Abstract
Diagnosis of gastrointestinal GVHD (GI-GVHD) is based on clinical symptoms and histologic findings. No biomarkers predicting responses to treatment are routinely available even though 30% to 50% of patients will not respond to corticosteroids. In this study, we aimed to evaluate fecal calprotectin, α-1-antitrypsin (α1-AT), and elastase at the time of first symptoms as diagnostic and prognostic tools for GI-GVHD in 72 consecutive patients, of whom 51 developed GI-GVHD. The prognostic value of markers was evaluated by their association with complete response (CR) and steroid-resistant (SR) GVHD. Calprotectin and α1-AT concentrations increased with GI-GVHD initial stages but patients with initial stage 1 GI-GVHD had similar marker levels to patients without GI-GVHD, so sensitivity to diagnose GI-GVHD was weak. In contrast, calprotectin and α1-AT were predictors for SR-GVHD and CR. Multiple regression modeling identified calprotectin and α1-AT concentration as independently predicting SR-GVHD together with initial stage > 2 GI-GVHD. Our results showed that fecal calprotectin and α1-AT levels at the time of diagnosis are predictive for responses to treatment but are not diagnostic markers for initial stage 1 to 3 GI-GVHD.
Introduction
Allogeneic HSCT is a potentially curative treatment for a wide range of hematologic diseases. Its main complication, GVHD, affects 50% to 70% of patients and represents an important source of morbidity and mortality.1 First-line therapy for acute GVHD is corticosteroids but only 40% to 70% of patients will respond to this treatment. Patients with steroid-resistant acute GVHD (SR-GVHD) have a very poor outcome with long-term survival not exceeding 30%.2,3 The evolution of acute GVHD during the initial weeks will ultimately define the severity of GVHD (maximum grade III-IV) and/or the absence of response to corticosteroids.2,4-6 Currently, very few markers predict the severity of GVHD at disease onset.7-10 In this study, we investigate the significance of 3 fecal markers in patients with gastrointestinal (GI) GVHD: calprotectin, elastase, and α-1-antitrypsin (α1-AT). None of these markers are specific for a particular disease but they are symptomatic of a pathogenesis. Their usual and routine applications are: (1) help in diagnosis in chronic diarrhea and (2) a measure of disease activity in patients with diarrhea. The fecal calprotectin level reflects mucosal intestinal inflammation of any origin (infectious or noninfectious). Its measurement has been widely used in patients with inflammatory bowel disease (IBD), that is, ulcerative colitis and Crohn disease as a diagnostic tool and disease activity marker.11 The sensitivity and specificity of calprotectin to discriminate an IBD from other noninflammatory bowel diseases, such as irritable bowel syndrome, range from 80% to 95% with a usual threshold of between 50 and 100 μg/g stool.11 Calprotectin levels are also able to predict responses to treatment and the probability of relapse in patients with IBD.
α1-AT is a serine protease inhibiting proinflammatory mediators, which increase in cases of inflammation, including the “cytokine storm” described early after HSCT. Specifically, it has been established that α1-AT is able to have an inhibitory effect on allogeneic activation via inhibition of proteinase 3.12 As α1-AT increases in any cases of inflammation, it is common to consider it as a surrogate of inflammation. Routinely, α1-AT clearance is used to diagnose protein-losing enteropathies related to various erosive mucosal damage, such as IBD and gut malignancy, or nonerosive mucosal damage, such as celiac disease and tropical sprue.13 GI-GVHD is included in the erosive mucosal gut disease family, as usually confirmed by histology. Several decades ago, Weisdorf et al reported increased fecal α1-AT concentration in patients with GI-GVHD until symptoms disappeared, whereas patients without GI-GVHD had normal values, except during GI symptoms related to conditioning regimen toxicity.2 These findings have recently been confirmed in 7 children with GI-GVHD by Hagen et al.14 Fecal elastase has a high sensitivity to diagnose exocrine pancreatic insufficiency. However, villous atrophy of any origin leading to a decrease in cholecystokinin secretion may lead to pancreatic dysfunction. Cholecystokinin and pancreatic secretion are then restored to normal with intestinal mucosal regeneration.15 The aim of our study was to evaluate the capability of these fecal markers to diagnose GI-GVHD or to predict the prognosis of patients with GI-GVHD.
Methods
Study design
A prospective study, approved by the local ethical committee of the Hôpital Saint-Louis, was designed to test clinical and biologic parameters potentially associated with GVHD diagnosis and prognosis. All consecutive patients who gave their consent to participate in this noninterventional study and who presented a first GVHD episode that included GI symptoms had stools collected to test fecal markers. The study began in September 2008 and inclusions were closed in December 2010. For data analysis, follow-up was stopped in March 2011. Clinical and biologic data of acute GVHD were collected at inclusion (onset), and on day 3, 5, 7, 10, and 14 thereafter. GVHD status was then prospectively updated until last follow-up. As it was a noninterventional protocol, no invasive tests other than routine exams were planned. Acute GVHD was diagnosed according to the Gluckberg criteria independently from the date of transplantation (early or late onset),4,5 and GI-GVHD was suspected when diarrhea, anorexia, nausea, and vomiting occurred after HSCT. According to our local policy, acute GVHD was treated by corticosteroids (doses 1-2 mg/kg/d). Stools were collected as soon as possible after the first GI symptoms (at a median of 2 days after the first symptoms, first quartile 1.5, fourth quartile 4.5 days) corresponding to a median of 22 days from transplantation. Standard coprology (to detect bacteria or parasites), PCR to detect adenovirus and rotavirus, and the research of Clostridium difficile and its toxin in stools were systematically performed. In addition, the concentrations of calprotectin, α1-AT, and elastase were determined in stool samples. GI endoscopies for histologic analysis were performed according to the physician's discretion, if the patient gave his informed consent and in absence of contraindication: severe thrombocytopenia, suspicion of ileus, or intestinal perforation. Four-month cumulative incidence of complete response (CR) of GI-GVHD was defined as the disappearance of all clinical signs related to GI-GVHD. Consequently, if a patient did not achieve CR 4 months after the onset of GVHD, he was not considered in CR. SR-GVHD was defined by either the absence of remission at day 14, stability at day 7, or progression at day 3. During GVHD evaluation for SR (14 days), if the patient required a second line of treatment, he was considered SR.
Analysis of fecal markers
Fecal samples were collected in plastic containers and sent to the laboratory within 48 hours. Fecal α1-AT assay was performed immediately, and an aliquot of the stool samples was frozen at −80°C for calprotectin and elastase measurements.
The concentration of fecal calprotectin was assayed in duplicate by a “sandwich”-type ELISA which uses a polyclonal Ab system (Calprest; Eurospital). The assay was performed according to the manufacturer's instructions. Using this assay, the measurement range was 15-5000 μg/g and 50 μg/g stool was considered to be the upper normal limit as already published and routinely accepted in other digestive diseases.11,16
The concentration of fecal elastase was determined in duplicate using a “sandwich”-type enzyme immunoassay (Schebo-Biotech) which combines the use of 2 mAbs binding to 2 distinct epitopes specific to human pancreatic elastase 1. Using this assay, the detection limit was 15 μg/g stool and 200 μg/g stool was considered as the lower normal limit as already published and routinely accepted in other digestive diseases.15,17
The concentration of fecal α1-AT was measured using an immunonephelemetric method adapted on the BN ProSpec system (Siemens). Stool samples were diluted 1:5 in 0.15M NaCl then shaken vigorously by the means of a vortex until complete homogenization occurred. The homogenate was centrifuged at 10 000g for 15 minutes at 4°C and the supernatant was used for analysis which was performed at 2 different final dilutions (1:5 and 1:500) to avoid any prozone phenomena. Using this method, the measurement range was 0.01-20 mg/g stools. Results were expressed in milligrams per gram of dry stool and 1.5 mg/g dry stool was considered the upper normal limit. This threshold has been established in the laboratory based on other protein-losing diseases.
Statistical analysis
Data are presented as count (percentage) and median (range). The association of markers with histopathologic findings was tested by the Wilcoxon rank-sum test. The prognostic value of markers was evaluated for patients with GI-GVHD at inclusion by the association of each marker with complete response in gut and SR-GVHD. Resistance to steroids was considered as a binary variable, whereas achievement of a CR was considered to be a censored variable and was analyzed in a competing risks framework. Factors associated with resistance to steroids were analyzed first by bivariate analyses using Fisher exact tests before a multiple logistic regression model was used for adjusted analyses. Validity of the logistic model was examined using Le Cessie and Van Houwelingen's goodness-of-fit test.18 Model calibration was assessed by the calibration slope19 and the bootstrap bias-corrected calibration slope; model discrimination was assessed by the c index20 (identical to the area under the receiver operating characteristic [ROC] curve) after bootstrap correction for overoptimism.21 When selecting the variables in a model based on results obtained in the same sample, the resulting model is prone to better discriminate the observed data than future observations. Bootstrap correction removes this overoptimistic part of the model properties, and enables a c index to be closer to what would be obtained in external data. To compare the discrimination of different models, the categoryless net reclassification improvement (NRI) was also used.22 A model can be regarded as providing a score, related to a model-based probability of event. When a model is compared with another one, each patient can be considered as upward reclassified by the new model (his/her score is higher with the new model than with the old one) or downward reclassified (lower score with the new model). The categoryless NRI is then equal to twice the difference in the probabilities of upward reclassification for event patients (here, patients refractory to steroids) and nonevent patients (nonrefractory to steroids). If the new model better discriminates events and nonevents, then it should increase the score for events and decrease the score for nonevents, thus leading to a positive NRI.23 For censored variables, cumulative incidence functions were estimated using standard methodology,24 and compared with the Gray test.25 The adjusted analyses were then performed using the Fine-Gray proportional subdistribution hazards model. The assumption of proportional subdistribution hazards was tested by the analog of the Grambsch and Therneau lack-of-fit test.26
Although recent work showed that the usual “rule of thumb” of 10 events per variable may be too stringent for adjusted logistic and survival models, the number of cases of steroid resistance and, to a lesser extent, the number of CRs precluded the use of more standard model selection procedures with backward variable elimination. We therefore performed a stepwise (forward and backward) model selection procedure restricted to models with no more than 4 parameters, excluding the intercept but including potential interaction, that were then tested. The model selection was based on the Akaike information criterion (AIC). First, a model without fecal markers was built, then a model with fecal markers but not stages. Finally, the covariates retained in these 2 first models were mixed to evaluate the prediction related to fecal markers.
Overall survival (OS) was examined and risk factors for OS were analyzed using Cox proportional cause-specific hazards model. The survival models were restricted to 6 months because clinical factors were more discriminant at this time point.
All tests were 2-sided and P values ≤ .05 were considered to indicate significant association. Analyses were performed using the R statistical software (Version 2.10.1; R Foundation for Statistical Computing).
Results
Patient characteristics
During the study period, 60 patients with GI symptoms and 12 patients presenting an acute GVHD without GI symptoms (controls) had their stool sampled and analyzed. Median follow-up was 289 days. The median age at the time of transplantation was 44 years (range, 8-66). Sixty-one percent of patients were male. The stem cell source was peripheral blood in 54 cases (75%), BM in 14 cases (18%) and cord blood in 5 cases (7%). Donors were an HLA-matched sibling in 28 cases (39%), a matched unrelated (10 of 10 HLA allelic identities) donor in 27 cases (38%), and a mismatched unrelated donor in 17 cases (24%). Myeloablative conditioning regimen was used in 28 patients (39%). GVHD prophylaxis was cyclosporine plus methotrexate in 22 patients (31%) and cyclosporine plus mycophenolate mofetil in 47 patients (65%). Details of patient characteristics are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Calprotectin, α1-AT, and elastase concentrations and GI-GVHD diagnosis
Among the 60 patients with GI symptoms after HSCT, 51 diagnoses of GI-GVHD were recorded, which justified high-dose corticosteroid therapy. GI-GVHD stages at the time of first symptoms were stage 1 in 23 patients, stage 2 in 14 patients, and stage 3 in 14 patients. No patient presented an initial stage 4 GI-GVHD. The 9 other patients presented gut symptoms attributed to GI viral infections or colitis related to C difficile. The 12 controls had no GI symptoms but were diagnosed with acute GVHD not involving the gut. Fecal biomarkers were compared in these 3 groups of patients.
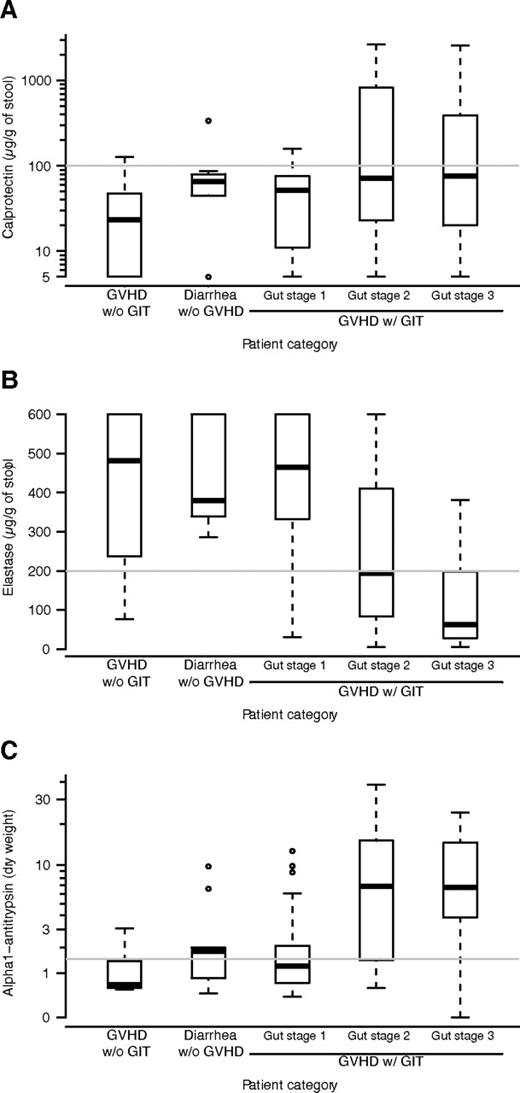
The concentration of calprotectin was higher in patients presenting GI symptoms compared with the controls (Figure 1A). Nevertheless, there was no statistical evidence that calprotectin had a different distribution in the 3 patient groups (P = .081, Kruskal-Wallis test) and sensitivity to detect any acute GI-GVHD was only 30% (Table 1). Specificity for GI-GVHD diagnosis was 90%.
Relationship between fecal markers and diagnosis of GI-GVHD. The panels show concentration of calprotectin (A), elasatse (B), and alpha1-antitrypsin (C) by clinic in this order: patients with no GI symptoms, patients with diarrhea not related to GVHD, patients with stage 1 GI-GVHD, patients with stage 2 GI-GVHD, and patients with stage 3 GI-GVHD. Box and whisker plots display the median, 25th, and 75th percentile of the distribution (box), and whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box.
Relationship between fecal markers and diagnosis of GI-GVHD. The panels show concentration of calprotectin (A), elasatse (B), and alpha1-antitrypsin (C) by clinic in this order: patients with no GI symptoms, patients with diarrhea not related to GVHD, patients with stage 1 GI-GVHD, patients with stage 2 GI-GVHD, and patients with stage 3 GI-GVHD. Box and whisker plots display the median, 25th, and 75th percentile of the distribution (box), and whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box.
Sensitivity and specificity of fecal markers to diagnose GI-GVHD
| . | AUROC (95% CI) . | Threshold . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . |
|---|---|---|---|---|
| Gut GVHD any stage | ||||
| Calprotectin | 0.623 (0.491-0.756) | > 100 μg/g | 31 (19-46) | 90 (70-99) |
| Elastase | 0.683 (0.560-0.805) | < 200 μg/g | 43 (29-58) | 90 (70-99) |
| α1-antitrypsin | 0.699 (0.577-0.822) | > 1.5 mg/g | 59 (44-72) | 62 (38-82) |
| Gut GVHD stage 2-3 | ||||
| Calprotectin | 0.661 (0.524-0.798) | > 100 μg/g | 46 (28-66) | 89 (75-96) |
| Elastase | 0.824 (0.728-0.920) | < 200 μg/g | 64 (44-81) | 86 (73-95) |
| α1-antitrypsin | 0.808 (0.698-0.917) | > 1.5 mg/g | 79 (59-62) | 64 (48-78) |
| . | AUROC (95% CI) . | Threshold . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . |
|---|---|---|---|---|
| Gut GVHD any stage | ||||
| Calprotectin | 0.623 (0.491-0.756) | > 100 μg/g | 31 (19-46) | 90 (70-99) |
| Elastase | 0.683 (0.560-0.805) | < 200 μg/g | 43 (29-58) | 90 (70-99) |
| α1-antitrypsin | 0.699 (0.577-0.822) | > 1.5 mg/g | 59 (44-72) | 62 (38-82) |
| Gut GVHD stage 2-3 | ||||
| Calprotectin | 0.661 (0.524-0.798) | > 100 μg/g | 46 (28-66) | 89 (75-96) |
| Elastase | 0.824 (0.728-0.920) | < 200 μg/g | 64 (44-81) | 86 (73-95) |
| α1-antitrypsin | 0.808 (0.698-0.917) | > 1.5 mg/g | 79 (59-62) | 64 (48-78) |
GI indicates gastrointestinal; AUROC, area under the Receiver Operating Characteristics curve; and CI, confidence interval.
The concentration of elastase was lower in patients with initial stage 2-3 GI-GVHD compared with the other patient groups (Figure 1B). The statistical evidence for a different distribution was mild (P = .051), but a difference was observed between GVHD stage 2 or 3 and the other groups (P < .0001 overall). Consequently, sensitivity to detect GI-GVHD was 43% and increased to 64% when detecting stage 2-3 GI-GVHD (Table 1). Specificity was 90%.
The concentration of α1-AT was higher in patients with initial stage 2-3 GI-GVHD than in patients with initial stage 1 GI-GVHD, with GI infection or in the controls (Figure 1C). The statistical evidence for a different distribution was once again mild, but a difference was observed between GVHD stage 2 or 3 and the other groups (P < .0001 overall). Consequently, sensitivity to detect GI-GVHD was 59% and increased to 79% when detecting stage 2-3 GI-GVHD (Table 1). Overall specificity was 62%.
Histologic findings and correlation with fecal markers
GI pathologic data were available for 32 (63%) of the 51 patients with GI-GVHD. Histologic abnormalities were described as follows: (1) apoptosis, (2) inflammatory cellular infiltrate of the chorion, (3) vascular damage, and (4) epithelial abrasion. Each of the 4 histologic features was analyzed to test a correlation with each fecal biomarker in stools. Apoptosis and the presence of an inflammatory cellular infiltrate were the 2 most frequent histologic findings in our series (Table 2). Vascular damage or epithelial abrasions were found in 4 and 11 biopsies, respectively. The presence of epithelial abrasions correlated with higher levels of calprotectin (Table 2) and vascular damage was more frequently observed in patients with abnormal fecal markers without reaching statistical significance (too few patients). Conversely, the absence of apoptosis correlated with abnormal levels for the 3 fecal markers (Table 2).
Relationship between histology and fecal biomarkers in patients with GI-GVHD
| Histology . | N . | Calprotectin . | Elastase . | α1-antitrypsin . |
|---|---|---|---|---|
| Apoptosis | ||||
| Absent | 8 | 408 (322-597) | 66 (28-188) | 10.6 (5.3-14.6) |
| Present | 24 | 37 (15-101) | 332 (144-450) | 2.1 (0.9-8.9) |
| P | .002 | .029 | .037 | |
| Infiltrate | ||||
| Absent | 2 | 5; 828 | 30; 84 | 0; 15.8 |
| Present | 29 | 61 (30-170) | 279 (142-439) | 3.9 (1.2-9.0) |
| P | ||||
| Vascular damage | ||||
| Absent | 28 | 54 (22-196) | 266 (138-441) | 3.5 (1.1-9.1) |
| Present | 4 | 276 (163-936) | 33 (15-106) | 11.2 (6.1-17.0) |
| P | .063 | .046 | .19 | |
| Epithelial abrasion | ||||
| Absent | 21 | 37 (18-141) | 279 (124-446) | 1.7 (0.9-8.8) |
| Present | 11 | 302 (83-472) | 157 (38-370) | 7.7 (3.9-14.7) |
| P | .017 | .22 | .074 |
| Histology . | N . | Calprotectin . | Elastase . | α1-antitrypsin . |
|---|---|---|---|---|
| Apoptosis | ||||
| Absent | 8 | 408 (322-597) | 66 (28-188) | 10.6 (5.3-14.6) |
| Present | 24 | 37 (15-101) | 332 (144-450) | 2.1 (0.9-8.9) |
| P | .002 | .029 | .037 | |
| Infiltrate | ||||
| Absent | 2 | 5; 828 | 30; 84 | 0; 15.8 |
| Present | 29 | 61 (30-170) | 279 (142-439) | 3.9 (1.2-9.0) |
| P | ||||
| Vascular damage | ||||
| Absent | 28 | 54 (22-196) | 266 (138-441) | 3.5 (1.1-9.1) |
| Present | 4 | 276 (163-936) | 33 (15-106) | 11.2 (6.1-17.0) |
| P | .063 | .046 | .19 | |
| Epithelial abrasion | ||||
| Absent | 21 | 37 (18-141) | 279 (124-446) | 1.7 (0.9-8.8) |
| Present | 11 | 302 (83-472) | 157 (38-370) | 7.7 (3.9-14.7) |
| P | .017 | .22 | .074 |
GI indicates gastrointestinal.
Correlation between fecal biomarkers at disease onset and response to steroids
Among the 51 patients with GI-GVHD, 27 (53%) were considered resistant to corticosteroids on day 14. A high concentration of calprotectin (≥ 100 μg/g stool) was strongly associated with steroid resistance. Specifically, patients presenting abnormal calprotectin levels at inclusion had a higher cumulative incidence of SR-GVHD (93% vs 33%, P = .00001). Likewise, an abnormally high concentration of α1-AT ≥ 1.5 mg/g dry stool was closely associated with a high cumulative incidence of SR-GVHD (72% vs 21%, P = .0009). However, no significant association was found between elastase concentration and SR-GVHD. Other parameters predicting SR-GVHD were hypoalbuminemia (81% vs 30%, P = .001), the initial GI-GVHD stage (1: 29%, 2: 54%, and 3: 86%, P = .004), WHO performance status (1-2: 41% vs 3-4: 79%, P = .027), epithelial abrasion (82% vs 35%, P = .023), and the absence of apoptosis (100% vs 35%, P = .002; Table 3).
Markers potentially associated with CR to corticosteroids and steroid-refractory GI-GVHD (univariate analysis)
| Markers . | N . | Cumulative incidence of CR, % (95% CI) . | P . | Probability for SR-GVHD (%) . | P . |
|---|---|---|---|---|---|
| Calprotectin | |||||
| < 100 μg/g | 35 | 86 (68-94) | 12 (34) | ||
| ≥ 100 μg/g | 16 | 56 (24-79) | .05 | 15 (94) | < .0001 |
| Elastase | |||||
| > 200 μg/g | 29 | 86 (65-95) | 14 (48) | ||
| ≤ 200 μg/g | 22 | 64 (38-81) | .12 | 13 (59) | .57 |
| α1-antitrypsin | |||||
| < 1.5 mg/g | 21 | 90 (59-98) | 5 (24) | ||
| ≥ 1.5 mg/g | 30 | 67 (46-81) | .0007 | 22 (73) | .0007 |
| Apoptosis | |||||
| Absent | 8 | 62 (17-88) | 8 (100) | ||
| Present | 24 | 83 (59-94) | .20 | 9 (38) | .003 |
| Vascular damage | |||||
| Absent | 28 | 86 (63-95) | 13 (46) | ||
| Present | 4 | 25 (0-73) | .084 | 4 (100) | .10 |
| Abrasions | |||||
| Absent | 21 | 90 (61-98) | 8 (38) | ||
| Present | 11 | 55 (20-80) | .16 | 9 (82) | .028 |
| Albumin | |||||
| > 30 g/L | 24 | 87 (63-96) | 7 (29) | ||
| ≤ 30 g/L | 23 | 61 (35-79) | .006 | 19 (83) | .0004 |
| GI-GVHD stage | |||||
| 1 | 23 | 87 (61-96) | 7 (30) | ||
| 2 | 14 | 79 (40-94) | 8 (57) | .004 | |
| 3 | 14 | 57 (26-79) | .15 | 12 (86) | |
| WHO PS | |||||
| 1-2 | 36 | 86 (68-94) | 15 (42) | ||
| 3-4 | 15 | 53 (20-78) | .028 | 12 (80) | .016 |
| Markers . | N . | Cumulative incidence of CR, % (95% CI) . | P . | Probability for SR-GVHD (%) . | P . |
|---|---|---|---|---|---|
| Calprotectin | |||||
| < 100 μg/g | 35 | 86 (68-94) | 12 (34) | ||
| ≥ 100 μg/g | 16 | 56 (24-79) | .05 | 15 (94) | < .0001 |
| Elastase | |||||
| > 200 μg/g | 29 | 86 (65-95) | 14 (48) | ||
| ≤ 200 μg/g | 22 | 64 (38-81) | .12 | 13 (59) | .57 |
| α1-antitrypsin | |||||
| < 1.5 mg/g | 21 | 90 (59-98) | 5 (24) | ||
| ≥ 1.5 mg/g | 30 | 67 (46-81) | .0007 | 22 (73) | .0007 |
| Apoptosis | |||||
| Absent | 8 | 62 (17-88) | 8 (100) | ||
| Present | 24 | 83 (59-94) | .20 | 9 (38) | .003 |
| Vascular damage | |||||
| Absent | 28 | 86 (63-95) | 13 (46) | ||
| Present | 4 | 25 (0-73) | .084 | 4 (100) | .10 |
| Abrasions | |||||
| Absent | 21 | 90 (61-98) | 8 (38) | ||
| Present | 11 | 55 (20-80) | .16 | 9 (82) | .028 |
| Albumin | |||||
| > 30 g/L | 24 | 87 (63-96) | 7 (29) | ||
| ≤ 30 g/L | 23 | 61 (35-79) | .006 | 19 (83) | .0004 |
| GI-GVHD stage | |||||
| 1 | 23 | 87 (61-96) | 7 (30) | ||
| 2 | 14 | 79 (40-94) | 8 (57) | .004 | |
| 3 | 14 | 57 (26-79) | .15 | 12 (86) | |
| WHO PS | |||||
| 1-2 | 36 | 86 (68-94) | 15 (42) | ||
| 3-4 | 15 | 53 (20-78) | .028 | 12 (80) | .016 |
GI indicates gastrointestinal; CR, complete response; CI, confidence interval; SR, steroid resistant; N, number of evaluated patients; and PS, performance status.
The model selection procedure identified a model with both α1-AT–calprotectin and initial GI-GVHD stage 3 as providing the best prediction of SR-GVHD, although marginal testing did not yield a significant odds ratio for α1-AT (model 3 in Table 4). To measure the advantage of the fecal markers in predicting SR-GVHD, we compared 3 multiple models and their c index for discrimination: model 1 with initial GI-GVHD stages only, model 2 with fecal markers only, and the complete model 3 with GI-GVHD stages and fecal markers (Table 4). Model 3 with calprotectin and α1-AT plus GI-GVHD fitted the data much better than model 1 (P = .0007). Both the c index and the corrected c index were higher and the NRI was 0.85 (95% confidence interval [CI], 0.11-1.19), indicating a significantly better (P = .001) prediction of SR-GVHD when adding the fecal biomarkers to the initial stage of GI-GVHD. Model 2, excluding the initial stage of GI-GVHD, was built to know whether fecal markers could be predictive for SR-GVHD, in the absence of the initial stage of GI-GVHD (hypothesizing that GI-GVHD stage would not be available for any reasons). Again, model 2 better fitted the data than model 1, indicating a better prediction of SR-GVHD with both these markers than with the initial stage of GI-GVHD only. When data concerning the initial stage of GI-GVHD were available, model 3 had the best c index but the corrected c index was no better than model 2, indicating that while the prediction is better when the physician knows the clinical stage and fecal markers in the original sample, it is also less robust than that obtained with fecal markers alone (Table 4). When using calprotectin only, the c index and corrected c index were 0.757 and 0.761, respectively. The NRI of model 2 compared with a model with calprotectin only was 0.96 (95% CI, 0.30-1.94) indicating a markedly better prediction (P = .021).
Multiple regression models on prediction of steroid-refractory GVHD
| . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Gut GVHD stage 1 | 1 | 1 | ||||
| Gut GVHD stage 2 | 3.06 (0.77-12.1) | .11 | 1.28 (0.22-7.52) | .78 | ||
| Gut GVHD stage 3 | 13.7 (2.41-78.2) | .003 | 6.88 (0.95-49.5) | .056 | ||
| Calprotectin ≥ 100 μg/g | 16.1 (1.76-147.6) | .014 | 16.8 (1.70-165.3) | .016 | ||
| α1-AT ≥ 1.5 mg/g dw | 4.10 (0.98-17.1) | .053 | 2.84 (0.60-13.4) | .19 | ||
| Model LR | 11.65 | 21.95 | 26.22*† | |||
| c index | 0.747 | 0.833 | 0.863 | |||
| Corrected c index | 0.733 | 0.827 | 0.826 | |||
| . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Gut GVHD stage 1 | 1 | 1 | ||||
| Gut GVHD stage 2 | 3.06 (0.77-12.1) | .11 | 1.28 (0.22-7.52) | .78 | ||
| Gut GVHD stage 3 | 13.7 (2.41-78.2) | .003 | 6.88 (0.95-49.5) | .056 | ||
| Calprotectin ≥ 100 μg/g | 16.1 (1.76-147.6) | .014 | 16.8 (1.70-165.3) | .016 | ||
| α1-AT ≥ 1.5 mg/g dw | 4.10 (0.98-17.1) | .053 | 2.84 (0.60-13.4) | .19 | ||
| Model LR | 11.65 | 21.95 | 26.22*† | |||
| c index | 0.747 | 0.833 | 0.863 | |||
| Corrected c index | 0.733 | 0.827 | 0.826 | |||
OR indicates odds ratio; CI, confidence interval; AT, antitrypsin; LR, likelihood ratio; and dw, dry weight.
P = .0007 vs model 1.
P = .12 vs model 2.
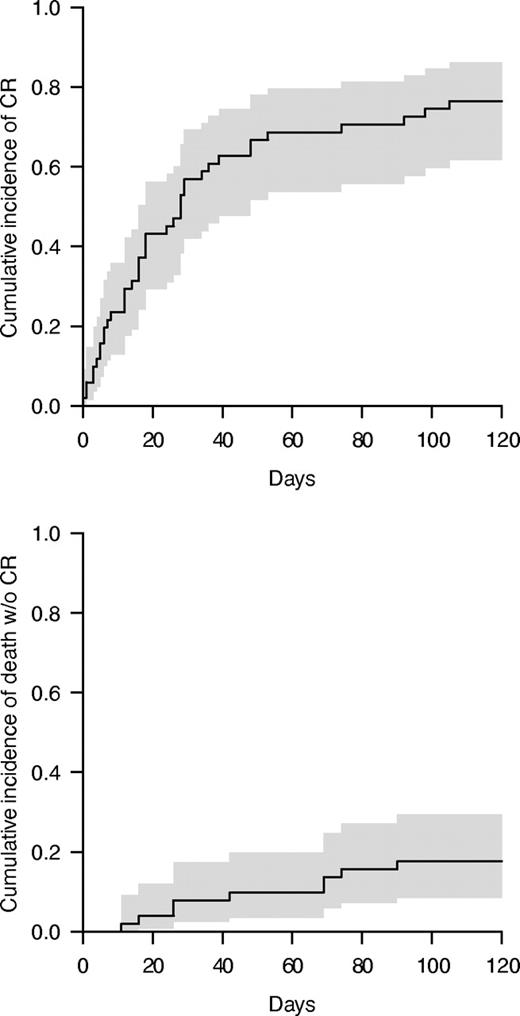
Among the 51 patients with GI-GVHD, 39 achieved a CR 4 months after GVHD was diagnosed, including some patients diagnosed with SR-GVHD during the first 14 days of treatment. Cumulative incidence of CR at 4 months was 76% (95% CI, 62-86; Figure 2). A significant association was observed between both calprotectin and α1-AT concentration and the probability of achieving a CR to steroids. More specifically, patients with a calprotectin concentration > 100 μg/g stool at the time of first symptoms had a significantly lower cumulative incidence of CR (56% vs 86%, P = .005). Similarly, a concentration of α1-AT ≥ 1.5 mg/g dry stool was also associated with a lower cumulative incidence of CR (90% [95% CI, 59-98] vs 67% [95% CI, 46-81]), P = .00007). In contrast, nonsignificant association was observed between fecal elastase concentration and CR. Other parameters affecting the probability of CR were albuminemia ≤ 30 g/L at the onset of GI symptoms (87% vs 61%, P = .006) and WHO performance status (1-2: 86% vs 3-4: 53%, P = .028). There was a nonsignificant higher CR rate in patients with no histologic vascular damage (86% vs 25%, P = .084) or epithelial abrasions (90% vs 55%, P = .16).
Cumulative incidence of CR in patients with GI-GVHD. The curve is represented in gray with a 95% CI. The curve on the top represents the cumulative incidence of response and the down curve represents the competing risk (death).
Cumulative incidence of CR in patients with GI-GVHD. The curve is represented in gray with a 95% CI. The curve on the top represents the cumulative incidence of response and the down curve represents the competing risk (death).
Multiple regression modeling identified a calprotectin concentration ≥ 100μg/g stools and an α1-AT concentration ≥ 1.5mg/g (dry weight) as being independently associated with a lower probability of CR (hazard ratio [HR], 0.47 [95% CI, 0.24-0.92, P = .028] and 0.47 [95% CI, 0.23-0.96, P = .037], respectively).
Giving calprotectin and α1-AT the same weighting, the probability of SR-GVHD and the cumulative incidence of CR by GI-GVHD initial stage were also analyzed (Table 5). When patients with initial stage 1 to 3 had both normal markers, the cumulative incidence of CR and probability of SR-GVHD were 90% and 17%, respectively. In contrast, when both markers were increased, the cumulative incidence of CR and probability of SR-GVHD were 53% and 93%, respectively. This prediction does not appear to be similar among patients with GI-GVHD initial stage 1, 2, or 3. For stage 1, fecal markers were not predictive for CR, whereas the rate of SR-GVHD increased with the number of abnormal markers (21%, 43%, and 50%). For stage 2 and 3, the cumulative incidence of CR declined with the number of abnormal fecal markers, whereas the SR-GVHD rate increased without reaching significance for stage 3 (details are shown in Table 5).
Rates of SR and cumulative incidence of CR by the initial stage of GI GVHD
| Marker . | Stage 1 . | Stage 2 . | Stage 3 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | CR, % . | SR, % . | N . | CR, % . | SR, % . | N . | CR, % . | SR, % . | |
| All patients | 23 | 87 | 30 | 14 | 79 | 57 | 14 | 57 | 86 |
| Calprotectin, μg/g stool | |||||||||
| < 100 | 20 | 85 | 25 | 8 | 88 | 25 | 7 | 86 | 71 |
| ≥ 100 | 3 | 100 | 67 | 6 | 67 | 100 | 7 | 29 | 100 |
| P | .25 | .21 | .27 | .021 | .022 | .46 | |||
| α1-AT, mg/g dw | |||||||||
| < 1.5 | 15 | 87 | 27 | 4 | 100 | 0 | 2 | 100 | 50 |
| ≥ 1.5 | 8 | 88 | 38 | 10 | 70 | 80 | 12 | 50 | 92 |
| P | .25 | .66 | .001 | .015 | .23 | .27 | |||
| No. of positive markers | |||||||||
| 0 | 14 | 86 | 21 | 4 | 100 | 0 | 2 | 100 | 50 |
| 1 | 7 | 86 | 43 | 4 | 75 | 50 | 5 | 80 | 80 |
| 2 | 2 | 100 | 50 | 6 | 67 | 100 | 7 | 29 | 100 |
| P | .49 | .40 | .005 | .004 | .068 | .23 | |||
| Marker . | Stage 1 . | Stage 2 . | Stage 3 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N . | CR, % . | SR, % . | N . | CR, % . | SR, % . | N . | CR, % . | SR, % . | |
| All patients | 23 | 87 | 30 | 14 | 79 | 57 | 14 | 57 | 86 |
| Calprotectin, μg/g stool | |||||||||
| < 100 | 20 | 85 | 25 | 8 | 88 | 25 | 7 | 86 | 71 |
| ≥ 100 | 3 | 100 | 67 | 6 | 67 | 100 | 7 | 29 | 100 |
| P | .25 | .21 | .27 | .021 | .022 | .46 | |||
| α1-AT, mg/g dw | |||||||||
| < 1.5 | 15 | 87 | 27 | 4 | 100 | 0 | 2 | 100 | 50 |
| ≥ 1.5 | 8 | 88 | 38 | 10 | 70 | 80 | 12 | 50 | 92 |
| P | .25 | .66 | .001 | .015 | .23 | .27 | |||
| No. of positive markers | |||||||||
| 0 | 14 | 86 | 21 | 4 | 100 | 0 | 2 | 100 | 50 |
| 1 | 7 | 86 | 43 | 4 | 75 | 50 | 5 | 80 | 80 |
| 2 | 2 | 100 | 50 | 6 | 67 | 100 | 7 | 29 | 100 |
| P | .49 | .40 | .005 | .004 | .068 | .23 | |||
Rates of SR are compared with Fisher exact tests and cumulative incidences of CR by Gray tests.
SR indicates stereo resistant; CR, complete response; GI, gastrointestinal; AT, antitrypsin; and dw, dry weight.
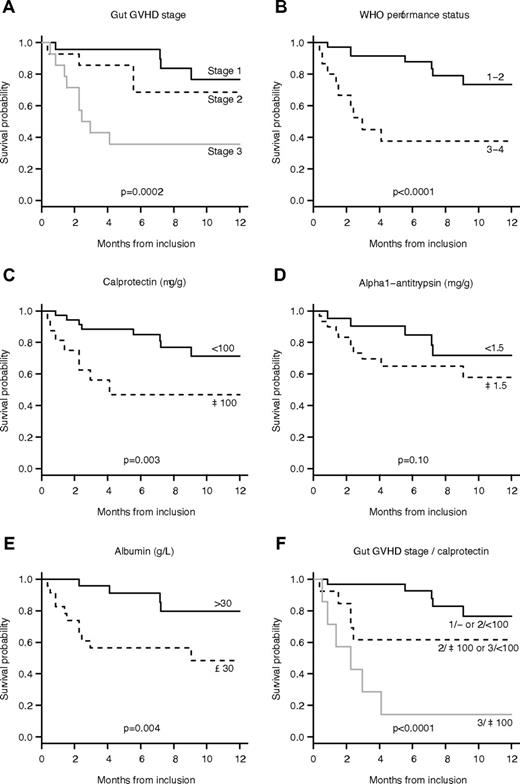
OS in our series was 78% (95% CI, 68-91) at 3 months and 64% (95% CI, 50-81) at 12 months (Figure 3). Initial GVHD stage (HR 4.20 per one stage increase; 95% CI, 1.82-9.71, P = .0002), concentrations of calprotectin (HR 4.78 when 100 μg/g or more; 95% CI, 1.55-14.8, P = .003), and elastase (HR 3.21 when > 200 μg/g; 95% CI, 1.00-10.4, P = .040), albumin (HR 6.79 when 30 g/L or more; 95% CI, 1.48-31.1, P = .004), and WHO performance status (HR 7.70 when 3 or 4; 95% CI, 2.36-25.2, P < .0001) were associated with 6-month survival. A multiple model was built including the initial stage of GI-GVHD and the level of calprotectin. To analyze the respective yield of both variables, interactions were integrated to the model and patients with similar prognoses were regrouped (supplemental Table 2). We ended up with a model with only 3 categories: stage 1 or stage 2 with normal calprotectin, stage 2 with elevated calprotectin, or stage 3 with normal calprotectin, and stage 3 with elevated calprotectin, which had improved fit and discriminative ability compared with the other models. OS according to these groups are shown in Figure 3.
OS of the 51 patients with GI-GVHD. (A) The OS rate by GI-GVHD stage. (B) The OS rate by WHO performance status staging. (C) The OS rate by the concentration of calprotectin. (D) The OS rate by the concentration of α1-AT concentration. (E) The survival rate by the concentration of albumin. (F) The survival rate by GI-GVHD stage and calprotectin levels with 3 groups: (1) stage 1 and stage 2 with a normal concentration of calprotectin; (2) stage 2 with a high concentration of calprotectin and stage 3 with a normal concentration of calprotectin; (3) stage 3 with a high concentration of calprotectin.
OS of the 51 patients with GI-GVHD. (A) The OS rate by GI-GVHD stage. (B) The OS rate by WHO performance status staging. (C) The OS rate by the concentration of calprotectin. (D) The OS rate by the concentration of α1-AT concentration. (E) The survival rate by the concentration of albumin. (F) The survival rate by GI-GVHD stage and calprotectin levels with 3 groups: (1) stage 1 and stage 2 with a normal concentration of calprotectin; (2) stage 2 with a high concentration of calprotectin and stage 3 with a normal concentration of calprotectin; (3) stage 3 with a high concentration of calprotectin.
Discussion
This study evaluates the clinical implication of 3 fecal proteins, already routinely used in some digestive diseases, in the setting of HSCT: calprotectin, α1-AT, and elastase, markers, respectively for intestinal inflammation, protein-losing enteropathy and exocrine pancreatic dysfunction. In patients with GI-GVHD, abnormal levels of all 3 of these markers were observed in the current study. However, the major advantage of these noninvasive markers was not their capability to diagnose GI-GVHD but rather their ability to predict the response to steroids, a major prognostic factor for these patients.
In our study, patients with GI-GVHD often had a high level of calprotectin with a cutoff at 100 μg/g (cutoff at 50 μg/g did not increase sensitivity [data not shown]). Stage 2-3 GI-GVHD at the time of diagnosis correlated with higher calprotectin levels, but sensitivity for GI-GVHD diagnosis, especially for stage 1, was not good. This observation is easily understandable because a typical GI infection or inflammation of any origin can increase calprotectin.
Similar to the level of calprotectin, the concentration of α1-AT was near normal in patients with initial stage 1 GI-GVHD and was more frequently elevated in patients with initial stage 2 or higher GI-GVHD, confirming the findings of Weisdorf et al14 and Hagen et al.27
Interpretation of elastase levels should be moderated by its interaction with gut disease. Although fecal elastase is a marker of exocrine pancreatic dysfunction, pancreatic secretion is impaired not only in patients with primary pancreatic insufficiency but also in those with a decreased production of cholecystokinin (the predominant hormonal regulator of postprandial pancreatic enzyme secretion). As such, a low concentration of elastase can reflect either primary pancreatic insufficiency and/or the lack of pancreatic enzyme stimulation in patients with transient intestinal damage (as revealed by abnormal calprotectin and/or α1-AT), regardless of the cause.28 Consequently, elastase levels may be interpreted only in patients with normal concentrations of calprotectin and α1-AT, who represent a minority of patients. For this reason, fecal elastase did not appear to be a reliable marker. Other serum biomarkers have been reported to show a remarkable correlation with GVHD initial stages and response to immunosuppressive treatment. Serum cytokeratin-18 fragments, a surrogate marker of epithelial apoptosis, is associated with the initial stage of GVHD and SR-GVHD.10,29 When the kinetic measure of cytokeratin-18 fragments was performed, the values increased with GVHD stages and decreased at GVHD resolution (steroid withdrawal). Similar observations have been reported in the same study for thrombomodulin, Fas ligand, and angiopoietin-2 levels and angiopoietin-2/VEGF ratio. As our study focused on the value of markers at diagnosis, no kinetic measure was performed and the correlation between the markers and different time points was not examined.
To conclude on the correlation between GI-GVHD and fecal markers at the time of first symptoms, concentrations of α1-AT or calprotectin gradually increase with GI-GVHD initial stages but are not sensitive markers for GI-GVHD, especially for initial stage 1.
The second part of our analyses sought to discover whether these markers can predict the severity of GI-GVHD when they are measured at the onset of symptoms. Two parameters concerning response to first-line corticosteroids were analyzed: achievement of a CR at 4 months and diagnosis of a SR-GVHD. Univariate analysis showed that fecal calprotectin or α1-AT levels are predictive for SR-GVHD and GI-GVHD CR, as are the initial stage of GI-GVHD, performance status, albuminemia, epithelial gut abrasions, and vascular damage on histology, as already reported by others.7,30
Recently, another biomarker, the angiopoietin-2 in serum, has also been reported to predict SR-GVHD. Luft et al have also demonstrated a statistical correlation between the level of angiopoietin-2 in serum before transplantation and the evolution to an SR-GVHD.10
One of the aims of this study was to test the benefits of measuring fecal markers over other prognostic markers. The statistical analysis shows that a multiple model including the levels of calprotectin and α1-AT (with or without GI-GVHD stage) was more robust and discriminating than a model including only the stage of GI-GVHD, demonstrating that these markers appear to be at least as good a predictor as the initial stage of GI-GVHD. In clinical practice, this finding has a major impact because the staging of GI-GVHD based on the quantification of stools sometimes remains uncertain for many reasons: outpatients, mixtures of urine and stools, loss of stools, and so on. In such cases (where the stage of GI-GVHD is unknown or uncertain), fecal markers can give similar or even better information concerning the response to steroids.
Histology is also a reliable marker for diagnosis and prognosis and cannot be replaced by fecal markers.30 Nevertheless, we observed in our study that 37% of patients did not benefit from an endoscopy because of medical contraindications or patient refusal. In patients without histology, fecal markers which can easily be obtained from all patients may also predict the severity of GI-GVHD, even though they are nonspecific markers, unable to discriminate GVHD from other causes (of diarrhea) such as infectious gastroenteritis. Furthermore, these markers are immediately available in the majority of hospitals because they are regularly used in other digestive diseases.
We also examined the accuracy of calprotectin and α1-AT prediction by GI-GVHD stage at the time of diagnosis. For stage 3, the probability of SR-GVHD gradually increased with the number of abnormal fecal markers without reaching significance; more patients are needed to reach this significance. In contrast, the cumulative incidence of CR significantly decreases with the number of abnormal fecal markers in stage 3 but not in stage 1. Our hypothesis to interpret these results is that patients with stage 1, even with a SR-GVHD, finally obtained a CR, which was evaluated 4 months after the observation of the first symptoms. Interestingly, the prediction of fecal markers was particularly true in patients with stage 2 GI-GVHD: the probability of SR-GVHD was 100% if the 2 markers were high and 0% if the 2 markers were low. In clinical practice, patients with initial stage 2 GI-GVHD can evolve to a higher stage or become SR. The prediction of SR-GVHD in patients with initial stage 2 GI-GVHD can consequently be very useful for the management of the immunosuppressive treatment.
Finally, as expected because of the known poor outcome of patients with SR-GVHD, OS was poorer in patients presenting higher levels of calprotectin. Similar observations have been reported with other biomarker predicting SR-GVHD, that is, the level of angiopoietin-2/VEGF ratio and low levels of Fas ligand, which predict higher nonrelapse mortality.10 In our study, a multiple model was built including the initial stage of GI-GVHD and calprotectin level showing that patients with stage 2 GI-GVHD can be split into 2 groups: (1) patients with stage 2 and normal levels of calprotectin have similar survival rates to those with stage 1; and (2) patients with stage 2 and high levels of calprotectin have similar survival rates to those with stage 3 and normal calprotectin levels. The results confirm that these fecal markers are particularly useful in patients with stage 2 GI-GVHD, allowing patients with the worst prognoses to be identified, but because of the limited sample size, this model requires further validation.
We conclude that calprotectin and α1-AT may be considered to be new noninvasive fecal markers of severe GI-GVHD. These markers seem remarkable by their very early modification in GI-GVHD history, allowing a prognosis to be predicted when the first symptoms are observed. Further studies are now needed to confirm and test these markers among larger populations and to confirm whether they can replace and/or complement other well-known markers such as GVHD stage, hypoalbuminemia, or histology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Association “Centpoursanglavie.”
This work was supported by Association “Centpoursanglavie,” which provided financial support. P.R.-O. received funding from the Fundación Alfonso Martin Escudero (Spain).
Authorship
Contribution: P.R.-O., R.P., M.C., and M.R. collected the data, conducted the statistical analysis, and edited the manuscript; P.R.-O., R.P.d.L., Y.B., A.X., A.A., P.R., and M.R. collected the data and edited the manuscript; A.J. was in charge of histology; N.K. performed functional coprology of patients; and G.S. and M.R. designed the study, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Gérard Socié, Hematology/Transplantation, Hospital Saint Louis, 1 Ave Claude Vellefaux, 75475, Paris CEDEX 10, France; e-mail: gerard.socie@paris7.jussieu.fr.
References
Author notes
G.S. and M.R. contributed equally and should be considered as senior authors.