an increased TF activity with the use of a 2-stage clotting assay. However, levels of TF mRNA or Ag were not measured.19 The second study similarly reported the ability of Abs purified from patients with HIT to induce TF activity in PBMCs in vitro in the presence of PF4 and heparin.21 The researchers observed increased TF mRNA in monocytes after whole blood was incubated with HIT Abs, heparin, and PF4. Interestingly, TF induction in whole blood depended on the presence of both heparin and PF4, whereas that in PBMCs did not require the presence of heparin. A recent report by Rauova et al found that PF4 binds to GAGs present on the surface of monocytes and that these PF4/GAG complexes are recognized by Abs against the PF4/heparin complex.43 Through a series of in vivo experiments that used a mouse model of HIT, these investigators also found that depletion of monocytes leads to decreased thrombosis, suggesting that monocytes play a role in HIT-associated thrombosis. Our findings complement and extend these recent observations. None of these studies evaluated MP release from monocytes. Our study differs from these previous reports in that we evaluated TF mRNA levels in isolated monocytes exposed to HIT Ab complexes and measured TF protein expression by flow cytometry. Finally, we have also found that the interaction of HIT Ab complexes with monocytes leads to the release of TF+ MPs and that the stimulatory effect of the HIT Ab complex depends on the FcγRI receptor.
Interestingly, we found that preformed HIT Ab complexes did not induce TF expression in PBMCs. Although complexes of PF4 and heparin lead to an immune response and the development of Abs against PF4/heparin, our data indicate that preassembled PF4/heparin/Ab complexes do not induce TF expression in PBMCs. This, coupled with the minimal effect on TF induction by KKO alone, suggests that induction of monocyte TF in HIT involves assembly of the immune complex on the cell surface rather than interactions between monocytes and circulating preassembled immune complexes.
The formation of antigenic PF4-heparin complexes is primarily determined by electrostatic forces.44 Indeed, the ability of the HIT Ab complex to activate platelets depends on the molar ratios of both heparin and PF4. An excess of either component results in decreased platelet activation, probably as a result of dissociation of the antigenic complexes from the platelet surface. In fact, the ability of excess heparin to displace the HIT complex from the platelet surface and thereby inhibit platelet activation is routinely used as a confirmatory step in the laboratory diagnosis of HIT. We evaluated whether the induction of TF expression by the HIT Ab complex similarly depended on the optimal concentrations of heparin and PF4. Our results show that this is indeed the case because the addition of both excess heparin and PF4 lead to less induction of TF activity. However, this effect was more marked for heparin than for PF4 with excess PF4 still resulting in some degree of TF expression. This may be related to the increased affinity of PF4 to bind to GAGs on the monocyte surface compared with the platelet surface, as has been reported.45 This is also probably the reason why PF4 and Ab can lead to induction of monocyte TF in the absence of exogenous heparin as has been shown by another group.19 In this setting, the binding of PF4 to GAGs on the surface of the monocytes resembles the PF4-heparin interaction and can still lead to complex formation and Ab presentation to the FcγRI receptor.
Binding of the HIT Ab complex to the platelet FcγRIIa receptor1,10 results in activation and clearance of Ab-bound platelets from the circulation. Interestingly, we found that blocking FcγRIIa did not have any significant effect on the induction of TF expression in monocytes. In contrast, inhibition of FcγRI in monocytes almost completely inhibited induction of TF expression and the release of TF+ MPs. These results indicate that FcγRI is the primary receptor to mediate monocyte activation and TF expression by the HIT complex. There are several explanations for the involvement of different FcRs in platelets and monocytes. FcγRI is not expressed on platelets but is abundantly expressed in monocytes. FcγRI is also a high-affinity receptor compared with FcγRII and FcγRIII, which, although also expressed in monocytes, are low-affinity FcRs. We found that both PBMCs and monocytes incubated with Ab alone (either KKO or HIT plasma) exhibited a small increase in TF activity. This may reflect different sensitivities of the different assays used to measure TF expression because incubation with Ab alone did not result in induction of TF mRNA.
We evaluated the role of MAP kinases in TF induction after activation of FcγRI by HIT Ab complexes. Incubation of monocytes with the HIT Ab complex for 30 minutes at 37°C resulted in phosphorylation of ERK1/2. Further, preincubation of monocytes with an Ab against FcγRI led to reduced phospho-ERK1/2 levels. We also evaluated the effect of inhibition of MEK-1. Preincubation of monocytes with PD98059 completely inhibited TF activity in monocytes and MP TF activity. Taken together, these data indicate that the HIT Ab complex induction of TF in monocytes and the release of TF+ MPs is mediated via activation of FcγRI and the MEK1-ERK1/2 intracellular signaling pathway.
MPs contain both phosphatidylserine (PS) and Ags, such as TF, that reflect their cell of origin. Monocyte-derived MPs are particularly procoagulant, given that they may express both PS and TF on their surface. We and others have also reported an increased number of TF+ MPs in the plasma of patients with cancer with venous thromboembolism.32,35,39 These reports support a role for TF+ MPs in the development of thrombosis. The ability of the HIT Ab complex to induce TF expression in monocytes and the subsequent release of TF+ MPs suggests that monocyte TF may contribute significantly to the development of thrombosis in HIT.
We have considered the possibility of platelet contamination in the PBMC and monocyte cultures and activated platelets contributing to our results. Platelet contamination is more of a concern in PBMC preparations than in purified monocytes, whereby the extent of platelet contamination is minimal (< 5% of the cells in our experience) as measured by flow cytometry. Although our model to recapitulate HIT in vitro uses PBMCs, our findings were confirmed with the use of purified monocytes (data not shown). In addition, use of a blocking Ab against FcγRIIa did not significantly alter TF induction (Figure 5A). If platelet activation contributed significantly to our results, blocking FcγRIIa may have been expected to decrease TF induction. We do not therefore feel that platelet contamination in monocytes or PBMCs adversely affected our findings. A final consideration is that LPS contamination in our reagents could contribute to TF induction in monocytes. We routinely check for LPS contamination in all lots of heparin, PF4, and KKO, and, if LPS is detected, the contaminated lots are not used. Further, in time course experiments with PBMCs, LPS induction of TF expression peaked at 8 hours and started to trend toward baseline by 24 hours, whereas the HIT Ab complex–induced TF expression occurred with slower kinetics with an upward trend at the 24-hour time point (data not shown). These results suggest that LPS contamination is not contributing to our findings.
In summary, we have found that the HIT Ab complex induces monocyte TF expression and the release of TF+ MPs. To induce monocyte TF expression, the HIT Ab complex needs to be assembled on the cell surface. Our findings lend further support for a role of TF, monocytes, and MPs in the development of thrombosis in HIT. The HIT Ab complex binds to FcγRI and activates the MEK-ERK1/2 signaling pathway. Whether additional receptors or coreceptors contribute to this interaction needs to be evaluated in future studies.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant K08 HL098567, R.S.K.) and an HTRS Mentored Research Award (R.S.K.).
National Institutes of Health
Authorship
Contribution: R.S.K. was involved in developing the hypothesis and experimental design, analyzed data, and prepared the manuscript; S.L.G. and W.J. performed many of the experiments and contributed to preparation of the manuscript; T.M. performed the mRNA measurements; G.M.A. collaborated on the research, provided several reagents, and prepared the manuscript; R.P., N.S.K., and N.M. were involved in developing the experimental design and preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raj S. Kasthuri, Division of Hematology/Oncology, Department of Medicine, University of North Carolina at Chapel Hill, CB#7035, 321B Mary Ellen Jones Bldg, 98 Manning Dr, Chapel Hill NC 27599; e-mail: raj_kasthuri@med.unc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal