In this issue of Blood, Markey and colleagues demonstrate that immunodeficiency associated with graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (SCT) is caused by functional impairment of dendritic cells.1
Graft-versus-host disease and infection are the major complications of allogeneic SCT, and their close relationship has been recognized. One of the primary characteristics of GVHD is a profound and long-lasting immunodeficiency that allows development of severe opportunistic infections, while the role of systemic immunosuppressive drugs used for prevention and treatment of GVHD is well appreciated. It has been assumed that GVHD-mediated immunodeficiency is primarily attributed to defects within the T-cell compartment. GVHD induces abnormalities in nonalloreactive bystander T-cell populations and limits their ability to mount effective immunity in transplant recipients.2
Markey and colleagues challenge this paradigm focusing on antigen-presenting cells (APCs) using immunologic techniques to assess antigen presentation.1 In their mouse model of bone marrow transplantation (BMT), adoptively transferred, donor-derived TEa transgenic T cells exclusively respond to host alloantigens expressed on donor-derived APCs in the context of major histocompatibility complex (MHC) class II. Thus, assessment of TEa cell proliferation represents a stringent test of antigen-presentation capacity of donor-derived APCs, particularly dendritic cells (DCs). They observed that TEa cell proliferation was markedly reduced when transferred into mice that had developed GVHD after BMT, suggesting an impairment of antigen presentation of donor-derived DCs. DCs are composed of distinct subsets; CD8− DCs are specialized for antigen presentation on MHC class II (see figure).3 Markey et al found that development of CD8− DCs and their antigen presentation within MHC class II was specifically impaired during GVHD, while antigen presentation on MHC class I remained almost intact. Net effect of profound inflammatory milieu induced by GVHD is likely responsible for the defect in DC development and function.
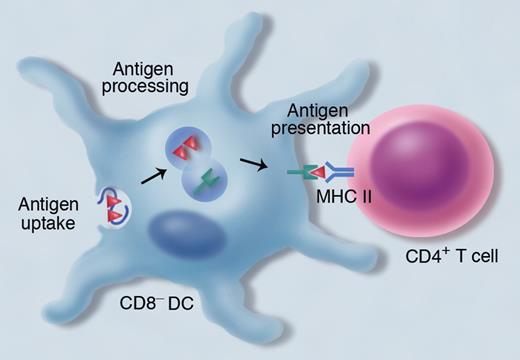
CD8− dendritic cells (DCs) are crucial for the presentation of exogenous antigens within major histocompatibility complex (MHC) class II to CD4+ T cells. Exogenous antigens are endocytosed into vesicles, where the antigens are processed and the peptides bind to MHC class II. Professional illustration by Marie Dauenheimer.
CD8− dendritic cells (DCs) are crucial for the presentation of exogenous antigens within major histocompatibility complex (MHC) class II to CD4+ T cells. Exogenous antigens are endocytosed into vesicles, where the antigens are processed and the peptides bind to MHC class II. Professional illustration by Marie Dauenheimer.
Although TEa is an alloantigen, similar results were obtained when ovalbumin-specific OT-II T cells were used as a reporter tool. Therefore, the results obtained in this study are likely to be extrapolated to other exogenous antigens that are acquired and presented by APCs in the same manner.
The article by Markey and colleagues reminds us of a seminal study demonstrating the failure of peripheral homeostatic proliferation of postthymic T cells during GVHD reported a decade ago by Dulude and colleagues.4 Interestingly, however; T cells isolated from GVHD mice expanded normally when transferred into thymectomized secondary hosts, whereas normal T cells failed to expand when injected into mice with GVHD. These results suggest that the diminished peripheral T-cell pool in GVHD is not due to an intrinsic T-cell defect but to an extrinsic microenvironment abnormality such as a defect in functional peripheral T-cell niches. Peptides/MHC complex and cytokines are crucial for homeostatic and antigen-driven proliferation and survival of naive and memory T cells.5 Thus, resident DCs represent one of the most crucial elements of T-cell niches. Markey and colleagues elegantly demonstrate that reconstitution and functions of DCs were markedly reduced during GVHD. This study together with the Dulude et al study thus suggests that qualitative and quantitative defects in T-cell immunity during GVHD after allogeneic SCT are caused by a problem of soil (DC compartment) rather than seed (T-cell compartment).
CD4+ T cells, a key player for conducting adoptive immunity, are the most affected element in immune reconstitution during GVHD but the mechanisms remain to be elucidated. DCs regulate the size of the peripheral CD4+ T-cell pool through their MHC class II and T-cell receptor interaction and IL-7 production.6 Thus, it is tempting to speculate that GVHD-mediated defect in development and function of CD8− DCs may be responsible for delayed reconstitution of functional CD4+ T cells. If so, donor DC infusion rather than donor lymphocyte infusion (DLI) may be a promising strategy to facilitate effective immune reconstitution without exacerbating GVHD, as Markey et al demonstrated. However, it is not currently obvious whether intrinsic T-cell defects that clearly exist in patients with GVHD are secondary to DC defects or due to other mechanisms independent of DC defects, such as chronically inflamed environment or bystander effects of apoptosis or exhaustion of alloreactive T cells.2,7 In addition, it remains to be elucidated why defects are limited to CD8− DCs (versus CD8+ DCs) and antigen presentation in the context of MHC class II (versus MHC class I).
Better understanding of the complex interactions between T-cell and DC reconstitution in the setting of GVHD has important clinical implications. Immune reconstitution after allogeneic SCT is a dynamic and complex process involving alterations in structural organization of lymphoid tissues and cytokine/chemokine environment, as well as changes in lymphoid and DC populations. Certainly, further studies are required for better understanding of the effects of GVHD on such a net process of immune reconstitution after allogeneic SCT for establishing novel strategies to promote reconstitution of effective T-cell immunity after allogeneic SCT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal