Abstract
CD47 on erythrocytes inhibits phagocytosis through interaction with the inhibitory immunoreceptor SIRPα expressed by macrophages. Thus, the CD47-SIRPα interaction constitutes a negative signal for erythrocyte phagocytosis. However, we report here that CD47 does not only function as a “do not eat me” signal for uptake but can also act as an “eat me” signal. In particular, a subset of old erythrocytes present in whole blood was shown to bind and to be phagocytosed via CD47-SIRPα interactions. Furthermore, we provide evidence that experimental aging of erythrocytes induces a conformational change in CD47 that switches the molecule from an inhibitory signal into an activating one. Preincubation of experimentally aged erythrocytes with human serum before the binding assay was required for this activation. We also demonstrate that aged erythrocytes have the capacity to bind the CD47-binding partner thrombospondin-1 (TSP-1) and that treatment of aged erythrocytes with a TSP-1–derived peptide enabled their phagocytosis by human red pulp macrophages. Finally, CD47 on erythrocytes that had been stored for prolonged time was shown to undergo a conformational change and bind TSP-1. These findings reveal a more complex role for CD47-SIRPα interactions in erythrocyte phagocytosis, with CD47 acting as a molecular switch for controlling erythrocyte phagocytosis.
Introduction
Erythrocyte clearance has been studied for many years in the context of normal turnover,1,2 enhanced clearance resulting from defects in erythrocyte metabolism or changes in membrane composition,3-5 and in blood transfusion.6 A large number of factors that may influence erythrocyte clearance have been proposed, ranging from autoantibody binding to band 3 after conformational changes resulting from aging (the “senescent cell antigen”),7,8 to the expression of phosphatidylserine on the outer leaflet of the erythrocyte membrane.9 In general, it is thought that erythrocytes are cleared after accumulating so-called “eat me” signals and are then phagocytosed by macrophages of the reticuloendothelial system, predominantly in the spleen and liver.1,10,11 In contrast, one of the membrane proteins expressed by erythrocytes, CD47, has been shown to inhibit phagocytosis of erythrocytes by macrophages of the reticuloendothelial system.12-16 CD47 exerts its inhibitory effect through binding to SIRPα on the macrophage, which induces inhibitory signaling by the immunoreceptor tyrosine-based inhibition motifs (ITIMs) residing in the cytoplasmic tail of SIRPα.17,18 On ligation of SIRPα by CD47, the SIRPα ITIMs recruit and activate tyrosine phosphatases SHP-1 and SHP-2, and this regulates, generally in a negative fashion, downstream signaling pathways and effector functions. The inhibitory effect of CD47 on erythrocyte clearance can be illustrated by transfusion of CD47-deficient erythrocytes into a wild-type recipient, which leads to rapid phagocytosis of the CD47-deficient erythrocytes by red pulp macrophages in the spleen.12,19 Thus, phagocytosis of erythrocytes is supposed to be the result of “eat me” signals already present that override the inhibitory signal of CD47. Although it has been suggested that the expression of CD47 decreases during in vitro erythrocyte aging under blood bank conditions, there are other studies that suggest that CD47 levels remain unaltered.20,21 Nevertheless, it is well known that transfusion of red cell concentrates in patients leads to a rapid clearance of 10% to 25% of the transfused cells depending on storage time.22,23 This could contribute to some of the complications associated with blood transfusion, including transfusion-related acute lung injury.24 The mechanism behind this rapid clearance of a proportion of the red cells has not been clarified.
Intriguingly, evidence exists that the CD47-SIRPα interaction can also promote phagocytosis of apoptotic cells, although the mechanism behind this phenomenon is unknown.25,26 Furthermore, work by Kusakari et al has indicated that SIRPα can mediate trans-endocytosis of CD47-containing membrane of adjacent cells,27 illustrating that SIRPα signaling can lead to other phenomena besides inhibition of phagocytosis. Based on these findings, we hypothesized that SIRPα might play a role in the removal of aged erythrocytes through CD47 binding. Using SIRPα-transfected cell lines, we observed that aged erythrocytes bind to SIRPα through CD47. We obtained data that strongly suggest that CD47 undergoes a conformational change during aging evoked by oxidative stress. Because of this conformational change, CD47 is then recognized, after binding of thrombospondin-1,28,29 as an “eat me” signal by SIRPα. We observed phagocytosis of experimentally aged erythrocytes by primary human red pulp macrophages through this mechanism, suggesting that this mechanism is operational in vivo. In line with our findings, we observed that erythrocytes that had been stored for a prolonged period displayed a conformational change in CD47 when diluted into fresh whole blood, and subsequently bound thrombospondin-1. This result suggests a potential role for the CD47-SIRPα axis in the rapid phagocytosis of donor erythrocytes after transfusion. Collectively, our data identify CD47 as a molecular switch for erythrocyte phagocytosis.
Methods
Reagents
Mouse anti–human CD47 (clone B6H12)–allophycocyanin (APC), CFSE, and IMDM were from Invitrogen. Mouse anti–human CD47 (clone 2D3)–FITC was obtained from eBioscience. Streptavidin-APC was from BD Biosciences. Deoxyribonuclease II (DNase) and ascorbic acid were from Sigma-Aldrich. Purified collagenase was obtained from Worthington. Human serum albumin and pasteurised plasma-protein solution were obtained from Sanquin. FCS, penicillin/streptomycin, and l-glutamine were obtained from PAA. DMEM/F12 culture medium was from Lonza Germany.
Purification of erythrocytes from whole blood
Venous blood was collected from healthy donors, after obtaining informed consent. Blood studies were approved by the Sanquin Research institutional medical ethical committee in accordance with the standards laid down in the 1964 Declaration of Helsinki. Erythrocytes were isolated from fresh heparinized whole blood by centrifugation at 270g for 15 minutes. After removing the platelet-rich plasma and the peripheral blood mononuclear cells, the erythrocytes were washed 2 times with saline-adenine-glucose-mannitol (150mM NaCl, 1.25mM adenine, 50mM glucose, 29mM mannitol; Fresenius) and resuspended in saline-adenine-glucose-mannitol. Final cell concentration was determined with an Advia 2120 (Siemens Medical Solutions Diagnostics).
Culturing and transfection of CHO cells
Chinese Hamster Ovary (CHO) cells were cultured in DMEM/F12 culture medium, supplemented with 10% (volume/volume) FCS, 1% penicillin/streptomycin, and 2mM l-glutamine. The constructs were cloned by means of BamHI and EcoRI and put in pcDNA3 and transfected in CHO cells with fugene (Roche) according to the manufacturer's protocol. Subsequently, the CHO cells were sorted on a BD FACSAria II (BD Biosciences) as described in the next paragraph. CHO cells with human constructs were stained with an antibody recognizing SIRPα1 (mouse anti–human SIRPα, clone OX118). The rat constructs were detected with a mouse anti–rat SIRPα, clone ED9.30 The CHO cells were incubated with 10 μg/mL primary antibody in HEPES buffer (132mM NaCl, 20mM HEPES, 6mM KCl, 1mM MgSO4, 1.2mM K2HPO4, all from Sigma-Aldrich) supplemented with 0.5% (volume/volume) human serum albumin, 1mM CaCl2 (Sigma-Aldrich), and 10mM glucose (Sigma-Aldrich; HEPES+) for 30 minutes on ice. After washing, the cells were incubated with 10 μg/mL goat anti–mouse IgG-Alexa-488 (Invitrogen) for 30 minutes on ice. The cells were washed, and the cells with the highest mean fluorescence intensity were sorted and taken into culture. To keep the constructs in the CHO cells, 3.75 mg/mL Geneticin (Invitrogen) was added to the culture medium of transfected CHO cells.
Binding of erythrocytes to CHO cells
CHO cells were cultured in a 6-well plate (Corning) to confluence, after which they were cultured for 2 more days. Erythrocytes isolated from fresh heparinized whole blood were washed and resuspended in HEPES+ to a final concentration of 2 × 107 cells/mL. To block CD47 on the erythrocytes, the cells were resuspended to a final concentration of 1 × 108 cells/mL in HEPES+ with 10 μg/mL CD47- F(ab′)2 fragments (made from clone B6H12), after which they were washed and resuspended in HEPES+ to a final concentration of 2 × 107 cells/mL. The CHO cells were washed with PBS, after which 4 × 107 erythrocytes were added to 10-cm2 dishes. The cells were incubated overnight in a 37°C incubator. After washing the wells with PBS, erythrocyte binding was analyzed by wide field microscopy (Evos, AMG micro).
Confocal microscopy
CHO cells were cultured as described in “Culturing and transfection of CHO cells,” with the following modifications. CHO cells were cultured in a 24-well plate with glass bottom (Zell Kontakt) instead of a 6-well plate. After the overnight incubation and removal of the unbound cells by washing, the cells were stained with 10 μg/mL unconjugated mouse anti–human glycophorin A (clone CD235a, Sanquin Reagents) in HEPES+. The cells were washed with HEPES+ and incubated with 10 μg/mL goat anti–mouse IgG-Alexa-488 in HEPES+. For intracellular staining of phagocytosed erythrocytes, all extracellular erythrocytes were lysed with lysis buffer, an ice-cold isotonic NH4CL solution (155mM NH4CL, 10mM KHCO3, 0.1mM EDTA, pH 7.4). After 5 minutes of incubation on ice and removing the supernatant, this step was repeated once. Thereafter the cells were fixed overnight in 4% (weight/volume) ice-cold paraformaldehyde in PBS. Subsequently, the cells were washed in PBS and incubated with 7% BSA (Sigma-Aldrich) in PBS for 30 minutes. The cells were washed in PBS and permeabilized with methanol/acetone (1:1 ratio, volume/volume) for 30 minutes. Staining was performed by incubating the permeabilized cells with 10 μg/mL unconjugated mouse IgG anti–human glycophorin A in 7% BSA. After washing, the cells were stained with goat anti–mouse IgG-Alexa-647 (Invitrogen). The cells were analyzed using a confocal microscope (Axiovert 100M; Zeiss) and green events larger than 3 μm were counted by Image Pro-Plus Version 6 (Media Cybernetics).
Bead binding assay
CHO cells were cultured as described in “Culturing and transfection of CHO cells” for confocal microscopy. The wells were washed with PBS, and 1 mL HEPES+ was added to each well. Subsequently, 5 × 106 beads were added to each well and incubated for 30 minutes in a 37°C incubator. The wells were washed thoroughly with PBS to remove unbound beads. Subsequently, bead binding was analyzed by fluorescence microscopy (Evos) and bound beads were counted.
CD47 analysis after experimental aging
Conformational changes of CD47 were analyzed with an APC-conjugated conformation-independent antibody (clone B6H12) and a FITC-conjugated conformation-dependent antibody (clone 2D3). Experimental aging was induced by incubating erythrocytes with CuSO4 and ascorbic acid as described in “Experimental aging of erythrocytes.” After washing the cells, a double-staining was performed with 10 μg/mL of both CD47 antibodies diluted in HEPES+. The samples were washed and analyzed with a flow cytometer with high throughput system (LSRII, BD Biosciences) and analyzed with computer software (FACSDiva Version 6.1, BD Biosciences).
TSP-1 peptide binding and staining for flow cytometry
Binding of the thrombospondin-1 (TSP-1) peptide was analyzed as follows. Erythrocytes, untreated or after induction of oxidative damage, were resuspended in HEPES+ and 50 μg/mL biotinylated TSP-1 peptide (4N1K, amino acid sequence; KRFYVVMWKK) or 50 μg/mL biotinylated control peptide (4NGG, amino acid sequence; KRFYGGMWKK) was added. Subsequently, the cells were incubated for 30 minutes on ice, washed and incubated for 30 minutes with 1:250 steptavidin-APC on ice. After washing the cells and resuspending in HEPES+, the cells were analyzed by a flow cytometer with high throughput system and analyzed with FACSDiva Version 6.1.2 software.
TSP-1 binding
TSP-1 binding was analyzed by staining with the mouse anti–human TSP antibody A6.1 (Abcam). This was done by washing and diluting erythrocytes with HEPES+ to a final concentration of 1 × 108 cells/mL and incubating them with 10 μg/mL A6.1. Subsequently, the cells were incubated for 30 minutes on ice, washed and incubated with 10 μg/mL goat anti–mouse IgG-Alexa-488 for 30 minutes on ice. After washing the cells and resuspending in HEPES+, the cells were analyzed by a flow cytometer with high throughput system and analyzed with FACSDiva Version 6.1.2 software.
Experimental aging of erythrocytes
Erythrocytes isolated from whole blood were spun down and resuspended in PBS supplemented with 0.2mM CuSO4 and 5mM ascorbic acid to a final concentration of 0.4 × 108 cells/mL.15 After incubating the cells at 37°C for 60 minutes in a shaking heating block, the cells were washed 3 times with PBS and resuspended in HEPES+.
Experimental aging of CD47-beads
To induce oxidative damage in CD47-coupled beads, CD47 beads were diluted to a final concentration of 0.3 × 108 beads/mL in PBS supplemented with 0.2mM CuSO4 and 5mM ascorbic acid. After incubating the beads at 37°C for 60 minutes in a shaking heating block, the beads were washed 3 times with PBS and resuspended in HEPES+.
Whole blood dilution
Erythrocytes obtained from a stored red cell concentrate, prepared as described elsewhere,31 were washed twice with HEPES buffer and diluted to a hematocrit of 40%. The erythrocytes were diluted 1:10 with heparinized whole blood obtained from AB+ donors and incubated overnight at 37°C. To detect the donor erythrocytes, the cells were stained with antibodies against the minor blood group antigens (Ortho Clinical Diagnostics). This was done by diluting the blood to a final concentration of 1 × 108 cells/mL in HEPES+ and adding undiluted human anti–human in a ratio of 5:1. After incubation for 30 minutes at room temperature, the cells were washed extensively before resuspending in 10 μg/mL goat anti–human IgG-Alexa-568 (Invitrogen) to a final concentration of 1 × 107 cells/mL and incubating for 30 minutes at room temperature. The cells were subsequently washed and resuspended in HEPES+ and analyzed on a flow cytometer (BD Biosciences) with high throughput system. Data analysis was performed with FACSDiva Version 6.1.2 software (BD Biosciences).
Statistical analysis
Data were analyzed using Graphpad Prism Version 5.01 for Windows software. Statistical analysis was performed by t test when 2 groups were compared or 1-way ANOVA with Tukey Multiple Comparison Test when more than 2 groups were compared (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
SIRPα transfected CHO cells bind erythrocytes from whole blood
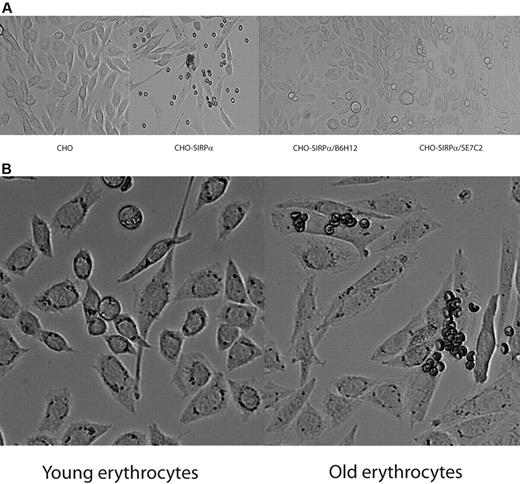
To identify a possible interaction between CD47, expressed on erythrocytes, and SIRPα, we transfected CHO cells with full-length human SIRPα. Next, we incubated erythrocytes overnight with the CHO cells. We observed that, after thorough washing, a small fraction of the erythrocytes was bound to the CHO SIRPα cells (Figure 1A). We did not observe binding of erythrocytes to untransfected CHO cells (Figure 1A). To establish that the observed interaction between the erythrocytes and the CHO SIRPα cells was mediated through CD47 on the erythrocytes and SIRPα on the CHO cells, we preincubated the erythrocytes with a CD47 blocking antibody, B6H12 (Figure 1A). The CD47 blocking antibody inhibited the binding of the erythrocytes to the CHO SIRPα cells. In addition, pretreatment of the CHO SIRPα cells with a blocking antibody against SIRPα (SE7C2) also completely inhibited binding of erythrocytes to the CHO SIRPα cells (Figure 1A), thus implicating that the binding of the erythrocytes to the CHO SIRPα cells was mediated by CD47-SIRPα interactions. To gain more insight into the nature of the erythrocytes with SIRPα binding capacity, we separated the erythrocytes on the basis of their density into younger and older cells by Percoll fractionation32 and compared their binding to CHO SIRPα cells. Strikingly, the fraction containing the youngest erythrocytes did not show any binding to the CHO SIRPα cells, whereas the fraction containing the oldest cells showed the highest amount of erythrocytes bound (Figure 1B), indicating that only a small fraction of the aged erythrocytes have the capacity to bind SIRPα through CD47.
Binding of erythrocytes to CHO cells transfected with SIRPα. (A) A fraction of erythrocytes obtained from fresh blood binds to CHO SIRPα cells, whereas no binding was observed to mock-transfected cells. Binding of erythrocytes to CHO SIRPα cells is dependent on CD47 and SIRPα. (B) The erythrocytes from fresh blood that bind to CHO SIRPα cells reside in the fraction of old erythrocytes. Data are representative of 4 experiments.
Binding of erythrocytes to CHO cells transfected with SIRPα. (A) A fraction of erythrocytes obtained from fresh blood binds to CHO SIRPα cells, whereas no binding was observed to mock-transfected cells. Binding of erythrocytes to CHO SIRPα cells is dependent on CD47 and SIRPα. (B) The erythrocytes from fresh blood that bind to CHO SIRPα cells reside in the fraction of old erythrocytes. Data are representative of 4 experiments.
CHO SIRPα cells bind and phagocytose erythrocytes
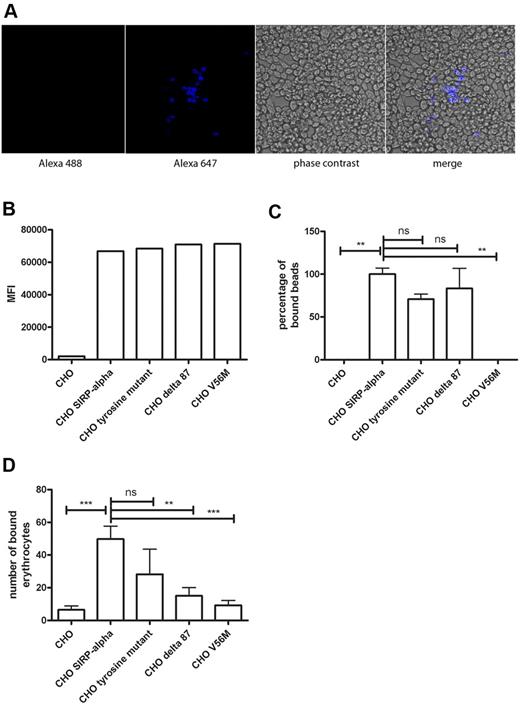
To determine whether bound erythrocytes were phagocytosed by CHO SIRPα cells, we performed live imaging of CHO SIRPα cells that had bound erythrocytes. This demonstrated that a significant proportion of the erythrocytes that had been bound by the CHO SIRPα cells was phagocytosed (supplemental Video 1). Furthermore, when after an overnight incubation, bound erythrocytes were lysed and the CHO SIRPα cells were permeabilized and intracellularly stained for glycophorin A, phagocytosed erythrocytes were detected by confocal microscopy (Figure 2A).
CHO SIRPα cells bind and phagocytose erythrocytes. (A) Phagocytosed erythrocytes were detected by intracellular glycophorin A staining in combination with a secondary goat anti–mouse Alexa-647 antibody. (B) Expression levels of different SIRPα constructs expressed in CHO cells, as detected by flow cytometry. (C) Binding of CD47 beads to CHO cells transfected with different constructs of SIRPα in the absence of serum. (D) Erythrocyte binding to CHO cells transfected with different SIRPα constructs in the absence of serum. Data are representative of 3 experiments. ns indicates not significant. **P < .01; ***P < .001.
CHO SIRPα cells bind and phagocytose erythrocytes. (A) Phagocytosed erythrocytes were detected by intracellular glycophorin A staining in combination with a secondary goat anti–mouse Alexa-647 antibody. (B) Expression levels of different SIRPα constructs expressed in CHO cells, as detected by flow cytometry. (C) Binding of CD47 beads to CHO cells transfected with different constructs of SIRPα in the absence of serum. (D) Erythrocyte binding to CHO cells transfected with different SIRPα constructs in the absence of serum. Data are representative of 3 experiments. ns indicates not significant. **P < .01; ***P < .001.
SIRPα binding of CD47 on aged erythrocytes is dependent on the SIRPα cytoplasmic tail
We investigated whether the binding of aged erythrocytes to SIRPα was dependent on the intracellular tail of SIRPα. We expressed several mutants of SIRPα in CHO cells, including a mutant in which the 4 tyrosine residues of the ITIM motifs (tyrosine mutant) were mutated to phenylalanine. Another mutant lacked 87 amino acids of the C-terminus of SIRPα (Δ87 mutant) and therefore has only a small cytoplasmic domain of approximately 23 amino acids remaining. In addition, the V56M point mutation within the N-terminal V-set Ig domain that abolishes CD47 binding, as shown by us and others,33,34 was tested for its ability to bind old erythrocytes through CD47 recognition. These mutants were expressed at a similar level as the SIRPα construct from which they were derived (Figure 2B). The mutants displayed nonsignificant differences in binding of recombinant human CD47 coupled to beads compared with wild-type SIRPα (Figure 2C), except the V56M mutant, which did not display binding of CD47 beads above background level. Furthermore, the binding of erythrocytes freshly isolated from whole blood to the tyrosine mutant was not significantly different from the binding to the construct with the wild-type intracellular tail (P < .05; Figure 2D). However, both the Δ87 mutant as well as the V56M mutant showed significantly reduced binding of erythrocytes in comparison with the construct with the wild-type intracellular tail (P < .05; Figure 2D). So, in contrast to binding of beads coated with CD47, deletion of part of the cytoplasmic tail of SIRPα affects the binding of aged erythrocytes to SIRPα.
CD47 on experimentally aged erythrocytes is recognized by SIRPα
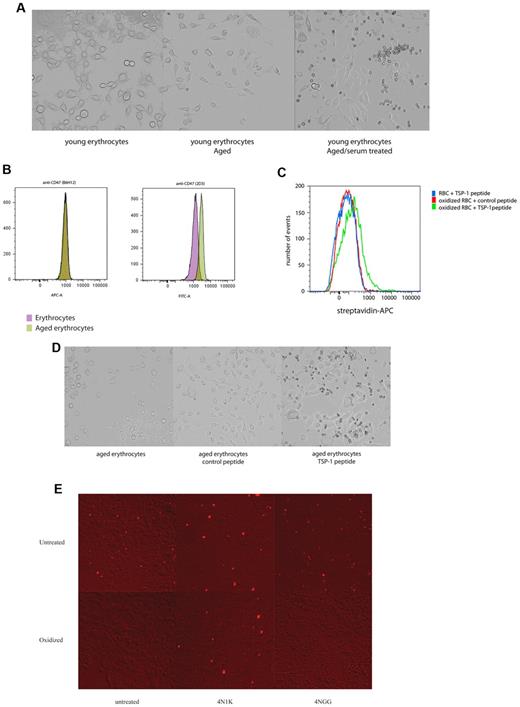
During the erythrocyte life span, oxidative damage accumulates on erythrocyte membrane proteins, which contributes to their aging and their subsequent clearance. We therefore investigated whether erythrocytes that were aged artificially by oxidation15 would acquire the capacity to bind SIRPα. For this purpose, young erythrocytes (obtained by density centrifugation), which possess minimal SIRPα-binding capacity, were used. When the erythrocytes were incubated with the CHO SIRPα cells after oxidation, no binding was observed (Figure 3A). However, when the experimentally aged erythrocytes were preincubated with human serum, substantial binding did occur (Figure 3A). Based on these finding, we hypothesized that oxidation induces a conformational change in CD47 that would lead to binding of a serum factor that would subsequently support the binding of SIRPα. First, we tested whether CD47 undergoes conformational changes because of oxidation. Two antibodies directed against different CD47 epitopes, used previously to detect potential conformational differences in CD47 on sickle erythrocytes,35 were used to detect differences in CD47 conformation on aging. One of the antibodies, B6H12, recognizing a constant epitope, showed similar levels of binding to oxidized and nonoxidized erythrocytes (Figure 3B), indicating that the total level of surface expression was not altered between these erythrocyte preparations. Strikingly, binding of the second antibody, 2D3, which has previously been shown to recognize a variable epitope on CD47,35 showed enhanced binding to oxidized erythrocytes, indicating that CD47, or at least part of the CD47 molecules present on erythrocytes, had indeed undergone a conformational change (Figure 3B).
CD47 on experimentally senescent erythrocytes is recognized by SIRPα. (A) Experimental aging of erythrocytes in combination with serum treatment induces binding to CHO SIRPα cells. (B) CD47 staining with the conformation-independent antibody B6H12 and 2D3, an antibody that detects a conformation-dependent epitope of CD47 on experimentally aged erythrocytes, shows a conformational change of CD47 after experimental aging. (C) Experimental aging of erythrocytes results in binding of the TSP-1-derived peptide 4N1K. No binding was observed for the control peptide, 4NGG. Furthermore, no streptavidin-APC staining was observed for fresh erythrcoytes and oxidized erythrocytes when no peptide was added or when fresh erythrocytes were incubated with the 4NGG peptide (data not shown). (D) Aging of young erythrocytes in combination with 4N1K peptide incubation results in binding to CHO SIRPα cells. (E) Oxidation of CD47 beads inhibits binding to CHO SIRPα cells. The binding of oxidized CD47 beads could subsequently be induced in combination with the 4N1K peptide, but not with the control peptide, 4NGG. Data are representative of 3 experiments.
CD47 on experimentally senescent erythrocytes is recognized by SIRPα. (A) Experimental aging of erythrocytes in combination with serum treatment induces binding to CHO SIRPα cells. (B) CD47 staining with the conformation-independent antibody B6H12 and 2D3, an antibody that detects a conformation-dependent epitope of CD47 on experimentally aged erythrocytes, shows a conformational change of CD47 after experimental aging. (C) Experimental aging of erythrocytes results in binding of the TSP-1-derived peptide 4N1K. No binding was observed for the control peptide, 4NGG. Furthermore, no streptavidin-APC staining was observed for fresh erythrcoytes and oxidized erythrocytes when no peptide was added or when fresh erythrocytes were incubated with the 4NGG peptide (data not shown). (D) Aging of young erythrocytes in combination with 4N1K peptide incubation results in binding to CHO SIRPα cells. (E) Oxidation of CD47 beads inhibits binding to CHO SIRPα cells. The binding of oxidized CD47 beads could subsequently be induced in combination with the 4N1K peptide, but not with the control peptide, 4NGG. Data are representative of 3 experiments.
One of the best known binding partners of CD47 is TSP-1, a protein derived from thrombocytes.28,29,36,37 Interestingly, TSP-1 binding to CD47 on erythrocytes induces erythrocyte death, a response that can be mimicked by a C-terminal peptide derived from TSP-1, 4N1K.38 Moreover, changes in CD47 conformation on sickle cell disease erythrocytes induce binding of TSP-1 to CD47.35 Control and oxidized erythrocytes were incubated with either biotinylated 4N1K peptide or a biotinylated control peptide, 4NGG. Experimentally aged erythrocytes bound the 4N1K peptide, but not the 4NGG peptide, indicating that the conformational change induced by experimental aging results in increased binding of 4N1K to CD47 (Figure 3C). Next, the ability of the 4N1K peptide to induce binding of experimentally aged erythrocytes to CHO SIRPα cells was tested. Oxidized erythrocytes were incubated with either the 4NGG peptide or the 4N1K peptide. Only when the 4N1K peptide was used, binding was observed (Figure 3D). In contrast, untreated erythrocytes did not bind to CHO SIRPα cells, even when they were incubated with the 4N1K peptide (data not shown).
To investigate whether the conformational change in CD47 induced by oxidative damage represents a direct effect on the extracellular domain of CD47 or an indirect effect via other membrane proteins present in the CD47 complex which might be oxidized, beads coated with the extracellular domain of CD47 were oxidized and binding to CHO SIRPα cells was tested. When the beads were not oxidized, 4N1K and 4NGG had no effect on bead binding to CHO SIRPα cells (Figure 3E). However, after oxidation, the beads were unable to bind to the CHO SIRPα cells. The binding to CHO SIRPα cells was restored by the preincubation with the 4N1K peptide, but not by the 4NGG peptide. We did not observe any binding to CHO cells that were not transfected with SIRPα (data not shown).
Experimentally aged erythrocytes are phagocytosed by human macrophages after 4N1K opsonization
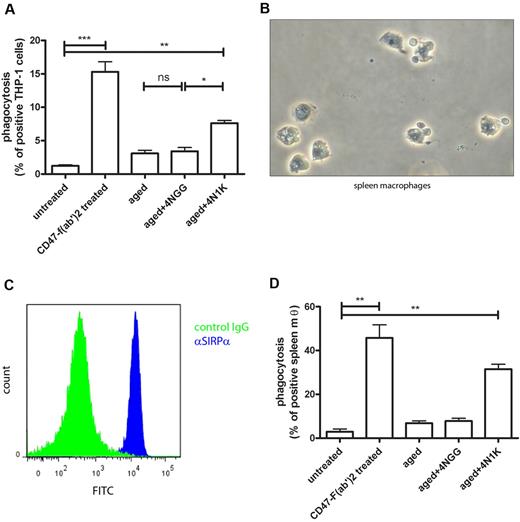
To investigate whether the interaction between CD47 on experimentally aged erythrocytes and SIRPα can also trigger phagocytosis by human macrophages, we first tested the ability of the human monocytic cell line THP-1 to phagocytose experimentally aged erythrocytes. The inhibitory role of CD47 on erythrocyte phagocytosis by THP-1 can be demonstrated by pretreating the erythrocytes with F(ab′)2 fragment of the B6H12 antibody directed against CD47 that blocks CD47-SIRPα interactions.39 As anticipated, erythrocytes were phagocytosed when the interaction between CD47 and SIRPα was blocked by the F(ab′)2 fragment directed against CD47 (Figure 4A), indicating that CD47 normally functions as a “do not eat me” signal on erythrocytes. However, when we incubated experimentally aged erythrocytes after opsonization with the 4NGG peptide or the 4N1K peptide with the THP-1 cells, only erythrocytes that were both oxidized and incubated with the 4N1K were phagocytosed, strongly suggesting that, under these conditions, the CD47-SIRPα interaction is changed in such a manner that phagocytosis is promoted (Figure 4A).
Experimentally aged erythrocytes are phagocytosed by macrophages after 4N1K binding. (A) Aging of fresh erythrocytes in combination with 4N1K peptide incubation induces phagocytosis by differentiated THP-1 cells (n = 3). (B) Phase-contrast image of primary human red pulp macrophages. Arrowheads indicate the erythrocytes still associated with the red pulp macrophages on isolation. (C) Expression of SIRPα on primary human red pulp macrophages, as detected by flow cytometry. (D) Aging of fresh erythrocytes in combination with 4N1K peptide incubation induces phagocytosis by primary human red pulp macrophages (n = 2). ns indicates not significant. *P < .05; **P < .01; ***P < .001.
Experimentally aged erythrocytes are phagocytosed by macrophages after 4N1K binding. (A) Aging of fresh erythrocytes in combination with 4N1K peptide incubation induces phagocytosis by differentiated THP-1 cells (n = 3). (B) Phase-contrast image of primary human red pulp macrophages. Arrowheads indicate the erythrocytes still associated with the red pulp macrophages on isolation. (C) Expression of SIRPα on primary human red pulp macrophages, as detected by flow cytometry. (D) Aging of fresh erythrocytes in combination with 4N1K peptide incubation induces phagocytosis by primary human red pulp macrophages (n = 2). ns indicates not significant. *P < .05; **P < .01; ***P < .001.
Next, we tested the cell type that is responsible for the uptake of aged erythrocytes in vivo, the splenic red pulp macrophage (RPM; Figure 4B). Human RPM, like their murine counterparts, express SIRPα (Figure 4C). As for the THP-1 cells, we determined whether the CD47-SIRPα interaction between normal erythrocytes and spleen macrophages mimics that found in the murine system. As expected, we observed very little phagocytosis of fresh erythrocytes when incubated with human RPM (Figure 4D). Again, erythrocytes were effectively phagocytosed when they were pretreated with the F(ab′)2 fragment directed against CD47. Furthermore, experimentally aged erythrocytes opsonized with the 4N1K peptide were phagocytosed, whereas 4NGG peptide-treated cells were not, illustrating that primary human RPMs are able to phagocytose erythrocytes in a CD47-SIRPα–mediated fashion.
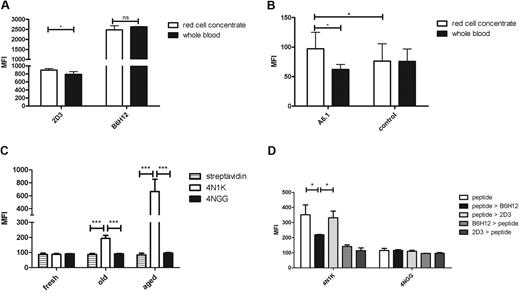
Prolonged storage of erythrocytes induces a conformational change in CD47 and leads to TSP-1 binding
For transfusion, it is accepted that up to 25% of the transfused cells is removed from the circulation in the first 24 hours after transfusion.22 Because we found that aged erythrocytes bound to CHO SIRPα cells and macrophages in a CD47-SIRPα-dependent fashion, we hypothesized that this mechanism might explain the loss of donor erythrocytes after transfusion. To determine changes in the conformation of CD47 and subsequent TSP-1 binding during red cell storage, we sampled stored erythrocytes during a period of 35 days. However, very little phenotypic changes were observed when different antigens on erythrocytes were analyzed in samples taken directly from the storage bag (P.B., unpublished data, June 2011). This was also true for the conformation of CD47. In particular, we did not observe any changes in the ratio between the binding of the B6H12 and the 2D3 antibody or any binding of TSP-1 protein (data not shown). Therefore, we mimicked the conditions of a blood transfusion ex vivo by diluting donor erythrocytes in freshly drawn whole blood of an AB+ recipient in a 1:10 ratio and incubated this overnight. The donor cells were then identified by detection of minor blood group antigens in the mixture and analyzed for binding of the 2 different CD47 antibodies as well as TSP-1 binding. Erythrocytes stored for 35 days displayed increased binding of the 2D3 antibody compared with the recipient erythrocytes (Figure 5A). In line with our previous finding that experimentally senescent erythrocytes, which also have an increase in the expression of the 2D3 epitope and bound the 4N1K peptide, the 35-day stored erythrocytes bound plasma-derived TSP-1 (Figure 5B). In addition, the 4N1K peptide bound the donor cells as well, whereas the control peptide, 4NGG, did not (Figure 5C). These data strongly suggest that on transfusion at least part of the donor cells will bind TSP-1 because of conformational changes in CD47, which ultimately may lead to their clearance. To confirm that the 4N1K peptide indeed bound to CD47 present on the erythrocyte membrane, we performed a competition assay between the 4N1K peptide and both CD47 antibodies, B6H12 and 2D3. Therefore, 35-day stored erythrocytes were first incubated with either a CD47 antibody or the biotinylated TSP-1 peptides. Subsequently, after incubation with one of the CD47 antibodies, the erythrocytes were incubated with either the biotinylated 4N1K or biotinylated 4NGG peptides. Erythrocytes that were incubated with the biotinylated TSP-1 peptides were subsequently incubated with either B6H12 or 2D3. After these incubations, total 4N1K or 4NGG binding was determined by streptavidin binding. When we first incubated with the 4N1K peptide and subsequently with the CD47 antibodies, we observed that B6H12 could compete with the 4N1K peptide for binding to CD47 (Figure 5D). The 2D3 antibody did not compete with the 4N1K peptide. However, when we first incubated with the B6H12 or 2D3 antibodies and subsequently with the 4N1K peptide, we observed no binding of 4N1K, indicating that the affinity of the CD47 antibodies for CD47 is higher than the 4N1K peptide.
Storage of erythrocytes induces conformational changes in CD47 and TSP-1 binding. (A) CD47 on stored erythrocytes undergoes a conformational change, as detected by an increase in 2D3 binding, after overnight dilution in whole blood. (B) TSP-1 binding as detected by the A6.1 antibody on stored erythrocytes after overnight dilution in whole blood. (C) The 4N1K peptide binds to stored erythrocytes after overnight incubation in whole blood. The 4N1K peptide binding to aged erythocytes is shown for comparison. (D) Competition of CD47 antibody binding and 4N1K peptide binding was analyzed on stored erythrocytes after overnight. Stored erythrocytes were first incubated with either CD47 antibody or biotinylated TSP-1 peptides and subsequently incubated with biotinylated TSP-1 peptide and CD47 antibody, respectively. Subsequently, total TSP-1 peptide binding was determined by streptavidin binding (all experiments are n = 4). ns indicates not significant. *P < .05; ***P < .001.
Storage of erythrocytes induces conformational changes in CD47 and TSP-1 binding. (A) CD47 on stored erythrocytes undergoes a conformational change, as detected by an increase in 2D3 binding, after overnight dilution in whole blood. (B) TSP-1 binding as detected by the A6.1 antibody on stored erythrocytes after overnight dilution in whole blood. (C) The 4N1K peptide binds to stored erythrocytes after overnight incubation in whole blood. The 4N1K peptide binding to aged erythocytes is shown for comparison. (D) Competition of CD47 antibody binding and 4N1K peptide binding was analyzed on stored erythrocytes after overnight. Stored erythrocytes were first incubated with either CD47 antibody or biotinylated TSP-1 peptides and subsequently incubated with biotinylated TSP-1 peptide and CD47 antibody, respectively. Subsequently, total TSP-1 peptide binding was determined by streptavidin binding (all experiments are n = 4). ns indicates not significant. *P < .05; ***P < .001.
Discussion
Although CD47-SIRPα interaction is well known for its ability to inhibit phagocytosis of CD47-expressing cells,12,19 there have been several reports that provide evidence for a role for CD47 and SIRPα in promoting uptake of apoptotic cells.25,26 Here we describe the recognition and uptake of aged erythrocytes through CD47-SIRPα interaction as a new mechanism for erythrocyte clearance.
We obtained data that support a model in which a conformational change in CD47 is induced during aging, after which TSP-1 binds to CD47 and hereby creates a new binding site for SIRPα, which is then able to recognize CD47 as an “eat me” signal instead of a “do not eat me” signal. Importantly, oxidation of erythrocytes induced the conformational change in CD47 that was required for TSP-1 binding, suggesting that oxidative damage can be an important factor in generating this “eat me” signal on erythrocytes. The same oxidation abolished binding of CD47-coated beads to SIRPα CHO cells, suggesting that the conformational change observed in erythrocytes is primarily a direct effect on CD47 rather than an effect of oxidation of other membrane proteins. However, although the oxidation of beads abolished binding, we did not observe increased phagocytosis of erythrocytes after inducing experimental aging. This suggests that at least part of the CD47 present on aged erythrocytes can still interact with SIRPα, or that only part of the CD47 is modified by oxidation.
As for CD47 and SIRPα, TSP-1 has been described to promote phagocytosis of apoptotic cells.40 We were able to confirm that oxidation of erythrocytes and subsequent incubation with the TSP-1 peptide lead to uptake by phagocytes, including THP-1 cells and primary human splenic RPM. The results with the THP-1 cells as well as the splenic RPM indicate that oxidation alone, and the subsequent conformational change in CD47, do not lead to phagocytosis of the erythrocytes (Figure 4A-B). This means that, in the absence of the TSP-1 peptide, CD47 still delivers its inhibitory signal; blocking CD47 interaction with SIRPα through F(ab′)2 fragments leads to phagocytosis of erythrocytes, indicating that this signal is crucial in inhibiting phagocytosis of erythrocytes. After incubation with the TSP-1 peptide, phagocytosis is observed, indicating that erythrocytes are taken up only after CD47 has changed conformation and has bound TSP-1. Intriguingly, TSP-1 has also been shown to be able to induce erythrocyte phosphatidylserine exposure through CD47 after prolonged incubation.38 We therefore propose a model in which the conformational change in CD47 and subsequent TSP-1 binding are at the basis of the recognition of aged erythrocytes as well as the generation of additional “eat me” signals
How TSP-1 is able to induce phagocytosis of old erythrocytes through binding to CD47 and subsequent recognition by SIRPα is currently unknown. Although the results from the phagocytosis of aged erythrocytes treated with the TSP-1 peptide might suggest that TSP-1 is simply blocking the CD47-SIRPα interaction, this is not in line with the results from the CHO SIRPα binding. We clearly observed that TSP-1 was able to induce binding of aged erythrocytes to CHO SIRPα cells, which could not occur if TSP-1 would be blocking the CD47-SIRPα interaction. In line with this are the observations with oxidized beads coated with the extracellular domain of CD47. On oxidation, the CD47-coated bead binding to CHO SIRPα cells is abolished. However, when the oxidized beads are pretreated with the 4N1K peptide, we observed again binding to CHO SIRPα cells, but not to CHO cells that were not transfected with SIRPα. This suggests that TSP-1 does not block the interaction of CD47 with SIRPα but rather alters the interaction. TSP-1 has to bind close to the epitope of B6H12 on the Ig domain of CD47, as shown by the competition between B6H12 and the TSP-1 peptide. We therefore propose that TSP-1 binds to CD47 and thereby either alters the conformation of CD47, even further allowing new binding to sites to bind to SIRPα. It seems also possible that TSP-1 fixes CD47 in a conformation so that it is differently recognized by SIRPα. Further structure evidence will be necessary to give more insight into this interaction.
Our data indicate that CD47 regulates the recognition of erythrocytes that are destined to be cleared by macrophages in the spleen. We speculate that this mechanism may also be relevant for other cell types. In line with this thinking are previous publications in which CD47 on other cell types has also been indicated to be necessary for efficient phagocytosis of apoptotic cells.25,26
The signal transduction cascade that SIRPα uses to transmit inhibitory signals is well known. The cytoplasmic region of SIRPα contains 4 ITIMs, which on ligand binding can become phosphorylated, and mediate recruitment and activation of the tyrosine phosphatases SHP-1 and SHP-2.17 SHP-1 and SHP-2 can, in turn, dephosphorylate specific protein substrates and thereby regulate phagocytosis in a negative fashion. However, it is at present completely unknown how SIRPα signaling would lead to positive regulation of phagocytosis. Transfection of SIRPα in CHO cells (Figure 2A; supplemental Video 1) was sufficient for phagocytosis of aged erythrocytes, indicating that the signaling molecules required for SIRPα-dependent phagocytosis are present in these cells. CHO cells have been described to express SHP-1 and SHP-2,41,42 providing the possibility that these proteins also regulate phagocytosis through SIRPα. Currently, the signal transduction cascade leading to phagocytosis after SIRPα signaling is under investigation in CHO cells as well as in primary human RPM.
The data presented here may explain the enhanced clearance of erythrocytes in a number of circumstances. First, erythrocytes that have been stored for several weeks undergo a conformational change in CD47 when incubated in fresh blood and bind TSP-1, which may explain their high rate of clearance after transfusion (Figure 5). Second, sickle erythrocytes also display an increase in the binding of the conformation-dependent CD47 antibody 2D3 and bind TSP-1.43 This finding may explain in part the enhanced uptake of sickle erythrocytes in the spleen.
Although it seems clear that changes in CD47 conformation may be a critical determinant for erythrocyte survival, it is currently not known which phenomena drive this CD47 conformational switch and whether this occurs during normal red cell aging in vivo. As the conformational change in CD47 was only observed after incubation of long stored erythrocytes in whole blood, it is probable that other plasma components first induce the conformational change after which TSP-1 is able to bind to the changed CD47. As part of the band 3 complex in the erythrocyte membrane, it is also well possible that the change in CD47 that leads to the “eat me” configuration is the result of modifications in band 3 (ie, phosphorylation,44 cleavage and/or modification by transglutaminases45 ), which may occur during aging. In line with this are the observed differences in binding of erythrocytes and beads coated with the extracellular domain of CD47 to CHO cells transfected with SIRPα mutants. As the CD47 extracellular domain on the erythrocytes and beads is similar, this suggests that the transmembrane or intracellular part of CD47 also plays an important role in recognition by SIRPα. As CD47 is in a major protein complex with among others band 3, glycophorin A and CD44, an indirect effect on CD47 conformation by modification of other membrane proteins is well possible.
Another possibility is that CD47 becomes clustered on the erythrocyte membrane because of increased mobility, can then bind TSP-1, and is subsequently recognized by SIRPα as an “eat me” signal. Clustering of CD47 has been shown to occur on apoptotic erythrocytes,46 but the contribution of this phenomenon to inhibition or facilitation of clearance through SIRPα remains unknown. These different possibilities are currently under investigation.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof D. Roos for critical comments on the manuscript.
This work was supported by Sanquin grant PPOC-07-20.
Authorship
Contribution: P.B. performed experiments and wrote the manuscript; P.H.-S. performed experiments; D.d.K. and T.K.v.d.B. suggested key experiments and supervised the writing of the manuscript; and R.v.B. designed the study and supervised the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robin van Bruggen, Sanquin Research, Department of Blood Cell Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: r.vanbruggen@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal