To the editor:
Release and trafficking of cell membrane–derived vesicles is a constitutive cellular function important to cell-cell communication.1 Montecalvo and colleagues recently described the transfer of microRNA (miRNA) between dendritic cells.2,3 The authors report the selective incorporation of specific miRNA in vesicle compartments as a function of cellular-differentiation stage and demonstrate endogenous miRNA transfer to bystander cells via nano-sized vesicles with translational suppression of cognate mRNA targets. Even as the molecular mechanisms for protein or RNA incorporation into vesicles remain to be clarified, these elegant studies confirm earlier work demonstrating cell-cell transfer of coding and noncoding small RNA via vesicles with modulation of the target cell phenotype.1,4,5 At the same time they leave open to what extent vesicle trafficking occurs between nonimmune cells and whether it can be exploited for the programmed transfer of nonendogenous protein or RNA.
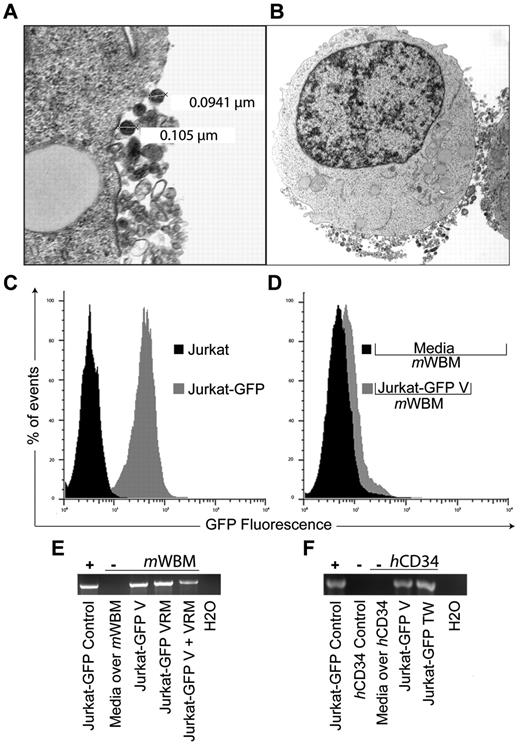
To test the hypothesis that protein or coding RNA can be deliberately transferred between nonimmune cells via vesicles, we genetically modified Jurkat T cells with a vesicular stomatitis virus G protein–pseudotyped lentivector bearing a green fluorescent protein expression cassette (Jurkat-GFP). Vesicle release by Jurkat cells has recently been studied by others and our transmission electron microcopy studies (Figure 1A) confirm that these were predominantly in the exosome size range (30-110 nm; Figure 1B). Jurkat-GFP cells were propagated in bulk, enriched by flow-cytometric sorting for > 90% purity, with stable proviral integration indicated by GFP expression over serial (> 20) passages (Figure 1C). Twenty-four-hour culture supernatant from Jurkat-GFP and parental Jurkat cells was collected and processed to generate cell-free vesicle preparations by standard sequential gradient centrifugation.6 To demonstrate successful transfer we exposed murine bone marrow cells (mWBM, C57B/6 strain) to Jurkat-GFP–derived purified vesicles (Jurkat-GFP-V) for 48 hours and detected GFP expression in mWBM by flow cytometry (Figure 1D). We tested vesicle transfer in 2 additional cell-cell contact-independent settings: as vesicle rich media (VRM) after removing debris from the harvest culture supernatant (2000g × 15 minutes) and in 0.4-μm transwell coculture (TW). After 48-hour coculture we observed successful amplification of GFP transcripts in DNAse-treated RNA from mWBM cells under all 3 conditions (Figure 1E). To highlight the potential relevance of our findings, these studies were repeated using human CD34+ (hCD34; StemCell Technologies) target cells, with identical results, showing cell contact–independent vesicle transfer of GFP transcript from Jurkat-GFP cells (Figure 1F).
GFP expression and mRNA transfer via exosome trafficking between non-immune cells. (A) Electron micrograph of vesicle release from a Jurkat cell. Cell preparations on UV activated carbon formvar 400 Mesh copper grids (Ted Pella 01 822-F), were imaged at 100 kV on a Philips CM120 TEM microscope. Images were collected on a Gatan 794CCD multiscan camera and converted into 8-bit gray-scale TIF, 28 000× magnification. OHSU-Electron Microscopy Resource (B) Jurkat cell with representative, exosome-sized, vesicles located at the limiting membrane at 7100× magnification. (C) Histogram representation of green fluorescent protein (GFP) expression by Jurkat cells (Jurkat-GFP) after replication deficient retrovirus vector transduction (vesicular stomatitis virus G protein pseudotype, MOI 1, GFP expression cassette) and non-transduced control (Jurkat). (D) Overlay histogram demonstrating GFP expression in murine whole bone marrow cells (mWBM) 48 hours after transwell (0.4 μm pore) exposure to Jurkat-GFP derived vesicles (Jurkat-GFP-V) or media control (Media over mWBM). Vesicles (V) were isolated from culture media by differential centrifugation at 300g × 10 minutes, 2 000g × 15 minutes, 10 000g × 20 minutes, and at 100 000g × 2 hours. The pellet was washed, ultracentrifuged at 100 000g for 2 hours and resuspended in PBS. (E) Reverse transcription PCR analysis indicating the presence or absence of GFP sequence in mWBM cells after indicated coculture conditions: 48-hour transwell (TW), concentrated vesicles (V) or vesicle rich media (VRM) from Jurkat-GFP cells versus media control. RNA was extracted using RNeasy (QIAGEN). Complementary DNA was synthesized using the SuperScript III First Strand Synthesis Kit (Invitrogen) with oligo-dT priming followed by PCR. (F) Detection of GFP transcripts in non-mobilized normal human CD34+ (hCD34) cells (Stem Cell Technologies). Culture condition, sample handling and reverse transcription PCR analysis as in panel E. Experiments were repeated with similar results.
GFP expression and mRNA transfer via exosome trafficking between non-immune cells. (A) Electron micrograph of vesicle release from a Jurkat cell. Cell preparations on UV activated carbon formvar 400 Mesh copper grids (Ted Pella 01 822-F), were imaged at 100 kV on a Philips CM120 TEM microscope. Images were collected on a Gatan 794CCD multiscan camera and converted into 8-bit gray-scale TIF, 28 000× magnification. OHSU-Electron Microscopy Resource (B) Jurkat cell with representative, exosome-sized, vesicles located at the limiting membrane at 7100× magnification. (C) Histogram representation of green fluorescent protein (GFP) expression by Jurkat cells (Jurkat-GFP) after replication deficient retrovirus vector transduction (vesicular stomatitis virus G protein pseudotype, MOI 1, GFP expression cassette) and non-transduced control (Jurkat). (D) Overlay histogram demonstrating GFP expression in murine whole bone marrow cells (mWBM) 48 hours after transwell (0.4 μm pore) exposure to Jurkat-GFP derived vesicles (Jurkat-GFP-V) or media control (Media over mWBM). Vesicles (V) were isolated from culture media by differential centrifugation at 300g × 10 minutes, 2 000g × 15 minutes, 10 000g × 20 minutes, and at 100 000g × 2 hours. The pellet was washed, ultracentrifuged at 100 000g for 2 hours and resuspended in PBS. (E) Reverse transcription PCR analysis indicating the presence or absence of GFP sequence in mWBM cells after indicated coculture conditions: 48-hour transwell (TW), concentrated vesicles (V) or vesicle rich media (VRM) from Jurkat-GFP cells versus media control. RNA was extracted using RNeasy (QIAGEN). Complementary DNA was synthesized using the SuperScript III First Strand Synthesis Kit (Invitrogen) with oligo-dT priming followed by PCR. (F) Detection of GFP transcripts in non-mobilized normal human CD34+ (hCD34) cells (Stem Cell Technologies). Culture condition, sample handling and reverse transcription PCR analysis as in panel E. Experiments were repeated with similar results.
Taken together, our experiments demonstrate the cell-cell contact-independent transfer of nonendogenous coding RNA from stably transduced Jurkat cells to murine and human hematopoietic targets after purified vesicle, vesicle-rich media, or transwell exposure. As a caveat, these observations do not yet resolve to what extent GFP protein transfer, versus mRNA translation, accounts for the observed expression in hematopoietic target cells. Notwithstanding, our report extends the observations by Montecalvo and colleagues by revealing the feasibility of vesicle trafficking for the programmed transfer of stably introduced transgenes between nonimmune cells. We believe the deliberate cell-cell vesicle transfer of nonendogenous RNA may be useful for the experimental, and potentially therapeutic, manipulation of hematopoietic and other stem cells.
Authorship
Acknowledgments: T.B.R. designed and performed research and analyzed data; A.M.S. performed research and analyzed data; and P.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Kurre, MD, Papé Family Pediatric Research Institute, Oregon Health & Science University, 701 SW Gaines Rd, CDRC-P, Portland, OR; e-mail: kurrepe@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal