Abstract
Alloreactivity after transplantation is associated with profound immune suppression, and consequent opportunistic infection results in high morbidity and mortality. This immune suppression is most profound during GVHD after bone marrow transplantation where an inflammatory cytokine storm dominates. Contrary to current dogma, which avers that this is a T-cell defect, we demonstrate that the impairment lies within conventional dendritic cells (cDCs). Significantly, exogenous antigens can only be presented by the CD8− cDC subset after bone marrow transplantation, and inflammation during GVHD specifically renders the MHC class II presentation pathway in this population incompetent. In contrast, both classic and cross-presentation within MHC class I remain largely intact. Importantly, this defect in antigen processing can be partially reversed by TNF inhibition or the adoptive transfer of donor cDCs generated in the absence of inflammation.
Introduction
Bone marrow transplantation (BMT) is well established as curative therapy for hematologic malignancies, including leukemia. BMT-related mortality remains high, however, with death occurring in up to 50% of recipients because of leukemic relapse, GVHD, and infection. Despite advances in the use of prophylactic antibiotic and antifungal therapies in patients with GVHD, infection remains a major cause of nonrelapse mortality. GVHD pathogenesis itself is characterized by 3 key elements: (1) the initial damage of host tissue by pretransplant conditioning, which leads to the release of proinflammatory cytokines and translocation of toll-like receptor (TLR) ligands from the gastrointestinal tract into the systemic circulation; (2) the activation of donor-derived T cells by contact with host and donor antigen-presenting cells (APCs); and (3) the subsequent expansion of effector T cells and production of further proinflammatory cytokines.1 Importantly, the hallmark pathologic feature of acute GVHD is extensive host tissue apoptosis.
Suppression of immune function is well recognized as one of the consequences of GVHD after BMT, with reports of suppressed immune function in humans and mice with GVHD appearing in the literature as early as the 1960s.2-6 Importantly, although immunosuppressive pharmacologic agents are administered to patients to prevent and treat GVHD, the observed level of immune suppression far exceeds that which may be expected because of their effects. The basis of this immunologic defect remains unclear, although it has primarily been attributed to defects within the T-cell compartment induced by the inflammatory cytokine milieu during GVHD.3 Defects in both cytotoxic T lymphocytes and T-helper cell function have been reported in GVHD, although these studies do not address the mechanism underlying the reported T-cell insufficiency.
Myeloid suppressor cells have also been recognized as potential contributors to post-BMT immune suppression, through both the production of regulatory cytokines and contact-dependent mechanisms.7-9 These studies were largely associative, however, with initial post-transplant immune suppression being correlated with high macrophage numbers in mouse spleen.8 Multiple preclinical studies have shown that that the prevalence of myeloid suppressor cells is greatest in the early posttransplantation period, and it is clear that immune suppression persists beyond this time. Therefore, this heterogeneous cell population is unlikely to be solely responsible for poor immune responses after BMT, especially those associated with chronic GVHD.
Using well-established models of GVHD after BMT, in combination with established immunologic techniques to assess antigen presentation, we have confirmed that presentation of both host-derived alloantigen and third-party antigen is markedly decreased in the presence of GVHD. This is not because of a defect within the T-cell compartment, but a defect in the presentation of antigen by dendritic cells (DCs) within MHC class II. The CD8− conventional DC (cDC) population, which is critical for the presentation of exogenous antigen to CD4 T cells via MHC class II in other models,10 is both developmentally and functionally altered during GVHD. This population can be further divided into the CD4+ and double-negative (DN) cDC populations; and interestingly, during GVHD we see failure of development of the CD4+cDC subset, along with a marked functional defect in the DN DCs that are present, specifically with respect to their capacity to present antigen within MHC class II.
Our systematic investigation of causative factors implicates early production of TNF in the GVHD microenvironment and, to a lesser extent, IFN-γ, in GVHD-induced immune suppression. We also demonstrate that supplementation of the DC pool with in vitro generated DN cDCs, generated in the absence of inflammatory stimuli, can restore the capacity to induce CD4 T-cell responses in vivo.
Methods
Mice
Female C57BL/6 (B6.WT, H-2b), PTprca (B6.PTprc, H-2b, CD45.1), B6D2F1 (H-2b/d), BALB/c (H-2d), C3H/Hej (H-2k), and DBA/1 (H-2q) mice were purchased from the Animal Resources Center. The following donor mice (on a B6 background) were bred and housed at the Queensland Institute of Medical Research (QIMR): IFN-γ−/−, IFN-γR−/−, perforin−/− (pfp−/−), and granzyme B−/−(grzb−/−), μMT, CD1d−/−, IL-17R−/− (originally provided by Amgen), IL-1R−/−, AR1−/−, MyD88/Trif−/−, β2m−/−, OT-I and OT-II Tg, MHC class II−/−, and Bcl2−/−. B6.bm1.Act-mOVA (H-2bm1) mice were originally provided by William Heath (Walter and Eliza Hall Institute [WEHI], Melbourne, Australia) and subsequently housed and bred in the QIMR animal facility. B6.CD11c.DTR transgenic (Tg) mice (where the diphtheria toxin [DT] receptor and enhanced green fluorescent protein are driven off the CD11c promoter) and DEREG (B6, H-2b) in which the DT receptor is driven off the FoxP3 promoter and in which the administration of DT leads to systemic depletion of donor FoxP3-expressing regulatory T cells, were bred and housed at QIMR. B6.TEa Tg mice were bred with luciferase-expressing B6 mice to generate TEa-luc+ animals. B6.CD11c.OVA mice were provided by R.J.S. BM from βc/βIL-3 deficient mice (which lack the receptor for IL-3, IL-5, and GM-CSF) was provided by H.S.R. All animal studies were performed in accordance with the QIMR Animal Ethics Comittee.
BMT
Mice were transplanted and T-cell depletion (TCD) of BM and purification of splenic CD3+ T cells were performed as previously described.11 All recipient mice were transplanted and irradiated on day 0, with irradiation doses as follows: BALB/c, 900 cGy; B6D2F1, 1100 cGy; and B6 and bm1.Act-mOVA, 1000 cGy. BALB/c mice were transplanted with 107 T cell–depleted (TCD) BM cells from B6 (allogeneic) or BALB/c (syngeneic) donors, with or without the addition of 0.2 × 106 CD3+ T cells, or CD4 or CD8 MACS-purified T cells. Wild-type (WT), Tg, or knockout donors on a B6 background were used as described. B6 mice were transplanted with 107 C3H/Hej TCD BM ± 1 × 106 CD3+ T cells. B6D2F1 mice received 5 × 106 TCD BM ± 1 × 106 CD3+ T cells. bm1.Act-mOVA mice received 5 × 106 B6.WT TCD BM ± 2 × 106 CD3+ T. β2m−/− TCD BM grafts were transferred as negative controls. MACS purification was performed according to the manufacturer's instructions (Miltenyi Biotec). Mice were scored weekly according to clinical parameters originally described elsewhere.12
Cell depletion and cytokine neutralization
DT (Sigma-Aldrich) administration for the depletion of cDC and regulatory T cells was performed as reported elsewhere.13,14 Depletion of selected cell subsets was always more than 90%. NK cells were depleted using α-NK1.1 mAb (PK136; 1 mg day 5, 0.5 mg on days 7 and 9). TNFR:Fc (Enbrel, Amgen) treatment was used to neutralize TNF and lymphotoxin-α as previously described.11 Briefly, recipient mice received 100 μg/dose on days 1, 3, 5, 7, and 9 after transplantation. To block IL-6 signaling, mice were treated with 500 μg of α-IL-6R antibody intraperitoneally on days 3, 5, and 9 (15A7; BioXcell). For TGF-β neutralization, mice were treated with anti–mouse TGF-β mAb on day 0, 2, 4, 6, and 8 with 100 μg/dose (1D11; produced in-house).15 IL-10R was blocked using a 500 μg dose of mAb (1B1.3a, produced in-house) on day −2, followed by 250 μg doses on days 0, 2, 4, 6, and 8. IDO activity was blocked using 1 mg/mL 1-methyltryptophan (1-MT, Sigma-Aldrich) in the mouse drinking water. Both control and 1-MT containing water was supplemented with artificial sweetener to improve palatability.
TEa Tg cell preparation and Modfit analysis
Spleen and lymph node–derived TEa Tg T cells were prepared for adoptive transfer by FACS sorting on the basis of Vα2 and Vβ6 TCR expression to more than 99% purity. Purified cells were labeled with CFSE and adoptively transferred by intravenous injection. Proliferation was measured by CFSE dilution and analyzed using Modfit Version 3.2 software (Verity Software House). Calculated proliferation index = Σ all cells/computed number of parent cells. OT-I, OT-II, and DBA/1 T cells for in vivo injection were prepared using CD4 or CD8 MACS beads (Miltenyi Biotec) followed by FACS purification if required.
Bioluminescent imaging
Mice were imaged using the Xenogen, IVIS 100 Bioluminescent Imaging System (Caliper Life Sciences) as described previously.16
Antibodies
FITC-conjugated CD3 (17A2); PE-conjugated CD45.2, (104) I-A/I-E (M5/114.15.2), CD40 (1C10), CD80 (16-10A1) CD86 (GL-1), ICOS-L (HK5.3), PDL-1 (10F.9G2), PDL-2 (TY25), B7-H3 (RTAA15), B7-H4 (clone 9); PE-Cy7 conjugated streptavidin, CD8α (53-6.7); and allophycocyanin-conjugated CD11c (N418), CD45.2 (104), and CD4 (GK1.5) were purchased from BioLegend. FITC-conjugated and biotinylated anti-Vβ6 (RR4-7), anti-Vβ5 (MR9.4), and PE-conjugated anti-Vα2 (B20.1) were purchased from BD Biosciences PharMingen. 120G8 (AbCys) was FITC-conjugated in-house. The monoclonal antibody against IAk–hen-egg lysozyme (HEL) was provided by J.A.V. The YAe antibody was purchased from eBioscience in both purified and biotinylated form (eBioY-Ae). Purified YAe antibody was used to block surface staining before intracellular staining for YAe complexes was performed using the BD Cytofix/Cytoperm kit (BD Biosciences). 7-aminoactinomycin D was routinely used in FACS analysis to exclude dead cells (Sigma-Aldrich).
Ex vivo mixed lymphocyte culture (MLC)
OT-I and II Tg T cells from spleen and lymph nodes were prepared by initial MACS purification on the basis of CD4 or CD8 (Miltenyi Biotec) followed by FACS sorting on the basis of CD3 expression (to > 99% purity). T-cell proliferation was assessed by adding 3H-thymidine for the final 12 to 18 hours of 84-hour cultures. Peptide controls were performed by adding SIINFEKL or OVA(323-339) peptide (New England Peptide) at the stated concentrations to cultures of sort-purified DC and OT-I or OT-II T cells.
DC functional analyses
Before staining for DC subset analyses and other phenotyping, splenocytes were enriched for the DC-containing fraction using Nycodenz density-gradient centrifugation.17 For DC purification for use in functional assays, FACS sorting was performed after Nycodenz density-gradient centrifugation; DCs were gated on the basis of CD11c expression and lack of 120G8 expression for whole cDC experiments, CD4 and CD8 were added for assays of DC subset function. To assess cellular uptake of whole ovalbumin (OVA) protein by pinocytosis, cDCs were sort-purified and incubated with OVA conjugated to AlexaFluor-488 (Invitrogen) for 1 hour at 37°C or on ice before assessment with flow cytometry (FACS Canto or Fortessa; BD Biosciences). To assess phagocytosis, Fluoresbrite YG microsphere beads (0.5 μm, Polysciences) were injected intravenously into BALB/c recipients of B6 transplants on day 10 after transplantation and allowed to circulate for 3 hours before measuring uptake by splenic cDC subsets.
DQ-OVA assay
A total of 106 light-dense cells (after density gradient centrifugation) were resuspended in 100 μL of cold 200 μg/mL DQ-OVA (Invitrogen) and incubated for 20 minutes at 4°C. Cells were washed and incubated for 30 minutes at 37°C before analysis by flow cytometry.
MHC complex formation assays
DCs were sort-purified and incubated with 1 to 2 mg/mL HEL (Sigma-Aldrich), for 60 minutes at 37°C, or 4°C for negative controls. YAe antibody (eBioscience) was used to stain splenic DC directly ex vivo.
DC expansion with Flt3-ligand
For in vivo expansion, BALB/c mice were transplanted as described in “BMT” and were injected subcutaneously with 10 μg recombinant human fms-like tyrosine kinase 3 ligand (Flt3L; Celldex) subcutaneously, daily from day 0 to day 9 after transplantation. For the in vitro generation of DCs for adoptive transfer, B6 BM was harvested and plated at 1 × 106/mL in IMDM with 10% FCS and 200 ng/mL Flt3L.
Cytokine analysis
Serum cytokines were determined using the BD Cytometric Bead Array system (BD Biosciences PharMingen) according to the manufacturer's protocol.
Statistical analysis
Data are shown as mean ± SEM. SEM was shown as all the data presented are reflective of both biologic and experimental variation. Statistical significance was determined using 2-tailed Mann-Whitney U tests because datasets were generally small (n < 10) and a normal distribution cannot be assumed. ANOVA was used to assess the panel of knockout animals and blocking strategies used (see Figure 2B). A Dunnet multiple comparison test was used to compare each test group with the WT control both in the non-GVHD and GVHD settings. Statistical analyses were performed using GraphPad Prism Version 5.02 software.
Results
Antigen presentation is suppressed in the presence of GVHD
To assess antigen (Ag) presentation in allogeneic transplantation in the presence or absence of GVHD, we used the B6 → BALB/c system in which alloantigen presentation can be measured by quantifying the proliferative response of the adoptively transferred TEa transgenic (Tg) T cells, as previously described.17 The B6 → BALB/c model of allogeneic BMT is one in which donor and recipient are mismatched at both major and minor histocompatibility loci, and GVHD is predominantly CD4 T-cell dependent.18 In these systems, adoptively transferred TEa Tg T cells respond exclusively to donor APCs presenting host MHC class II–derived peptide (I-Ed derived Eα52-68 peptide) in the context of I-Ab.19,20 Quantification of TEa proliferation by CFSE dilution therefore allows for precise measurement of alloantigen presentation. Importantly in these systems, the adoptively transferred TEa T cell is used as a tool for quantifying antigen presentation at specific time points using short-term (2-3 days) adoptive transfer. The use of the TEa T cells in these experiments has provided a method for measuring in vivo T-cell responses to exogenously acquired antigen; and although the Eα52-68 peptide21 measured by the TEa is a single representative antigen, we believe that the results obtained can be extrapolated to other antigens acquired and presented by APCs in the same fashion.
The TEa Tg T cell is not part of the original graft and therefore is not the agent responsible for the induction of GVHD in these models. We have recently demonstrated in a separate study that TEa Tg T cells do have the capacity to induce GVHD in response to donor APC presentation of host-derived peptide but in a highly attenuated fashion and only after 4 weeks in vivo.22 Therefore, the 72-hour window in which the naive TEa Tg T cells are present for this assay is not sufficient for any contribution to pathology in either the non-GVHD or GVHD recipient animals. Transgenic T-cell proliferation is a well-established method of determining antigen presentation in vivo, pioneered and validated extensively by Heath et al in numerous seminal publications.23-30
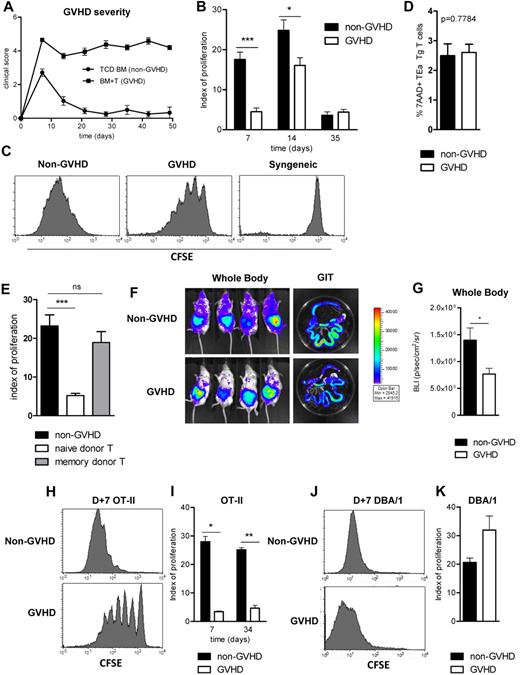
We initially used transplant systems in which WT donor grafts were either T-cell depleted or T-cell replete to generate an environment after BMT where GVHD was either absent or present. In this model, GVHD develops within the first week after transplantation and is maintained long-term, as demonstrated by the clinical GVHD scores shown in Figure 1A. Quantification of TEa Tg T-cell proliferation after injection at various time points after transplantation (Figure 1B) demonstrated significant differences in alloantigen presentation in the presence and absence of GVHD early before transplantation (day 7, P = .0006; and day 14, P = .026). Representative CFSE plots are shown in Figure 1C. Interestingly, after day 14, donor presentation of host-derived Ag declined and was similar between recipients of T cell–depleted and –replete grafts (day 35 shown). We hypothesized that this may be because of reducing availability of host-derived class II antigen as the effects of conditioning and its attendant damage to host-tissue resolve. Importantly, differences in TEa proliferation observed were not an artifact of differential TEa Tg T-cell death in the GVHD setting, as demonstrated in Figure 1D.
Immune responses are suppressed in the presence of GVHD. (A) A total of 107 B6.WT TCD BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT T cells (GVHD) were transferred into lethally irradiated (900 cGy, day 0) BALB/c recipients on day 0. GVHD clinical scores are shown. (B) TEa Tg T cells were sorted, CFSE labeled, and injected at days 7, 14, and 35 after transplantation. CFSE dilution in spleen was analyzed 3 days later, and proliferation indices are shown. Data are representative of 3 replicate experiments, with minimum n = 4/group/time point. *P = .05 to .01. ***P < .001. (C) Representative histograms of CFSE dilution in the TEa Tg T-cell population (day 10, after day 7 injection). Syngeneic control histograms are included (from BALB/c → BALB/c transplants). (D) Percentage of dead TEa Tg T cells on day 10 was measured with 7-aminoactinomycin D. Data are representative of 4 replicate experiments: n = 16 for the non-GVHD arm; n = 19 for the GVHD arm. (E) Naive and memory CD3+ donor T cells (sort purified on the basis of CD62L and Thy1 expression) were transferred on day 0. TEa T cells were injected on day 7 to measure antigen presentation. Data are representative of 2 replicate experiments: n = 7 for the TCD group; n = 9 for each naive and memory T-cell arm. ***P < .001. ns indicates not significant. (F) Grafts were performed as described, and luciferase-expressing TEa Tg T cells were transferred on day 7 after transplantation. Five days later, animals were injected with luciferin, and images were acquired. (G) Bioluminescent data are combined from 2 replicate experiments: n = 7 to 9/group. *P = .05 to .01. (H-I) 107 B6.CD11c.OVA BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT T cells (GVHD) were transferred into BALB/c recipients. On day 7 and 34 after transplantation, CFSE-labeled CD4+ OT-II T cells were transferred and proliferation was assessed 3 days later: day 7, P = .0286; n = 4/group; day 34, P = .0079; n = 5/group. Representative CFSE dilution histograms are shown in panel H. *P = .05 to .01. **P = .01 to .001. (J-K) B6.pfp−/− grafts (107 TCD B6.pfp−/− BM ± 0.2 × 106 B6.pfp−/−CD3+ T cells) were transferred into BALB/c recipients. On day 7 after transplantation, third-party DBA/1 CD4+ T cells were CFSE labeled, adoptively transferred, and analyzed 3 days later: n = 4/group. Representative CFSE dilution histograms are shown in panel J.
Immune responses are suppressed in the presence of GVHD. (A) A total of 107 B6.WT TCD BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT T cells (GVHD) were transferred into lethally irradiated (900 cGy, day 0) BALB/c recipients on day 0. GVHD clinical scores are shown. (B) TEa Tg T cells were sorted, CFSE labeled, and injected at days 7, 14, and 35 after transplantation. CFSE dilution in spleen was analyzed 3 days later, and proliferation indices are shown. Data are representative of 3 replicate experiments, with minimum n = 4/group/time point. *P = .05 to .01. ***P < .001. (C) Representative histograms of CFSE dilution in the TEa Tg T-cell population (day 10, after day 7 injection). Syngeneic control histograms are included (from BALB/c → BALB/c transplants). (D) Percentage of dead TEa Tg T cells on day 10 was measured with 7-aminoactinomycin D. Data are representative of 4 replicate experiments: n = 16 for the non-GVHD arm; n = 19 for the GVHD arm. (E) Naive and memory CD3+ donor T cells (sort purified on the basis of CD62L and Thy1 expression) were transferred on day 0. TEa T cells were injected on day 7 to measure antigen presentation. Data are representative of 2 replicate experiments: n = 7 for the TCD group; n = 9 for each naive and memory T-cell arm. ***P < .001. ns indicates not significant. (F) Grafts were performed as described, and luciferase-expressing TEa Tg T cells were transferred on day 7 after transplantation. Five days later, animals were injected with luciferin, and images were acquired. (G) Bioluminescent data are combined from 2 replicate experiments: n = 7 to 9/group. *P = .05 to .01. (H-I) 107 B6.CD11c.OVA BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT T cells (GVHD) were transferred into BALB/c recipients. On day 7 and 34 after transplantation, CFSE-labeled CD4+ OT-II T cells were transferred and proliferation was assessed 3 days later: day 7, P = .0286; n = 4/group; day 34, P = .0079; n = 5/group. Representative CFSE dilution histograms are shown in panel H. *P = .05 to .01. **P = .01 to .001. (J-K) B6.pfp−/− grafts (107 TCD B6.pfp−/− BM ± 0.2 × 106 B6.pfp−/−CD3+ T cells) were transferred into BALB/c recipients. On day 7 after transplantation, third-party DBA/1 CD4+ T cells were CFSE labeled, adoptively transferred, and analyzed 3 days later: n = 4/group. Representative CFSE dilution histograms are shown in panel J.
The decrease in antigen presentation during GVHD was dependent on the presence of naive donor T cells in the ingoing graft (Figure 1E). Importantly, memory T cells, which are known to have minimal ability to induce GVHD,31 did not impair antigen presentation, suggesting that this defect was a result of the GVHD process.
Next, to ensure that our assessment of antigen presentation within spleen during GVHD was reflective of all tissue sites, we performed experiments in which luciferase expressing (luc+) TEa T cells were transferred. An overall decrease in the bioluminescence imaging signal was recorded with whole-body and gastrointestinal tract imaging during GVHD (Figure 1F-G; P = .042), confirming that the results obtained from analysis of spleen reflected a systemic reduction in antigen presentation. Peripheral lymph nodes were also assessed at multiple time points, and TEa Tg T-cell proliferation was always found to be reflective of that in spleen (data not shown). Of additional interest, we observed that, in the presence and absence of GVHD, T-cell signals were greatest in the gastrointestinal tract, suggesting preferential activation of reporter TEa Tg T cells at this site.
To assess whether the defect seen in alloantigen presentation during GVHD reflected a broader deficiency in antigen presentation, B6.CD11c.OVA donor BM grafts, with or without T cells, were transplanted into irradiated BALB/c recipients, and the proliferation of adoptively transferred CFSE labeled OT-II cells (day 7) was used to measure Ag presentation by donor DCs after APC reconstitution.30,32 In these donor mice, OVA is driven off the CD11c promoter and thus is expressed by CD11chigh cDCs. The OVA protein is expressed in the membrane of CD11chigh DCs and OVA peptides, including the OVA(323-339) peptide recognized by OT-II Tg T cells, are presented by cDC in the context of MHC class II.33,34 As is the case with the TEa T cell, the transferred OT-II is a reporter tool only and is used to measure presentation of the OVA-derived peptide, not to induce GVHD in recipient animals. Importantly, the constitutive expression of OVA in this setting provides a nondiminishing antigen supply. In initial experiments, OT-II T cells were adoptively transferred 7 days after BMT as this represented the time point where the defect in alloantigen (alloAg) presentation during GVHD was greatest. As shown by representative CFSE plots in Figure 1H, presentation of processed OVA peptide by CD11c+ donor cells was markedly reduced in the presence of GVHD and maintained long after BMT (Figure 1I). Thus, GVHD is associated with long-term immunosuppression and diminished T-cell responses.
Having observed a reduction in magnitude of the antigen-specific response of both the TEa and OT-II Tg T cell in the presence of GVHD, we sought to examine whether the GVHD environment was inhibiting the transferred reporter population itself, independently of the antigen presentation required to generate Tg T-cell responses. We therefore adoptively transferred third party (DBA-1; H-2q) T cells into BMT recipients because this T-cell response is against disparate MHC itself, complexed with any peptide, either endogenous or exogenous (not a specific peptide as in the former experiments), and may be stimulated by both donor APC and any residual host APC that remain at the time of transfer. These experiments were performed to assess whether the GVHD environment was exerting a direct effect on T cells to dampen their proliferation. In contrast to the earlier data, GVHD did not suppress the proliferation of these adoptively transferred third-party CD4+ T cells (Figure 1J-K), confirming that the decrease in antigen-specific transgenic T-cell proliferation observed during GVHD is the result of a specific deficiency in the indirect presentation of exogenously acquired peptide.
GVHD-associated immune suppression is mediated by either CD4 or CD8 donor T cells and is independent of IFN-γ, cytolytic molecules and Treg
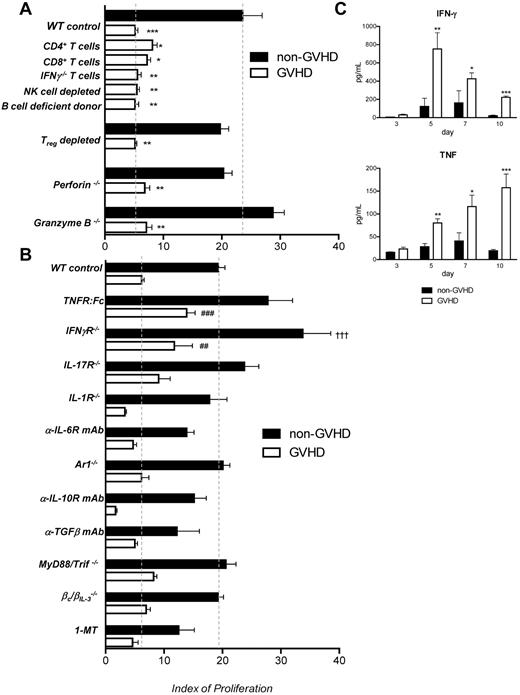
We next sought to establish the mechanisms by which GVHD impaired antigen presentation after BMT. Because the immunosuppressive effect of GVHD is dependent on the presence of alloreactive, naive donor T cells in the graft, we first investigated the contribution of donor T-cell subsets by performing transplantations in which CD4 or CD8 T cells alone were administered. As shown in Figure 2A, alloantigen presentation was suppressed to the same degree when either subset was transferred in the initial graft. This suggests that the impairment in the antigen presentation capacity of donor APC populations in GVHD may be the result of factors produced by both CD4 or CD8 T cells, or conversely be a result of GVHD and its effects on recipient target tissue itself (eg, GVHD in the gastrointestinal tract induced by either CD4 or CD8 donor T cells might equally allow the translocation of TLR ligands into the systemic circulation). IFN-γ is the key pathogenic mediator of acute GVHD, and donor T cells are the major source of this cytokine after transplantation. In addition, previous literature postulates that IFN-γ contributes to GVHD-associated immune suppression.9 Therefore, we transferred grafts in which donor T cells were IFN-γ deficient (Figure 2A), and resulting data excluded donor T cell–derived IFN-γ in isolation as a cause for the immune suppression seen early after BMT.
TNF and IFN-γ impact antigen presentation after BMT. (A) A total of 107 B6.WT TCD BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT CD3+ T cells (GVHD), or 0.2 × 106 CD4 or CD8 T cells, or 0.2 × 106 IFN-γ−/− CD3+ T cells were transferred into lethally irradiated (900 cGy, day 0) BALB/c recipients on day 0. Anti-NK1.1 antibody was used to deplete donor NK cells and was administered to recipients at day 1, 3, 5, 7, and 9 after transplantation (1 mg/dose). B cell-deficient (μMT) donors were used to assess the role of donor B cells. B6.DEREG grafts were transferred in parallel to B6.WT grafts, and all recipient mice were treated with DT after transplantation to deplete Treg cells. Grafts were B6.pfp−/−, B6.Grzb−/−, or B6.WT. TEa T cells were transferred at day 7 after transplantation, and proliferation was assessed as described. n = 15 in TCD, n = 16 in CD3+ control arm; n = 5 in CD4+/8+ and anti-NK1.1 treated groups; n = 4, μMT arm; n = 7, IFN-γ−/− T cell arm. For Treg depletion experiments, n = 4/group, B6.WT donors; n = 7 or 8/group, B6.DEREG donors; n = 4/group for pfp−/− and grzb−/− experiments. *Statistical significance between the relevant control non-GVHD arm and the GVHD test group: *P = .05 to .01. **P = .01 to .001. ***P < .001. (B) BM grafts deficient in cytokine receptors or incapable of cytokine production were used as labeled, and Ag presentation measured at day 10, as previously described. T cells were of WT.B6 origin. Blocking antibodies and 1-MT were used as described in “Cell depletion and cytokine neutralization,” and TEa T cells were transferred as described previously. Data are representative of 16 separate experiments. n = 3 (TGF-β TCD) − 36 (WT TCD and BM + T)/group. #Statistical significance between the test GVHD and WT GVHD arms: ##P = .01 to .001; ###P < .001. †Statistical significance between the test non-GVHD and WT non-GVHD arms: †††P < .001. (C) Serum cytokines were analyzed at day 3, 5, 7, and 10 after transplantation of either B6.WT TCD BM grafts (non-GVHD) or TCD BM + CD3+ T (GVHD).
TNF and IFN-γ impact antigen presentation after BMT. (A) A total of 107 B6.WT TCD BM (non-GVHD) + 0.2 × 106 CD3+ B6.WT CD3+ T cells (GVHD), or 0.2 × 106 CD4 or CD8 T cells, or 0.2 × 106 IFN-γ−/− CD3+ T cells were transferred into lethally irradiated (900 cGy, day 0) BALB/c recipients on day 0. Anti-NK1.1 antibody was used to deplete donor NK cells and was administered to recipients at day 1, 3, 5, 7, and 9 after transplantation (1 mg/dose). B cell-deficient (μMT) donors were used to assess the role of donor B cells. B6.DEREG grafts were transferred in parallel to B6.WT grafts, and all recipient mice were treated with DT after transplantation to deplete Treg cells. Grafts were B6.pfp−/−, B6.Grzb−/−, or B6.WT. TEa T cells were transferred at day 7 after transplantation, and proliferation was assessed as described. n = 15 in TCD, n = 16 in CD3+ control arm; n = 5 in CD4+/8+ and anti-NK1.1 treated groups; n = 4, μMT arm; n = 7, IFN-γ−/− T cell arm. For Treg depletion experiments, n = 4/group, B6.WT donors; n = 7 or 8/group, B6.DEREG donors; n = 4/group for pfp−/− and grzb−/− experiments. *Statistical significance between the relevant control non-GVHD arm and the GVHD test group: *P = .05 to .01. **P = .01 to .001. ***P < .001. (B) BM grafts deficient in cytokine receptors or incapable of cytokine production were used as labeled, and Ag presentation measured at day 10, as previously described. T cells were of WT.B6 origin. Blocking antibodies and 1-MT were used as described in “Cell depletion and cytokine neutralization,” and TEa T cells were transferred as described previously. Data are representative of 16 separate experiments. n = 3 (TGF-β TCD) − 36 (WT TCD and BM + T)/group. #Statistical significance between the test GVHD and WT GVHD arms: ##P = .01 to .001; ###P < .001. †Statistical significance between the test non-GVHD and WT non-GVHD arms: †††P < .001. (C) Serum cytokines were analyzed at day 3, 5, 7, and 10 after transplantation of either B6.WT TCD BM grafts (non-GVHD) or TCD BM + CD3+ T (GVHD).
The elimination of donor NK cells (with anti-NK1.1 antibody), B cells (using μMT donor mice), and NKT cells (CD1d−/− donors, not shown) had no effect on antigen presentation, confirming that these populations are not involved in GVHD-associated immune suppression (Figure 2A). As FoxP3+ regulatory T cells (Treg) have the capacity to attenuate immune responses, we performed transplantations using DEREG donors, in which donor Treg could be both excluded from the initial graft (by FACS depletion) and depleted after BMT via the administration of DT.13 The immune suppression associated with GVHD was not mediated by Treg, as no significant change in antigen presentation occurred in their absence (Figure 2A). Likewise, the classic (CD8-dependent) cytolytic molecules perforin and granzyme B were not involved in isolation in the GVHD-associated immune suppression (Figure 2A).
To clarify whether cytokines known to be important in GVHD played a role in the associated immune suppression by signaling through the bone marrow component of the graft, we used the relevant knockout animals or blocking reagents in the assessment of in vivo Ag presentation (Figure 2B). In the absence of IFN-γ and TNF signaling in APC (achieved via use of IFN-γR−/− donors and TNFR:Fc blockade, respectively), we observed increases in alloantigen presentation, although this was only specific to the GVHD setting in the case of TNF. Lack of IL-17R, IL-1R, IL-6R, and IFN-α receptor (AR1−/−) signaling in the donor BM did not reverse GVHD-associated immune suppression (Figure 2B).
Having eliminated these key proinflammatory cytokines, we next excluded a role for the regulatory cytokines IL-10 and TGF-β using neutralizing mAbs (Figure 2B). Likewise, signaling by IL-3, IL-5, and GM-CSF was not involved in GVHD-associated immune suppression because Ag presentation was equivalent when donors were WT or lacked the capacity to signal through IL-3/5/GM-CSF (βc/βIL-3−/−).35
TLR signaling has been implicated as a regulator of APC function and has a demonstrated ability to “paralyze” the ability of DCs to phagocytose and present newly encountered antigen.25 We therefore performed experiments using MyD88/Trif−/− donors. As shown in Figure 2B, this did not impact GVHD-associated immune suppression either, excluding a role for TLR signaling in this phenomenon. Finally, although IDO has an important role in regulating alloimmunity,36 inhibition using 1-MT had no effect on alloantigen presentation (Figure 2B).
These data demonstrate that only TNF contributed to the suppression of antigen presentation in a GVHD-specific fashion. In contrast, IFN-γ suppressed antigen presentation in both the presence and absence of GVHD. The systematic elimination of other cytokines known to be involved in GVHD pathogenesis revealed considerable redundancy in the ability of GVHD effector pathways to suppress antigen presentation. As expected, examination of serum cytokine levels early after transplantation demonstrated significant elevations in IFN-γ and TNF levels in animals with GVHD relative to those without (Figure 2C).
cDCs are the critical cell for alloantigen presentation in GVHD
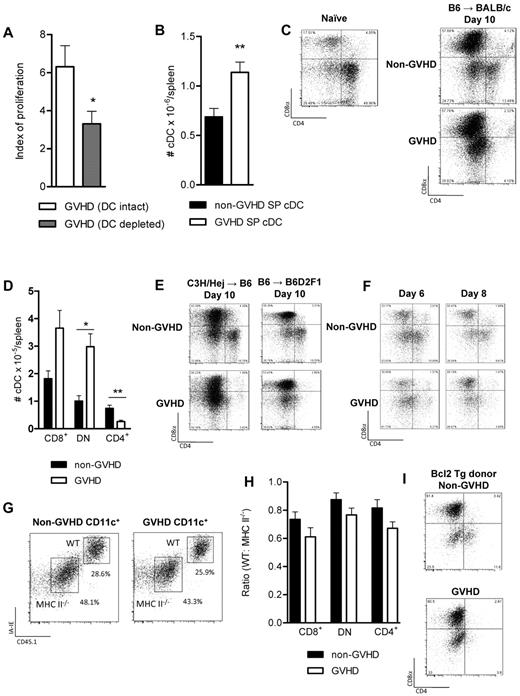
We next sought to examine the posttransplantation APC compartment directly. We have recently demonstrated that alloantigen presentation after T cell–depleted allogeneic BMT is critically dependent on cDCs.17 We therefore investigated whether cDCs were also an important population presenting alloAg during GVHD. These experiments were performed using BM grafts from CD11c.DTR donors, and cDCs were depleted after BMT using DT.17,37 Using this system, we confirmed that alloantigen presentation is dependent on donor cDC after T cell–replete BMT (Figure 3A). Furthermore, enumeration of splenic cDCs in recipients of WT grafts confirms that cDC numbers are not reduced in the setting of GVHD early after BMT (Figure 3B); so although numerical deficiency of these cells would reduce antigen presentation and T-cell proliferation, this does not explain our observations in GVHD.
cDC development is disrupted during GVHD. (A) BALB/c recipients of B6.CD11c.DTR BM + T donor grafts were treated with DT on days 12, 14, and 16 after transplantation to deplete donor cDCs. TEa Tg T cells were transferred on day 14, and proliferation was analyzed 3 days later as described. Data are combined from 2 replicate experiments: n = 8/group (saline) and n = 6/group (DT). *P = .0406. (B) Splenic DCs were enumerated at day 10 after transplantation (B6 → BALB/c model); n = 9/group. **P = .04. (C) cDC subset analysis was performed. FACS plots illustrate the CD8/CD4 expression profiles of naive WT.B6 CD11c+MHC class IIhigh cDCs and of day 10 post-transplantation cDCs (B6 → BALB/c system). (D) DCs were enriched by density gradient centrifugation, and phenotype was assessed by flow cytometry; n = 5/group. *P = .05 to .01. **P = .01 to .001. (E) BMTs were performed in the C3H/Hej → B6 and the B6 → B6D2F1 models (described in “BMT”), and DC subsets were analyzed at day 10 after transplantation. (F) cDC subsets at day 6 and 8 after transplantation. (G) Irradiated BALB/c mice (900 cGy) received 5 × 106 WT.PTPrca TCD BM (CD45.1) + 5 × 106 B6.MHC class II−/− TCD BM ± 0.2 × 106 WT.PTPrca CD4+ T cells on day 0. Splenic DCs were enriched by density gradient centrifugation and analyzed by flow cytometry on day 10. (H) Data demonstrate the ratio of CD45.1 (WT) to CD45.2 (MHC class II−/−) DCs. Combined data from 2 replicate experiments; n = 10/group. (I) TCD Bcl2 Tg donor BM was transferred with or without T cells, and DC subsets were assessed on day 10 after transplantation as described; n = 5/group.
cDC development is disrupted during GVHD. (A) BALB/c recipients of B6.CD11c.DTR BM + T donor grafts were treated with DT on days 12, 14, and 16 after transplantation to deplete donor cDCs. TEa Tg T cells were transferred on day 14, and proliferation was analyzed 3 days later as described. Data are combined from 2 replicate experiments: n = 8/group (saline) and n = 6/group (DT). *P = .0406. (B) Splenic DCs were enumerated at day 10 after transplantation (B6 → BALB/c model); n = 9/group. **P = .04. (C) cDC subset analysis was performed. FACS plots illustrate the CD8/CD4 expression profiles of naive WT.B6 CD11c+MHC class IIhigh cDCs and of day 10 post-transplantation cDCs (B6 → BALB/c system). (D) DCs were enriched by density gradient centrifugation, and phenotype was assessed by flow cytometry; n = 5/group. *P = .05 to .01. **P = .01 to .001. (E) BMTs were performed in the C3H/Hej → B6 and the B6 → B6D2F1 models (described in “BMT”), and DC subsets were analyzed at day 10 after transplantation. (F) cDC subsets at day 6 and 8 after transplantation. (G) Irradiated BALB/c mice (900 cGy) received 5 × 106 WT.PTPrca TCD BM (CD45.1) + 5 × 106 B6.MHC class II−/− TCD BM ± 0.2 × 106 WT.PTPrca CD4+ T cells on day 0. Splenic DCs were enriched by density gradient centrifugation and analyzed by flow cytometry on day 10. (H) Data demonstrate the ratio of CD45.1 (WT) to CD45.2 (MHC class II−/−) DCs. Combined data from 2 replicate experiments; n = 10/group. (I) TCD Bcl2 Tg donor BM was transferred with or without T cells, and DC subsets were assessed on day 10 after transplantation as described; n = 5/group.
The development of cDC subsets is corrupted during GVHD
In naïve animals, CD8− cDCs are the dominant population (Figure 3C). Early after BMT, we observed that cDC development was skewed toward the CD8+ subset, in both the presence and absence of GVHD. In addition to the CD8+ cDC dominance, during GVHD the CD8−CD4+ cDCs were absent. As shown in Figure 3D, the total number of CD8+ cDCs is not statistically different between animals with and without GVHD, and the animals with GVHD have a relative excess of the DN DC subset, and a notable absence of CD4+ cDCs. Data in additional models of BMT across major MHC boundaries confirm this observation (Figure 3E). We next performed time-course experiments to assess the development of cDC subsets early after BMT in mice with and without GVHD. Splenic DCs were analyzed on days 2, 4, 6, and 8 after transplantation. On days 2 and 4 after transplantation, donor cDCs had not yet reconstituted (not shown), but by day 6, an emerging population was present. Although CD4+ cDCs were present at this early time point in the non-GVHD mice, they failed to develop in the presence of GVHD (Figure 3F). To examine whether the expression of MHC class II by cDCs rendered them susceptible to killing by alloreactive donor T cells specific for the alloAg presented, BM from B6.MHC class II−/− and B6.WT donors was transplanted in equal recipient, with or without donor CD4+ T cells, into irradiated BALB/c reipient mice. As shown in Figure 3G and H, there is an increased prevalence of all DC subsets derived from the MHC class II-deficient portion of the original graft; however, this was independent of the presence of donor T cells. Importantly, the CD8− DC subset did not preferentially survive in the absence of MHC class II, confirming that this cDC population was not being killed by cytolysis secondary to cognate interaction with antigen-specific donor T cells. To assess whether other mechanisms may be playing a role in inducing apoptosis in the CD4 expressing DC population, we next used Bcl2 Tg donor mice, in which there is a failure of apoptosis. These experiments (Figure 3I) demonstrated a cDC developmental pattern equivalent to WT, further confirming that the CD4-expressing DCs fail to develop in GVHD, rather than developing and subsequently being killed by allogeneic peptide-specific donor T cells.
Ag presentation within MHC class II is specifically impaired in cDCs in the presence of GVHD
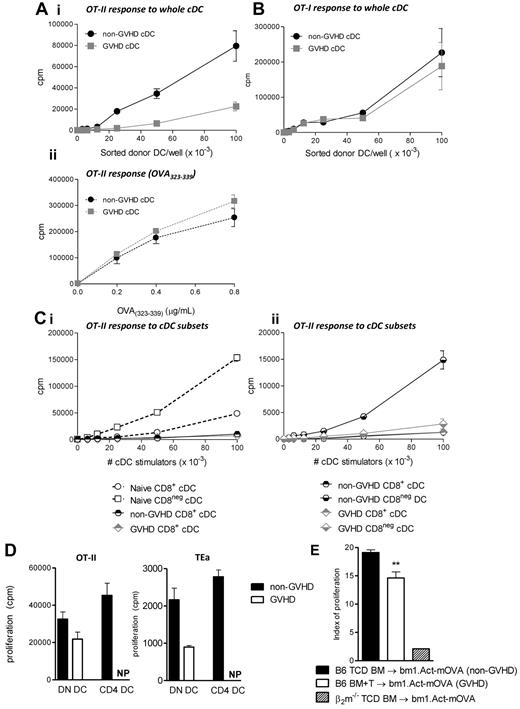
To quantify Ag presentation by cDC during GVHD, transplants were performed in which CD11c.OVA BM was transferred with or without T cells. In these mice, OVA-derived peptide is presented in the context of MHC class II, and because OVA is an endogenous protein for the cDCs, OVA-derived peptides are also presented in MHC class I. Ten days after BMT, donor cDCs (all subsets) were sort-purified and plated with OT-I and OT-II T cells to quantify Ag presentation within class I and class II, respectively. A clear defect in the capacity of cDCs to present OVA peptide within MHC class II was demonstrated when cDCs were isolated from animals with GVHD (Figure 4Ai). Of note, when cDCs were pulsed with the OVA(323-339) peptide (to saturate all available class II molecules), cDCs from GVHD and non-GVHD recipients induced equivalent proliferation in OT-II cells (Figure 4Aii), indicating that available functional MHC class II is equivalent. In contrast, antigen presentation within MHC class I (as determined by the ability to stimulate OT-I proliferation) was intact in the same cDCs during GVHD (Figure 4B). Thus, cDCs from animals with GVHD do not have a broad defect in Ag presentation but rather a specific defect in MHC class II Ag presentation.
cDC function is disrupted in GVHD, and this is specific to the MHC class II antigen presentation pathway. (Ai) B6.CD11c.OVA grafts (TCD BM ± CD3+ T cells) were transferred into irradiated BALB/c recipients. Ten days after transplantation, cDCs were sorted and cultured with OT-II Tg T cells. (Aii) OVA(323-339) was added to the culture wells as positive controls for MHC class II availability. Representative data shown from one of 4 replicate experiments. Negative controls, including T cells plated with peptide alone, and B6.WT DCs + OTI/II cells were performed to confirm specificity of the assay (data not shown). (B) Same as described in panel Ai, but DCs were plated with OT-I Tg T cells. (C) CD8+ and CD8− cDCs, from either naive (Ci) or post-transplantation animals (Cii), were sort purified and plated with OT-II T cells. Data are representative of 4 replicate experiments. (D) cDCs were further fractionated into DN or CD4+ subsets (non-GVHD animals) and DN only (GVHD animals). CD4+ cDCs were not present (NP) in the animals with GVHD and therefore could not be assessed. A total of 105 of the specified cDC subset were plated with 105 OT-II T cells or TEa T cells as shown. Data are representative of 3 replicate experiments. (E) A total of 5 × 106 B6 TCD BM (non-GVHD) + 2 × 106 CD3+ T cells (GVHD) were transferred into Bm1.Act-mOVA recipients, and CFSE-labeled OT-I T cells were adoptively transferred on day 7 after transplantation to measure the extent of cross-presentation by donor DCs. β2m−/− donor grafts were used as negative controls. Data are representative from 2 similar experiments: n = 8 in the BM only group; 9 in the BM + T group. **P = .01 to .001.
cDC function is disrupted in GVHD, and this is specific to the MHC class II antigen presentation pathway. (Ai) B6.CD11c.OVA grafts (TCD BM ± CD3+ T cells) were transferred into irradiated BALB/c recipients. Ten days after transplantation, cDCs were sorted and cultured with OT-II Tg T cells. (Aii) OVA(323-339) was added to the culture wells as positive controls for MHC class II availability. Representative data shown from one of 4 replicate experiments. Negative controls, including T cells plated with peptide alone, and B6.WT DCs + OTI/II cells were performed to confirm specificity of the assay (data not shown). (B) Same as described in panel Ai, but DCs were plated with OT-I Tg T cells. (C) CD8+ and CD8− cDCs, from either naive (Ci) or post-transplantation animals (Cii), were sort purified and plated with OT-II T cells. Data are representative of 4 replicate experiments. (D) cDCs were further fractionated into DN or CD4+ subsets (non-GVHD animals) and DN only (GVHD animals). CD4+ cDCs were not present (NP) in the animals with GVHD and therefore could not be assessed. A total of 105 of the specified cDC subset were plated with 105 OT-II T cells or TEa T cells as shown. Data are representative of 3 replicate experiments. (E) A total of 5 × 106 B6 TCD BM (non-GVHD) + 2 × 106 CD3+ T cells (GVHD) were transferred into Bm1.Act-mOVA recipients, and CFSE-labeled OT-I T cells were adoptively transferred on day 7 after transplantation to measure the extent of cross-presentation by donor DCs. β2m−/− donor grafts were used as negative controls. Data are representative from 2 similar experiments: n = 8 in the BM only group; 9 in the BM + T group. **P = .01 to .001.
Given the striking defect observed in cDC subset development, we next undertook functional assessment of the CD8+ and CD8− cDC fractions. CD8+ cDCs present antigen only minimally within MHC class II in the steady state and lose this function completely after BMT (Figure 4Ci). In contrast, the CD8− cDCs were responsible for the majority of antigen presentation within MHC class II in the absence of GVHD, and this function was dramatically impaired in the presence of GVHD (Figure 4Cii). We further separated the CD8− population into CD4+ and double-negative (DN) cDC subsets. In the absence of GVHD, DN cDCs and CD4+ cDCs present antigen at a similar level, inducing high levels of proliferation in both the TEa and the OT-II Tg T cells. In the presence of GVHD early after BMT when the CD4+ cDCs are absent, the capacity of the residual DN DCs to present antigen is diminished (Figure 4D). This suggests that the defect in antigen presentation seen during GVHD is the result of developmental failure within the CD8− cDC subset and an acquired defect in the residual DN DC populations.
Cross-presentation is only minimally decreased during GVHD
We were interested in whether the dominance of CD8+ cDCs would impact cross-presentation after BMT and therefore established a model that would specifically allow this presentation pathway to be assessed. We transferred B6.WT donor grafts into irradiated bm1.Act-mOVA recipient mice (in which a membrane-bound form of the ovalbumin molecule is driven off the β-actin promoter), and subsequently transferred CFSE labeled OT-I T cells to measure the presentation of host-derived SIINFEKL in donor MHC class I (because the OT-I cell cannot respond to OVA presented by recipient APC bearing H-2Kbm1). These data (Figure 4E) demonstrated that cross-presentation is significantly but only minimally decreased in the presence of GVHD, contrasting the dramatic defect in presentation of exogenous antigen within MHC class II (Figure 4A).
Costimulatory molecule expression is enhanced during GVHD, whereas antigen uptake remains intact
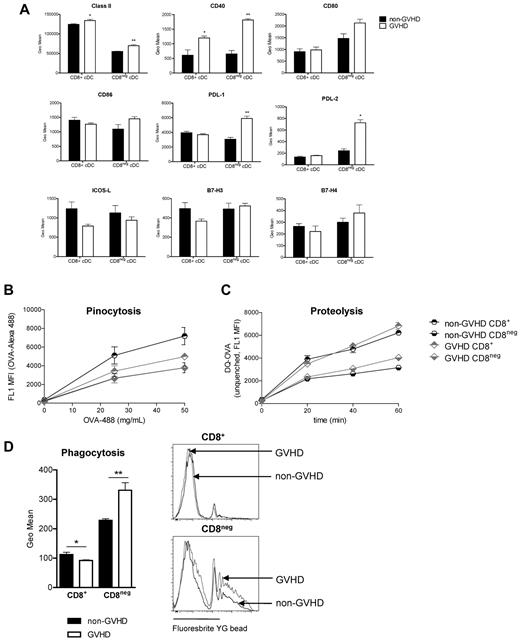
To ascertain the factors underlying poor function in the CD8− DC compartment in GVHD, we next assessed costimulatory molecule expression. Interestingly, CD8− cDCs from animals with GVHD expressed higher levels of MHC class II and CD40, together with positive and inhibitory costimulatory molecules CD80, CD86, PDL-1, and PDL-2 (Figure 5A), consistent with DCs that have matured within an inflammatory environment.25 Although this phenotype has been associated with impairments in antigen acquisition,38 the uptake and proteolysis of soluble antigen and the uptake of particulate antigen are intact in CD8− DCs during GVHD (Figure 5B-D). Equivalent levels of ICOS-L, B7-H3, and B7-H4 were seen (Figure 5A).
CD8− DCs express higher levels of costimulatory and coinhibitory molecules in GVHD. (A) cDCs were stained for costimulatory/coinhibitory molecules and analyzed by flow cytometry. Data are representative of 2 replicate experiments; n = 5/group in each. *P = .05 to .01. **P = .01 to .001. (B) DCs were sort-purified on day 10 after transplantation and incubated with OVA-AlexaFluor-488. Fluorescence data reflect soluble antigen uptake. (C) Sorted DCs (day 10) were incubated for 1 hour with DQ-OVA and uptake and processing assessed by level of fluorescence after 20, 40, and 60 minutes. (D) Fluorescent beads were injected intravenously at day 10 after transplantation. Geometric mean fluorescence intensity is shown; n = 5/group. *P = .05 to .01. **P = .01 to .001.
CD8− DCs express higher levels of costimulatory and coinhibitory molecules in GVHD. (A) cDCs were stained for costimulatory/coinhibitory molecules and analyzed by flow cytometry. Data are representative of 2 replicate experiments; n = 5/group in each. *P = .05 to .01. **P = .01 to .001. (B) DCs were sort-purified on day 10 after transplantation and incubated with OVA-AlexaFluor-488. Fluorescence data reflect soluble antigen uptake. (C) Sorted DCs (day 10) were incubated for 1 hour with DQ-OVA and uptake and processing assessed by level of fluorescence after 20, 40, and 60 minutes. (D) Fluorescent beads were injected intravenously at day 10 after transplantation. Geometric mean fluorescence intensity is shown; n = 5/group. *P = .05 to .01. **P = .01 to .001.
GVHD impairs the presentation of individual peptides derived from exogenous antigen
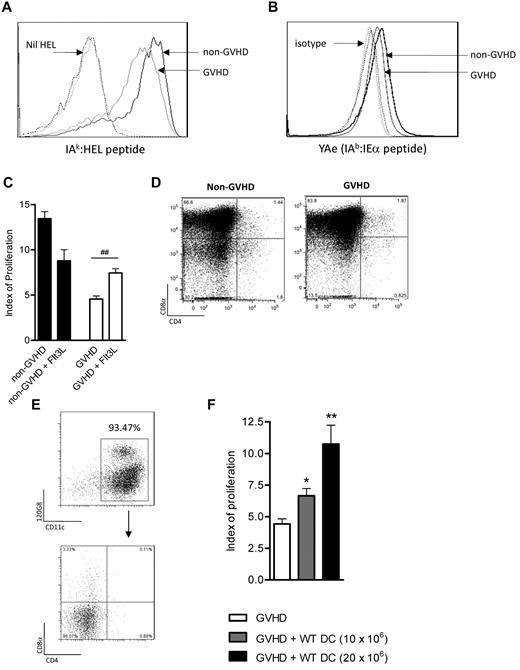
We next examined the presence of specific, measurable peptide/MHC complexes. Using the IAk-HEL monoclonal antibody (and grafts that were of C3H/Hej (H2Dk/IAk) origin), we were able to confirm that cDCs from animals with GVHD assembled lower levels of the IAk-HEL complex compared with non-GVHD cDCs (Figure 6A). DC subset-specific complex formation cannot be assessed in this system because cDCs down-regulate subset markers after the 20-hour culture period. Presentation of host-derived peptides by donor DCs was also assessed using the YAe antibody (Figure 6B), which measures the MHC I-Ab/I-Ed peptide complex recognized by the TEa T cell. A small but reproducible reduction was again noted. Thus, despite adequate class II expression (Figure 5A), the capacity of donor cDCs to present individual peptides is reduced during GVHD.
The assembly of peptide/MHC complexes within the cDC population is impaired during GVHD. (A) C3H/Hej grafts were transferred into B6 recipients as described in “Methods.” On day 10 after transplantation, DCs were sort-purified and pulsed with whole hen-egg lysosyme protein, and IAk-HEL levels were measured. Data are representative of 3 replicate experiments. Black solid line indicates non-GVHD; gray solid line, GVHD; and dotted lines, respective isotype controls. (B) Day 10 post-BMT DCs (B6 → BALB/c model) were assessed for expression of the YAe complex. Data are representative of 2 replicate experiments. Black solid line indicates non-GVHD; gray solid line, GVHD; and dotted lines, respective isotype controls. (C) BALB/c mice were transplanted and treated with Flt3L after transplantation. Data are combined from 2 replicate experiments, with n = 4 to 8/group shown. ##P = .01 to .001. (D) DC subset profiles of Flt3L-treated animals on day 10. (E) B6 DCs were generated in vitro as described in “Methods” and had the phenotype shown. (F) These cells were subsequently injected (day 6), followed by TEa T cells (day 7), as described. n = 9, 5, or 4/group. Data are representative of 2 replicate experiments in which either 10 or 20 × 106 BM DCs were transferred. *P = .05 to .01. **P = .01 to .001.
The assembly of peptide/MHC complexes within the cDC population is impaired during GVHD. (A) C3H/Hej grafts were transferred into B6 recipients as described in “Methods.” On day 10 after transplantation, DCs were sort-purified and pulsed with whole hen-egg lysosyme protein, and IAk-HEL levels were measured. Data are representative of 3 replicate experiments. Black solid line indicates non-GVHD; gray solid line, GVHD; and dotted lines, respective isotype controls. (B) Day 10 post-BMT DCs (B6 → BALB/c model) were assessed for expression of the YAe complex. Data are representative of 2 replicate experiments. Black solid line indicates non-GVHD; gray solid line, GVHD; and dotted lines, respective isotype controls. (C) BALB/c mice were transplanted and treated with Flt3L after transplantation. Data are combined from 2 replicate experiments, with n = 4 to 8/group shown. ##P = .01 to .001. (D) DC subset profiles of Flt3L-treated animals on day 10. (E) B6 DCs were generated in vitro as described in “Methods” and had the phenotype shown. (F) These cells were subsequently injected (day 6), followed by TEa T cells (day 7), as described. n = 9, 5, or 4/group. Data are representative of 2 replicate experiments in which either 10 or 20 × 106 BM DCs were transferred. *P = .05 to .01. **P = .01 to .001.
Adoptive transfer of BM DCs generated in the absence of inflammation can restore Ag presentation to the TEa T cell
As a strategy to expand cDCs during GVHD, we next assessed whether the in vivo treatment of post-transplantation animals with Flt3L could improve antigen presentation to the TEa T cell. As shown in Figure 6C, the Flt3L treatment led to a minor increase in Ag presentation (index of proliferation in GVHD groups 4.56 ± 0.33 [saline] vs 8.79 ± 1.22 [Flt3L]; P = .002). Of note, in vivo Flt3L treatment led to massive cDC expansion but preferentially increased the CD8+ subset such that they represented 78% to 90% of cDCs (Figure 6D).
As in vivo treatment did not serve to expand the most important (CD8−) DC subset for exogenous Ag presentation, we were next interested in whether adoptive transfer of a CD8−CD4− DC population, generated in the absence of the characteristic inflammatory storm of GVHD, could restore the defect in antigen presentation. We thus generated DCs in vitro (derived from B6 bone marrow in the presence of Flt3L). These DCs were 70% to 75% cDCs and 20% to 25% plasmacytoid DCs and lacked CD4 and CD8 (Figure 6E).When these donor DCs were adoptively transferred into mice 6 days after BMT, Ag presentation was significantly increased in a dose-dependent fashion (Figure 6F). This provides further evidence that donor DCs are defective during GVHD and highlights adoptive transfer of DC generated in vitro as a valid therapeutic strategy to restore immune competence after BMT.
Discussion
Immune dysfunction has been reported in both patients and mice with GVHD from as early as the 1960s.2-6 This phenomenon has been attributed largely to T-cell “paralysis” caused by the proinflammatory cytokine environment,4,39 but despite this, the role of specific cytokine signaling has remained unclear. Defects in cytotoxic T lymphocytes and T-helper cell function have already been reported,39,40 but this study is the first to confirm that defects within the cDC compartment make a critical contribution to the immune dysfunction observed during GVHD.
We observed a striking difference in the composition of the cDC subset compartment early after BMT compared with naive animals, such that the usual (4:1) CD8−/CD8+ ratio is inverted. Although this corrects over time in the absence of GVHD, in the presence of GVHD, the CD8− cDC subsets fail to develop normally. Importantly, the CD8− cDCs are the APC specialized for presenting antigen within the MHC class II pathway after BMT, consistent with published data in naive mice.10 These potent CD8− cDC display a phenotype consistent with high levels of activation. Within this population, the DN DCs are numerically dominant; and although the CD4+ cDCs are potent APCs, they are present in low numbers relative to the DN DC subset. No previous studies have reported a specialized function for DN DC, and the data shown here clearly demonstrate that they are crucial for exogenous antigen presentation after BMT. DC development after BMT is thus corrupted toward the population with the weakest capacity for presentation of exogenous antigen.
In the steady state, Flt-3L41 and GM-CSF (and to a lesser extent, M-CSF) are required for DC development and homeostasis.42 There is some redundancy in these signaling pathways, however, as DC development in mice deficient in GM-CSF receptor and CSF-1 receptor is only partially impaired.43 Our data generated using βc/βIL-3-deficient mice suggest that the lack of GM-CSF signaling does not change Ag presentation after BMT, nor does it influence the development of DC subsets. Inflammatory DCs are driven by “danger signals,” including TLR ligation and inflammatory cytokines,44,45 but these pathways did not appear to contribute to immune dysfunction during GVHD. Previously, IFN-γ signaling in the bone marrow compartment has been thought to be key in GVHD-associated immune suppression, with other authors noting, as we did, improved immune function in the absence of IFN-γ receptor signaling.9 However, it is clear from our data that the effect of IFN-γ is not specific to GVHD, with an improvement in antigen presentation also occurring in recipients of non-GVHD–inducing allografts. It is probable that the effect of the IFN-γ signaling is to increase the production of downstream cytokines (eg, TNF) and in concert with direct cytotoxic actions, increases the overall level of target tissue damage. While our panel of knockout and blocking antibody studies were able to reveal a role for TNF signaling in GVHD-associated immune suppression, this effect was only partial and clearly considerable redundancy exists. These studies demonstrate that the immune suppression induced by GVHD is highly reproducible, occurring even when other key pathogenic pathways are blocked. Importantly, during GVHD, DCs develop under the influence of extraordinary inflammatory stimuli.1 Our studies suggest that it is the sum of these effects within the lymphoid microenvironment that is probably responsible for the defects in DC development observed during GVHD rather than any single factor in isolation.
For DCs to effectively present antigen within MHC class II, they must first internalize, via phagocytosis, pinocytosis, or receptor-mediated uptake (eg, via DEC205) and then process protein antigen into appropriate peptide fragments (via the lysosome), before loading into the MHC class II molecule and transport to the cell surface.46 Cross-presentation also requires internalization of antigen but uses the early endosome rather than the lysosome for processing. Classic class I presentation requires the proteosomal processing of endogenous antigen before presentation. In this study, we have demonstrated a dramatic defect in MHC class II presentation during GVHD, with minimal effects on cross-presentation capacity and classic class I presentation. Although impaired antigen uptake may result in a selective defect of this nature,46 we have demonstrated that phagocytosis and pinocytosis, as well as initial proteolysis of soluble antigen are intact in purified DC subsets from mice with GVHD. In contrast, a scenario instead exists where the ability to present any single exogenous peptide in MHC class II within cDC is impaired, suggesting that the defect lies within antigen processing within the lysosome, loading onto MHC class II and/or transport onto the surface of the DCs. Although we identify a novel pathway that is defective in the GVHD setting and contributes to immune suppression, additional intrinsic T-cell defects clearly exist.8,40 It is not clear whether these intrinsic T-cell defects occur downstream of the APC impairment described here, or whether T-cell exhaustion is yet another side effect of the chronically inflamed microenvironment in GVHD. What is clear is that immunity to pathogens can be restored via the transfer of T cells primed in vitro,47,48 suggesting that antigen presentation in vivo may be a significant limiting factor.
This study has significant implications for clinical therapies aimed at improving immune competence in patients after BMT. First, TNF inhibition probably improves rather than impairs immune competence during clinical GVHD, providing a rationale for early rather than late use of TNF inhibitors in patients. Second, vaccination strategies after BMT, which require antigen presentation by DCs, are unlikely to be successful in the presence of GVHD. Instead, strategies that reduce alloreactivity will be most effective in promoting efficient antigen presentation because this will promote normal development and functional competence within the CD8− cDC subsets. Future work will need to focus on the preferential expansion of the CD8− cDC subset (the CD1b/c+ DC population in humans49 ), either in vitro before adoptive transfer or in vivo with recombinant GM-CSF.50 Interestingly, we have recently shown that donor APCs have only a very limited capacity to induce GVHD.22 This may be partly because of the inactivation of the MHC class II-dependent pathway as described here, and it suggests that the transfer of donor DCs is unlikely to exacerbate GVHD. For these reasons, approaches to overcome GVHD-associated immune suppression will best be achieved by the prevention or silencing of alloreactivity itself (eg, by alloreactive T-cell depletion) and/or cell based therapy to specifically restore the CD8− DC population when GVHD is present.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff at the QIMR animal facility and Grace Chojnowski and Paula Hall from the QIMR Flow Cytometry facility for their assistance; Lisa K. Denzin (Memorial Sloan- Kettering Cancer Center, New York, NY) for provision of the H2-O antibody, which was generated in her laboratory (although data not shown); Jonathan Bleier (QIMR) for discussions regarding the statistical analysis of data; Shizuo Akira (Osaka University, Japan) for the MyD88 and MyD88/Trif-deficient animals; and William Heath (WEHI, Melbourne, Australia) for providing the B6.bm1 and B6.bm1.Act-mOVA colonies.
This work was supported by the Leukemia Foundation of Australia and the National Health and Medical Research Council, Australia. G.R.H. is an National Health and Medical Research Council (NH&MRC) Australia Fellow and QLD Health Senior Clinical Research Fellow. K.P.A.M. is a Cancer Council Queensland Senior Research Fellow. K.A.M. is an NH&MRC Clinical Training Fellow. H.S.R. is a Peter Nelson Leukemia Research Fellow, funded by the Cancer Council SA. The contributions of J.V.-R., J.A.V., and A.M.L. to this work were made possible through Victorian State Government Operational Infrastructure Support and Australian Government NH&MRC Independent Research Institutes Infrastructure Support Scheme.
Authorship
Contribution: K.A.M. designed and performed experiments andwrote the manuscript; M.K. performed experiments and provided useful discussion; R.D.K., K.E.L., Y.A.W., S.D.O., N.C.R., A.L.J.D., A.V., R.J.R., and M.C. performed experiments; C.R.E. contributed reagents and provided useful discussion; R.J.S. provided essential reagents and useful discussion; H.S.R. and A.F.L. provided essential reagents; J.V.-R. performed experiments and provided useful discussion; A.M.L. and J.A.V. provided useful discussion; and G.R.H. and K.P.A.M. contributed to experimental design and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kate A. Markey, QIMR, 300 Herston Road, Herston, QLD, Australia, 4006; e-mail: kate.markey@qimr.edu.au; and Kelli P. A. MacDonald, QIMR, 300 Herston Road, Herston, QLD, Australia, 4006; e-mail: kelli.macdonald@qimr.edu.au.
References
Author notes
G.R.H. and K.P.A.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal