Abstract
FoxP3+ confers suppressive properties and is confined to regulatory T cells (Treg) that potently inhibit autoreactive immune responses. In the transplant setting, natural CD4+ Treg are critical in controlling alloreactivity and the establishment of tolerance. We now identify an important CD8+ population of FoxP3+ Treg that convert from CD8+ conventional donor T cells after allogeneic but not syngeneic bone marrow transplantation. These CD8+ Treg undergo conversion in the mesenteric lymph nodes under the influence of recipient dendritic cells and TGF-β. Importantly, this population is as important for protection from GVHD as the well-studied natural CD4+FoxP3+ population and is more potent in exerting class I–restricted and antigen-specific suppression in vitro and in vivo. Critically, CD8+FoxP3+ Treg are exquisitely sensitive to inhibition by cyclosporine but can be massively and specifically expanded in vivo to prevent GVHD by coadministering rapamycin and IL-2 antibody complexes. CD8+FoxP3+ Treg thus represent a new regulatory population with considerable potential to preferentially subvert MHC class I–restricted T-cell responses after bone marrow transplantation.
Introduction
The development of GVHD remains a major limitation to the therapeutic potential of bone marrow transplantation (BMT). GVHD occurs as a result of immunologic damage to the host tissue by conventional T and NK cells in the transplanted donor graft. Natural regulatory T cells (Treg) are defined by the expression of FoxP31 and are dedicated to suppression of immune responses. The importance of CD4+FoxP3+Treg in peripheral tolerance is well established, and the presence of naturally occurring CD4+Treg makes them an attractive target for adoptive transfer to control pathologic immune responses. Consequently, studies in GVHD using freshly isolated or ex vivo expanded donor CD4+Treg have demonstrated a delay or prevention of GVHD.2-5 Conversely, depletion of CD4+Treg from the donor graft results in an increased severity of GVHD.2 A limitation to using naive Treg in the adoptive transfer setting is the high ratio required compared with FoxP3− cells in the ingoing graft (1:1); thus, the number of Treg required is restrictive. This has resulted in difficulties transferring adoptive cell therapy into the clinic with multiple groups now focusing on ex vivo expansion protocols in an endeavor to overcome this limitation.6-8
In contrast to the extensive research being completed on naive CD4+Treg, studies investigating the role of Treg undergoing conversion in the periphery after BMT are lacking. The identification of FoxP3 as a Treg marker1 and subsequent development of reagents that allow for identification (B6.FoxP3-GFP),9 tracking (B6.FoxP3.LuciDTR)10 and depletion (B6.DEREG)11 of these cells has allowed for a more thorough investigation. We have used these reagents to characterize Treg populations after transplantation with the aim of identifying more potent and antigen-specific Treg that suppress GVHD at low numbers after BMT. In conjunction with the classic CD4+FoxP3+Treg population, we identified a novel CD8+FoxP3+Treg population that expands in the presence of an alloreactive response and is previously unrecognized after BMT. Importantly, this population displays preferential tropism for the gastrointestinal (GI) tract and inhibits class I–restricted immune responses. Furthermore, rapamycin (RAPA) and IL-2 antibody (Ab) complex treatment after transplantation increases the percentage of FoxP3-expressing cells within the CD8+ population to more than 30%. These cells thus represent a novel regulatory population highly suited to manipulation for the control of deleterious alloimmune responses after transplantation.
Methods
Mice
Female C57BL/6 (B6.WT, H-2b,CD45.2+), B6.SJL-Ptprca (PTPrca, H-2b,CD45.1+), BALB/c (H-2d, CD45.2+), or B6D2F1 (H-2b/d,CD45.2+) mice were purchased from the Animal Resource Center. B6.FoxP3-GFP,9 B6.DEREG,11 B6.C-H2bm1 (bm1), FoxP3.LuciDTR-410 (B6.FoxP3-luc+), B6.MHC class II-GFPxDBA2F1, B6.β2m−/−, B6.β-actin-luc+, C57BL/6J.VavP-Bcl212 (B6.Bcl-2), and B6.CD11c-DOGxDBA2F113 mice were supplied by the Queensland Institute of Medical Research animal facility. C3H.SW were purchased from The Jackson Laboratory and subsequently bred at Queensland Institute of Medical Research animal facility. The age of mice used ranged between 8 and 14 weeks. Mice were housed in microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation.
Antibodies
The following antibodies were purchased from BioLegend: phycoerythrin (PE)–conjugated anti-H2Db(KH95), CD80(16/10A1), CD86(GL-1), CD40(1C10), CD45.1(A20), α4β7 (DATK32), CD127(SB/199), CD62L(MEL-14), anti–IL-10(JES5-16E3), anti–IFN-γ(XM61.2), allophycocyanin-conjugated CD8α(53-6.7), CD4(RM4-5), anti-GITR(YGITR765), PE-Cy7–conjugated CD45.1(A20), biotin-conjugated anti-H2Dd(34-2-12), anti–CTLA-4(UC10-4B9), Pacific blue–conjugated anti-helios(22F6), Hamster anti-IgG(HTK888), and Alexa Flour-647 anti-FoxP3(150D). The following antibodies were purchased from BD Biosciences: PE-conjugated CD4(GK1.5), CD25(7D4), CD103(M290), CXCR5(2G8), CD54(3E2), PE-Cy7–conjugated CD8(53-6.7) and biotin-conjugated CD28(37.51). PE-conjugated anti-GARP(YG1C86) and IL-17(18F10) were purchased from eBioscience, and allophycocyanin-conjugated anti-Granzyme B(MHGB05) was purchased from Invitrogen. Anti–TGF-β14 (1D11), anti–IL-10R(1B1.3a), CD3(2c11), and anti-NK1.115 (PK136) were produced in house. Briefly, hybridoma cell culture supernatants were harvested and products precipitated using ammonium sulfate fractionation.16 Antibody was selected using GammaBind G Sepharose (Amersham Biosciences), dialysed with PBS using Membra-cel MD25 membrane, and concentrated using Amicon Ultra centrifugal filters (Millipore). Intracellular cytokine staining was performed using the FoxP3 cytofix/cytoperm kit (BioLegend) as per the manufacturer's instructions.
Cell preparation
T-cell depletion of BM was performed as previously described,17 and resulting cell suspensions contained less than 1% contamination of CD3+ T cells. T cells were purified using magnetic bead depletion as previously described,17 and subsequent CD3+ T-cell purities were greater than 80%. For in vitro assays, cell populations were purified using magnetic activated cells sorting (MACS) beads (CD4, CD8, or CD11c) and positive selection columns according to the manufacturer's instructions (Miltenyi Biotec) and then sorted on the basis of CD4+, CD8+, or MHC class II GFP and CD11c for dendritic cell (DC) isolation on Moflo cell sorter (Beckman Coulter) to more than 95% purity.
BMT
On day −1, mice received 1100 cGy (B6D2F1), 1000 cGy (B6 and bm1), or 900 cGy (BALB/c and C3H.SW) total body irradiation (137Cs source at 108 cGy/min) split into 2 doses separated by 3 hours. On day 0, recipients were transplanted with 107 BM cells with or without purified T cells from B6.FoxP3-GFP donors (2 × 106 T cells for B6D2F1 recipients, 5 × 106 T cells for C3H.SW recipients, or 0.5 × 106 T cells for BALB/c recipients). To examine the effect of irradiation on CD8+FoxP3+ Treg development, irradiated B6D2F1 recipients received 107 unfractionated B6.FoxP3-GFP splenocytes on day 0, and a nonirradiated B6D2F1 cohort received 1 mg anti-NK1.1 at day −2 and then 108 unfractionated B6.FoxP3-GFP splenocytes on day 0. For in vivo Treg tracking and depletion experiments, irradiated B6D2F1 recipients received B6.WT BM supplemented with 0.5 × 106 each of sort purified CD4 and CD8 donor T cells from B6.WT, B6.FoxP3-luc+ (tracking), or DEREG (depletion) donors, or combinations thereof, as described in the figure legends. For depletion experiments, DT (100 ηg intraperitoneally; Sigma-Aldrich) commenced on day 4 and was administered thrice weekly for the duration of the experiment. To determine the cellular origin of CD8 Treg, irradiated B6D2F1 recipients received B6.WT BM and 1 × 106 unfractionated CD3+ T cells from CD45.1+ B6.Ptprca donors supplemented with 1 × 106 unfractionated CD3+ T, 1 × 106 sort purified CD4+GFP−, 2 × 105 sort purified CD4+GFP+, or 1 × 106 sort purified CD8+GFP− T cells from B6.FoxP3-GFP donors. For depletion of host DCs, irradiated B6D2F1 and B6.CD11c.DOGxDBA2F1 transplant recipients received daily injections (day −2 to day 3) of saline or DT (160 ηg intraperitoneally; Sigma-Aldrich), respectively. For TGF-β and IL-10R blocking, recipients received control mAb (Mac49; 500 μg), anti-TGF (1D11; 500 μg), or anti–IL-10R (1B1.3a; 500 μg) on day 0 and day 2 of transplantation and donor FoxP3+ Treg populations analyzed at day 4. For in vivo expansion experiments, intraperitoneal injections of RAPA (1.5 mg/kg; Wyeth) were administered daily starting from day 0 as previously described.18 Recombinant murine IL-2 (rmIL-2, 1.5 μg; eBioscience) was incubated for 30 minutes with the antibody JES6-1A12 (50 μg) to form IL-2 Ab complexes and injected intraperitoneally on day 0 and day 4 of transplantation. For experiments that compared cyclosporine to RAPA treatment, cyclosporine (50 mg/kg; Novartis) was administered intraperitoneally daily as previously described.19
Assessment of GVHD
The degree of systemic GVHD was assessed using a cumulative scoring system that measures changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index 10). Mice were monitored daily, and those with GVHD clinical scores20 greater than or equal to 6 were culled and the date of death recorded as the next day in accordance with institutional animal ethics guidelines.
In vivo luminescence imaging
Recipients were injected with luciferin (0.5 mg) subcutaneously. Five minutes later, organs were harvested and imaged using the Xenogen imaging system (Xenogen IVIS 100; Caliper Life Sciences) to determine Treg trafficking. Data were analyzed with Living Image Version 4 software (Xenogen).
Cell labeling for proliferation analysis
For T-cell proliferation assays, purified T cells were suspended at 3 × 107 cells/mL in serum-free media and CFSE (Sigma-Aldrich) or Cell Trace violet proliferation dye (Invitrogen) added at 1 or 5μM final concentration, respectively. After incubation at 37°C for 10 to 20 minutes, cells were washed in media containing 2% FCS. Dye dilution was analyzed using a FacsCanto or FACSCantoII (BD Biosciences).
T-cell suppression assays
For nonspecific suppression assays, CFSE labeled BioMag purified B6.PTPrca T cells were cultured at 50 000/well with either MACS-purified B6D2F1 or WT.B6 DCs (5000/well) and CD3 (1 μg/mL). For antigen-specific suppression assays, CFSE labeled sort-purified B6.Ptprca CD4+ or CD8+ T cells were cultured at 50 000/well with either MACS purified DBA/2 or C3H.Hej DC (5000/well). B6.FoxP3-GFP+ Treg cells (10 000/well or as indicated in the figure legends) were sort purified from spleen, peripheral lymph node (pLN), and mesenteric lymph node (mLN) from B6D2F1 recipients of B6.Foxp3-GFP BM and T cell grafts at day 7 after transplantation. Recipients received RAPA and IL-2 Ab complex treatment in some experiments. Tissue culture supernatants were collected for cytokine analysis, and CFSE dilution of the B6.Ptprca T cells was assessed at 72 hours of culture for BioMag T responders, between 72 and 96 hours for purified CD8+ responders and 120 hours for CD4+ responders. For transwell suppression assays, transwell permeable supports (Corning Life Sciences) were used according to the manufacturer's protocol. Briefly, B6.Ptprca CD4 T cells (300 000) and DBA/2 DC (30 000) were cultured in the bottom of the plates, regulatory T cells (30 000) were added to either the bottom or the top of the wells, and the cultures processed as described earlier in this paragraph.
To investigate DC costimulatory molecule expression, B6.MHC class II-GFPxDBA2F1 mice were used and DCs sorted based on GFP and CD11c expression. B6.Ptprca sort purified CD4+ T cells (300 000) were cultured with 30 000 CD11c+GFP+ DCs, with or without 30 000 sort purified after transplantation FoxP3+Treg. After 72-hour culture, CD11c+GFP+ DCs were gated and assessed for costimulatory marker expression. To assess the ability of CD8+FoxP3+ Treg to kill DCs or responding effector T cells, CD4 T cells (50 000; sort purified to > 98%) and DCs (5000; MACS purified) from B6.WT, B6.b2m−/−, or B6.Bcl-2 mice were cultured with CD3 (1 μg/mL) and 25 000 CD8+FoxP3+ or CD4+FoxP3+ Treg that were isolated as described for nonspecific suppression assays.
Histopathology of GVHD target organs
At day 7 after transplantation, small bowel was harvested, formalin-preserved, embedded in paraffin, and processed to generate 5-μm-thick sections. H&E-stained sections of colon, small intestine, lung, and liver were examined in a blinded fashion (by A.D.C.) using a semiquantitative scoring system for GVHD as previously published.21 Images were acquired using an Olympus BX51 microscope (Olympus) and Evolution MP Version 5.0 Camera and QCapture software (QImaging).
Statistical analysis
Column graphs shown represent mean with error bars demonstrating the SEM. Statistical significance was determined using 2-tailed Mann-Whitney U tests with a P value cut-off of .05. Survival curves were generated as Kaplan-Meier estimates and compared by log-rank analysis. All statistical analyses were performed using Prism Version 5 software (GraphPad).
Results
Induction of a CD8+FoxP3+ population after allogeneic BMT
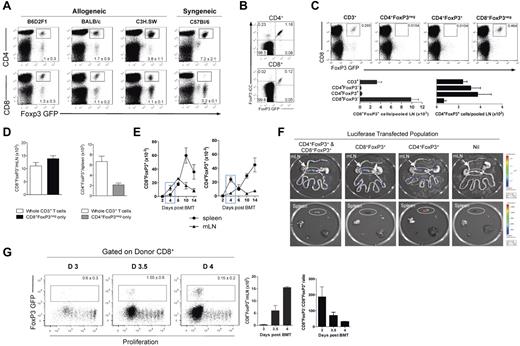
To investigate Treg populations after transplantation, we analyzed FoxP3.GFP expression in the spleen, mLN, and pLN at day 12 after BMT. We examined multiple different models of GVHD to MHC and/or multiple minor histocompatibility antigens (B6.FoxP3-GFP → B6D2F1, B6.FoxP3-GFP → BALB/c, B6.FoxP3-GFP → C3H.SW) and compared this with that in syngeneic recipients (B6.FoxP3-GFP → B6.WT). After allogeneic transplantation, a CD4+Foxp3+ Treg population was evident in all sites examined. Surprisingly, we also observed a CD8+Foxp3+ population in these BMT recipients (Figure 1A). Importantly, whereas CD4+Foxp3+ Treg were also evident after syngeneic transplantation, the CD8+Foxp3+ population was only seen after allogeneic BMT (Figure 1A). Thus, an alloreactive T-cell response appeared to be required for the generation of this cell population. To confirm GFP expression as a true representation of FoxP3 expression, intracellular staining of FoxP3 was assessed on splenocytes isolated at day 5 after BMT. Results indicated that all GFP-expressing CD4+ and CD8+ T cells coexpressed FoxP3 (Figure 1B). To determine the origin of the CD8+FoxP3+population, transplantations were performed where all recipients received a baseline graft containing B6 BM and T cells to induce GVHD, and in addition, sort-purified T-cell populations of B6.FoxP3-GFP transgenic cells (whole CD3+, CD4+FoxP3−, CD4+FoxP3+, or CD8+FoxP3− only). When FoxP3+ populations were analyzed after transplantation, CD8+FoxP3+ cells were observed only in those groups that received whole CD3+ or CD8+FoxP3− cells originating from transgenic B6.FoxP3-GFP donors. Thus, a population of T cells covert to FoxP3+ from the ingoing CD8+FoxP3− population (Figure 1C). To specifically compare the contribution of conversion of CD4+FoxP3+, recipients received either whole CD3+ T cells containing CD8+FoxP3−, CD4+FoxP3−, and CD4+FoxP3+ fractions, or either CD4+FoxP3− or CD8+FoxP3− cells only with numbers matched to that within the whole CD3+ graft. Results indicated that, within the spleen, conversion contributes to approximately 30% of the total CD4+FoxP3+ Treg pool at day 5 compared with 100% for CD8+FoxP3+ (Figure 1D).
CD8+FoxP3+ differentiate after allogeneic transplantation from CD8+FoxP3− in the mLN. (A) After lethal irradiation at day −1, BM and T cells from B6.FoxP3-GFP donors were transplanted into B6D2F1, BALB/c, C3H.SW, or C57Bl6 recipients. At day 12 after transplantation, CD4+FoxP3+ and CD8+FoxP3+ were analyzed in the spleen, pLN, and mLN. Representative plots of total live cells in the pLN are shown. Experiment was performed 3 times (n = 10, 7, 7, 6). (B) After lethal irradiation at day −1, BM and T cells from B6.FoxP3-GFP donors were transplanted into B6D2F1 recipients. Spleens were stained for intracellular FoxP3 at day 5 after transplantation. (C) After lethal irradiation at day −1, BM and T cells were transplanted from PTPrca donors at day 0. Sorted cell populations (CD3+, CD4+FoxP3−, CD4+FoxP3+, or CD8+FoxP3−) from B6.FoxP3-GFP donors were also transplanted with the donor graft. At day 12 after transplantation, CD8+FoxP3+ conversion was analyzed in the spleen and pLN. Representative plots of total live cells from peripheral LN are shown from 2 experiments; n = 6 per group. (D) Recipients were transplanted and analyzed as in panel C; however, CD8+FoxP3− and CD4+FoxP3− were matched to that within the whole CD3+ graft. Experiment was performed twice with results displayed as mean ± SEM; n = 6 per group. (E) At day 0, BM and T cells from B6.FoxP3-GFP donors were transplanted into lethally irradiated B6D2F1 recipients. At time points shown, spleen, and mLN were analyzed for CD4+FoxP3+ and CD8+FoxP3+cells. Combined data from 2 experiments; n = 6 per group. (F) After lethal irradiation on day −1, recipients were transplanted with the following grafts: (1) WT CD4+ T cells and WT CD8+ T cells, (2) WT CD4+ T cells and B6.FoxP3-luc+ CD8+ T cells, (3) B6.FoxP3-luc+ CD4+ T cells and WT CD8+ T cells, or (4) B6.FoxP3-luc+ CD4+ T cells and B6.FoxP3-luc+ CD8+ T cells. Recipients were culled at day 4 after transplantation, 5 minutes after luciferin injection, and the spleen, inguinal LN, liver, thymus, lung, and gut removed and imaged individually. Representative images from 2 experiments are shown (n = 6 per group). (G) Mice were transplanted as in panel E; however, donor T cells were labeled with Cell Trace violet proliferation dye and mLN analyzed for CD8 Treg conversion. Representative plots gated on live donor CD8+ T cells are shown, with numbers in graph showing mean ± SEM.
CD8+FoxP3+ differentiate after allogeneic transplantation from CD8+FoxP3− in the mLN. (A) After lethal irradiation at day −1, BM and T cells from B6.FoxP3-GFP donors were transplanted into B6D2F1, BALB/c, C3H.SW, or C57Bl6 recipients. At day 12 after transplantation, CD4+FoxP3+ and CD8+FoxP3+ were analyzed in the spleen, pLN, and mLN. Representative plots of total live cells in the pLN are shown. Experiment was performed 3 times (n = 10, 7, 7, 6). (B) After lethal irradiation at day −1, BM and T cells from B6.FoxP3-GFP donors were transplanted into B6D2F1 recipients. Spleens were stained for intracellular FoxP3 at day 5 after transplantation. (C) After lethal irradiation at day −1, BM and T cells were transplanted from PTPrca donors at day 0. Sorted cell populations (CD3+, CD4+FoxP3−, CD4+FoxP3+, or CD8+FoxP3−) from B6.FoxP3-GFP donors were also transplanted with the donor graft. At day 12 after transplantation, CD8+FoxP3+ conversion was analyzed in the spleen and pLN. Representative plots of total live cells from peripheral LN are shown from 2 experiments; n = 6 per group. (D) Recipients were transplanted and analyzed as in panel C; however, CD8+FoxP3− and CD4+FoxP3− were matched to that within the whole CD3+ graft. Experiment was performed twice with results displayed as mean ± SEM; n = 6 per group. (E) At day 0, BM and T cells from B6.FoxP3-GFP donors were transplanted into lethally irradiated B6D2F1 recipients. At time points shown, spleen, and mLN were analyzed for CD4+FoxP3+ and CD8+FoxP3+cells. Combined data from 2 experiments; n = 6 per group. (F) After lethal irradiation on day −1, recipients were transplanted with the following grafts: (1) WT CD4+ T cells and WT CD8+ T cells, (2) WT CD4+ T cells and B6.FoxP3-luc+ CD8+ T cells, (3) B6.FoxP3-luc+ CD4+ T cells and WT CD8+ T cells, or (4) B6.FoxP3-luc+ CD4+ T cells and B6.FoxP3-luc+ CD8+ T cells. Recipients were culled at day 4 after transplantation, 5 minutes after luciferin injection, and the spleen, inguinal LN, liver, thymus, lung, and gut removed and imaged individually. Representative images from 2 experiments are shown (n = 6 per group). (G) Mice were transplanted as in panel E; however, donor T cells were labeled with Cell Trace violet proliferation dye and mLN analyzed for CD8 Treg conversion. Representative plots gated on live donor CD8+ T cells are shown, with numbers in graph showing mean ± SEM.
To determine the anatomic sites where CD8+FoxP3+ cells were emerging, time-course experiments were performed. As shown in Figure 1E, CD8+FoxP3+ cells emerge by day 4 after transplantation in the mLN. In contrast, the CD4+FoxP3+ cells predominately emerge within the spleen after BMT (Figure 1E). Importantly, CD8+FoxP3+ remained in higher numbers compared with CD4+FoxP3+ in the mLN during the first 10 days after transplantation. To confirm the mLN as a key site for CD8+FoxP3+ expansion, B6.FoxP3-luc+ donors were used, in which luciferase expression is driven off the FoxP3 promoter. Based on our previous data indicating that all CD8+FoxP3+ cells underwent conversion from CD8+FoxP3− cells in the ingoing graft, we transplanted specific subsets of T cells that resulted in all FoxP3+, CD8+FoxP3+, or CD4+FoxP3+ cells only expressing the luciferase gene. When organs were imaged at day 4 after transplantation, CD8+FoxP3+ were indeed present only in the mLN early after BMT, whereas CD4+FoxP3+ were predominately in the spleen (Figure 1F). To determine which donor CD8+FoxP3− T cells were undergoing conversion into CD8+FoxP3+ T cells, violet dye was used to track proliferation. Interestingly, it was only the most actively proliferating CD8+ T cells that underwent conversion (Figure 1G). This conversion occurred very rapidly, with a 50-fold increase in the absolute number of CD8+FoxP3+ cells in the mLN within a 24-hour period between day 3 and day 4 after transplantation (Figure 1G). This was not simply because of overall T-cell expansion, as the CD8+FoxP3−:CD8+FoxP3+ ratio decreased over time (Figure 1G).
CD8+FoxP3+ cells display a distinct phenotype and potently suppress antigen-specific responses
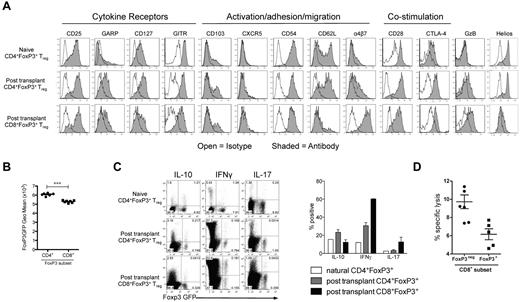
Phenotypic analysis of FoxP3+ cells generated after BMT was performed in the mLN and spleen, with similar results observed regardless of the site. CD8+FoxP3+ displayed reduced expression of GARP, GITR, FoxP3, CD62L, CD28, and CTLA/4 relative to CD4+FoxP3+ cells (Figure 2A-B). Conversely, there was an increase in expression of the integrin α4β7, a molecule critical in homing to the GI tract. As expected, CD8+FoxP3+ Treg did not express helios, a marker of thymic-derived CD4+ Treg (Figure 2A).22 In addition, the CD8+FoxP3+ Treg did not express γ-δ TCR or NK1.1 (data not shown), excluding the possibility they were an unusual gamma-delta, NK, or NKT cell. When cytokine production was investigated, CD8+FoxP3+ Treg produced low levels of IL-10 and IL-17, similar to CD4+FoxP3+ Treg at the same time point (Figure 2C). They produced higher levels of IFN-γ compared with CD4+FoxP3+ Treg, but the ratio of cells producing IFN-γ was similar to that seen within the CD8+FoxP3− compartment (Figure 2C). Donor CD8 T cells are known to be highly cytolytic during GVHD.23-29 However, the CD8+FoxP3+ Treg did not exhibit high levels of cytolytic activity against host targets relative to CD8+FoxP3− effectors (Figure 2D), suggesting that FoxP3 expression and suppression are independent of cytolysis.
CD8+FoxP3+ Treg display a distinct phenotype compared with CD4+FoxP3+ Treg. B6D2F1 recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. (A) At day 5 after transplantation, phenotyping was performed in the spleen and mLN. Representative plots from 2 experiments are shown from the spleen. (B) At day 5 after transplantation, B6.FoxP3-GFP mean fluorescence intensity was assessed. ***P < .001. Displayed results are from 6 mice, and similar results were confirmed at day 7 and day 12. (C) At day 7, spleens were removed and analyzed for expression of IL-10, IFN-γ, and IL-17 after phorbol myristate acetate and ionomycin stimulation. Concatenated plots from 2 replicate experiments are shown with quadrant statistics representing mean. Graph represents percentage of FoxP3 cells expressing the cytokine indicated. (D) Cytolytic activity of donor B6 FoxP3− and CD8 FoxP3+ T cells from transplanted B6D2F1 recipients in 51Cr release assays against host-type P815 mastocytoma. Results represent replicate wells from 2 experiments.
CD8+FoxP3+ Treg display a distinct phenotype compared with CD4+FoxP3+ Treg. B6D2F1 recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. (A) At day 5 after transplantation, phenotyping was performed in the spleen and mLN. Representative plots from 2 experiments are shown from the spleen. (B) At day 5 after transplantation, B6.FoxP3-GFP mean fluorescence intensity was assessed. ***P < .001. Displayed results are from 6 mice, and similar results were confirmed at day 7 and day 12. (C) At day 7, spleens were removed and analyzed for expression of IL-10, IFN-γ, and IL-17 after phorbol myristate acetate and ionomycin stimulation. Concatenated plots from 2 replicate experiments are shown with quadrant statistics representing mean. Graph represents percentage of FoxP3 cells expressing the cytokine indicated. (D) Cytolytic activity of donor B6 FoxP3− and CD8 FoxP3+ T cells from transplanted B6D2F1 recipients in 51Cr release assays against host-type P815 mastocytoma. Results represent replicate wells from 2 experiments.
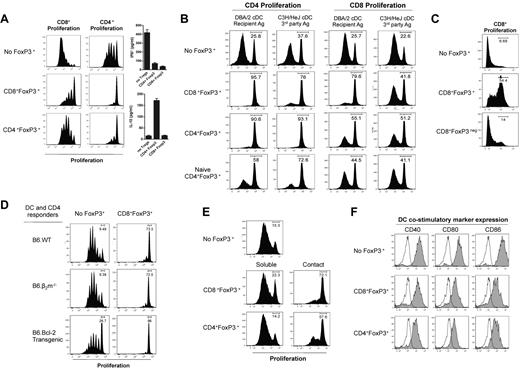
We next compared the capacity of the CD4+FoxP3+and CD8+FoxP3+ cells to suppress proliferation in vitro. The CD8+FoxP3+ cells suppressed CD4+ and CD8+ T-cell proliferation in response to Fc-bound CD3 to a greater extent than CD4+FoxP3+ Treg (Figure 3A). Whereas both FoxP3+ populations suppressed the generation of IFN-γ by proliferating T cells, IL-10 was only produced in the presence of CD4+FoxP3+ Treg (Figure 3A). We next analyzed whether the suppressive activity of CD8+FoxP3+ Treg was antigen-specific. CD4+ and CD8+ Treg were sort purified after transplantation and cultured with CFSE-labeled B6.CD45.1+ CD4+ or CD8+ responder T cells and either DBA/2 DCs, which express host MHC (H-2dd) or C3H/Hej DC which express a third party MHC (H-2dk). The CD4+FoxP3+ Treg suppressed proliferation similarly regardless of the stimulating DCs, whereas CD8+FoxP3+ Treg suppressed T-cell proliferation in an antigen-specific manner, preferentially in response to the DBA/2 DC, with limited activity against third party C3H/Hej DC (Figure 3B). Importantly, CD8+FoxP3+ Treg displayed superior suppressive capacity against DBA/2 DCs compared with CD4+FoxP3+ Treg and both were superior to freshly isolated naive CD4+FoxP3+ (Figure 3B). Previous studies investigating naive CD4+FoxP3+ commonly use a 1:1 ratio of Treg compared with responding T cells. We have used a 1:5 ratio in these studies; thus, suppression by naive CD4+FoxP3+ Treg is limited. Importantly, suppressive capacity within donor CD8+ cells was restricted to those expressing FoxP3 (hereafter referred to as CD8+FoxP3+ Treg), suggesting that suppression in the FoxP3+ CD8 T cells was not merely a consequence of cytotoxicity against DCs or responders mediated by effector CD8 T cells (Figure 3C). We confirmed this by performing suppression assays where both the stimulating antigen-presenting cells and responding T cells were either lacking MHC class I and so unable to be killed in an MHC class I–restricted fashion (β2m−/−)30 or overexpressed bcl-2, making them resistant to death.31,32 There was no reduction in the ability of CD8+FoxP3+ Treg to suppress proliferation in either of these conditions (Figure 3D), providing further evidence of true regulation by these cells. To further investigate the mechanism of suppression used by Treg generated after BMT, transwell assays were performed in which the DC-stimulated effector T cells were separated from the CD8+FoxP3+ Treg, which suggested that, like CD4+FoxP3+, CD8+FoxP3+ Treg require cell contact with the stimulating DCs to mediate their suppression (Figure 3E). In support of this, when expression of costimulatory markers on DCs were investigated, the presence of either CD4+FoxP3+ or CD8+FoxP3+ Treg inhibited up-regulation of CD40, CD80, and CD86 (Figure 3F).
CD8 Treg that undergo conversion after BMT exert contact-dependent, antigen-specific suppression and are more potent than CD4 Treg. B6D2F1 recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. At day 7 after transplantation, LN and spleens were removed, and CD4+Foxp3+, CD8+Foxp3+, and CD8+Foxp3− cells sort purified. These cells were cultured with the following responding cells: (A) CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. IL-10 and IFN-γ in the tissue culture supernatant were assessed. (B) Sorted CFSE-labeled CD45.1+CD4 or CD45.1+CD8 T cells and CD45.2+ recipient (DBA/2) or third party (C3H/Hej) DCs with CFSE dilution analyzed in CD45.1+ CD8 or CD4 responders. (C) CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. (D) Sorted Cell Trace-labeled responder CD4+ T cells and DCs from B6.WT, B6.β2m−/−, or B6.bcl-2 Tg mice with or without CD8+FoxP3+ Treg as shown and violet dilution quantified 72 to 96 hours later. (E) Sorted CFSE-labeled CD45.1+CD4+ T cells and DBA/2 DC in transwell plates with CFSE dilution quantified in CD45.1+ cells 3 days later. (F) FoxP3+ populations were sort purified and cultured with CD45.1+CD4+ T cells and B6.MHC class II-GFPxDBA2.F1 DC. Costimulatory molecules were analyzed on GFP+ DCs after 72 hours of culture with histograms shown (open represents isotype; and tinted, costimulatory marker). For all experiments, representative plots are shown from 2 duplicate experiments.
CD8 Treg that undergo conversion after BMT exert contact-dependent, antigen-specific suppression and are more potent than CD4 Treg. B6D2F1 recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. At day 7 after transplantation, LN and spleens were removed, and CD4+Foxp3+, CD8+Foxp3+, and CD8+Foxp3− cells sort purified. These cells were cultured with the following responding cells: (A) CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. IL-10 and IFN-γ in the tissue culture supernatant were assessed. (B) Sorted CFSE-labeled CD45.1+CD4 or CD45.1+CD8 T cells and CD45.2+ recipient (DBA/2) or third party (C3H/Hej) DCs with CFSE dilution analyzed in CD45.1+ CD8 or CD4 responders. (C) CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. (D) Sorted Cell Trace-labeled responder CD4+ T cells and DCs from B6.WT, B6.β2m−/−, or B6.bcl-2 Tg mice with or without CD8+FoxP3+ Treg as shown and violet dilution quantified 72 to 96 hours later. (E) Sorted CFSE-labeled CD45.1+CD4+ T cells and DBA/2 DC in transwell plates with CFSE dilution quantified in CD45.1+ cells 3 days later. (F) FoxP3+ populations were sort purified and cultured with CD45.1+CD4+ T cells and B6.MHC class II-GFPxDBA2.F1 DC. Costimulatory molecules were analyzed on GFP+ DCs after 72 hours of culture with histograms shown (open represents isotype; and tinted, costimulatory marker). For all experiments, representative plots are shown from 2 duplicate experiments.
Recipient DCs and TGF-β facilitate conversion of CD8 Treg
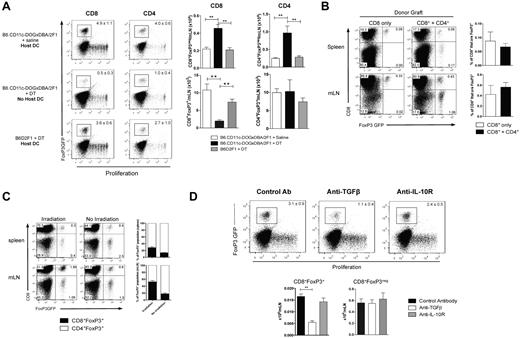
Because alloreactivity is required to drive CD8+FoxP3+conversion, we hypothesized that recipient antigen-presenting cells may play an important role in this process. To investigate this, DCs were depleted by administering DT to transgenic B6.CD11c-DOGxDBA2F1 recipients (in which the DT receptor is driven off the CD11c promoter). After the depletion of recipient DCs, a significant reduction in CD8+FoxP3+ Treg conversion was evident (Figure 4A). However, no significant changes were observed in CD4+FoxP3+ Treg in either the spleen or mLN at this early time point after transplantation (Figure 4A). To investigate whether CD4 T-cell help is required for CD8+FoxP3+ conversion, we used the B6.FoxP3-GFP → bm1 system, where GVHD is directed toward an isolated MHC class I disparity. This allowed us to investigate the effects of CD4 T cells in the donor graft, without having their presence or absence alter the development of GVHD. The absence of donor CD4 T cells did not impact on the generation of CD8+FoxP3+ Treg (Figure 4B), suggesting that they are not required for conversion of this Treg population after transplantation.
Host DCs and TGF-β are required for CD8 Treg conversion. (A) T cells from B6.FoxP3-GFP donors were labeled with Cell Trace violet dye and transplanted with BM into lethally irradiated B6.CD11c-DOGxDBA2.F1 recipients or B6D2F1 recipients. Recipients were treated with either DT or saline to deplete host DCs (160 ng/d, day −2 → day 3). At day 4 after transplantation, CD4+Foxp3+ and CD8+Foxp3+ conversion and proliferation were analyzed in the spleen and mLN. Representative plots of the mLN are shown, with numbers quantitated and displayed as mean ± SEM. ** P < .01. Combined data from 2 experiments; n = 6 per group. (B) BM and T cells (either CD8+ only or CD4+ + CD8+) from B6.FoxP3-GFP doors were transplanted into lethally irradiated bm1 recipients. At day 12 after transplantation, CD8+FoxP3+ Treg cells were analyzed in the spleen and mLN. Representative plots gated on total live cells are shown with combined data represented in the bar graphs displaying mean ± SEM; n = 3 per group. (C) B6D2F1 recipients received either irradiation at day −1 or NK1.1 at day −2 and splenocytes from B6.FoxP3-GFP donors at day 0. At day 5 after transplantation, CD8+FoxP3+ populations were analyzed in the spleen and mLN. Representative plots gated on total live cells are shown, and percent contribution displayed as mean ± SEM with 3 per group. (D) Lethally irradiated B6D2F1 recipients were transplanted with BM and T cells from B6.FoxP3-GFP donors. Recipients were treated with antibodies against either TGF-β or IL-10R. At day 4 after transplantation, CD4+Foxp3+ and CD8+Foxp3+ conversion and proliferation were analyzed in the spleen and mLN. Representative plots gated on CD8+ T cells in the mLN are shown, with numbers quantitated and displayed as mean ± SEM. Combined data from 2 experiments; n = 6 per group. **P < .01, control antibody versus anti–TGF-β
Host DCs and TGF-β are required for CD8 Treg conversion. (A) T cells from B6.FoxP3-GFP donors were labeled with Cell Trace violet dye and transplanted with BM into lethally irradiated B6.CD11c-DOGxDBA2.F1 recipients or B6D2F1 recipients. Recipients were treated with either DT or saline to deplete host DCs (160 ng/d, day −2 → day 3). At day 4 after transplantation, CD4+Foxp3+ and CD8+Foxp3+ conversion and proliferation were analyzed in the spleen and mLN. Representative plots of the mLN are shown, with numbers quantitated and displayed as mean ± SEM. ** P < .01. Combined data from 2 experiments; n = 6 per group. (B) BM and T cells (either CD8+ only or CD4+ + CD8+) from B6.FoxP3-GFP doors were transplanted into lethally irradiated bm1 recipients. At day 12 after transplantation, CD8+FoxP3+ Treg cells were analyzed in the spleen and mLN. Representative plots gated on total live cells are shown with combined data represented in the bar graphs displaying mean ± SEM; n = 3 per group. (C) B6D2F1 recipients received either irradiation at day −1 or NK1.1 at day −2 and splenocytes from B6.FoxP3-GFP donors at day 0. At day 5 after transplantation, CD8+FoxP3+ populations were analyzed in the spleen and mLN. Representative plots gated on total live cells are shown, and percent contribution displayed as mean ± SEM with 3 per group. (D) Lethally irradiated B6D2F1 recipients were transplanted with BM and T cells from B6.FoxP3-GFP donors. Recipients were treated with antibodies against either TGF-β or IL-10R. At day 4 after transplantation, CD4+Foxp3+ and CD8+Foxp3+ conversion and proliferation were analyzed in the spleen and mLN. Representative plots gated on CD8+ T cells in the mLN are shown, with numbers quantitated and displayed as mean ± SEM. Combined data from 2 experiments; n = 6 per group. **P < .01, control antibody versus anti–TGF-β
To determine whether the proinflammatory environment associated with myeloablative conditioning was contributing to CD8+FoxP3+ Treg conversion, transplantations were performed in the absence of conditioning. In this setting, CD8+FoxP3+ Treg conversion was still evident (Figure 4C). However, the conditioning regimen did alter the contribution of CD8+ or CD4+ subsets to the total FoxP3+ pool, with the CD8+FoxP3+ proportion increased after irradiation. To further elucidate which cytokines were involved in this conversion process, TGF-β and IL-10 were blocked after transplantation (because of their known importance in CD4+ Treg conversion and function). These studies confirmed that TGF-β, but not IL-10, was required for the conversion of CD8+FoxP3+ Treg (Figure 4D). The conversion of CD4+FoxP3+ Treg was not significantly reduced in the absence of either TGF-β or IL-10 signaling at this time point (data not shown), consistent with the origin of these cells predominantly from natural Treg in the transplanted graft.
CD8+FoxP3+ Treg inhibit GVHD
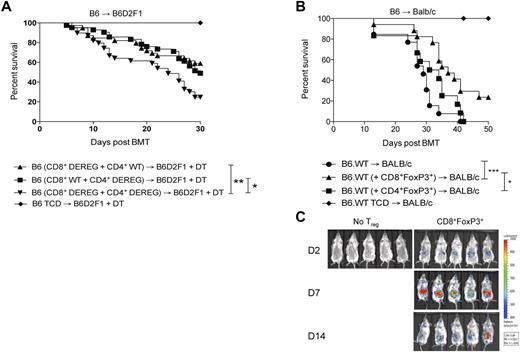
To determine the contribution of CD8+FoxP3+conversion to survival and GVHD severity after BMT, B6.DEREG mice were used as donors. In these animals, GFP expression and the DT receptor are driven of the FoxP3 promoter, allowing for specific depletion of FoxP3 expressing cells after DT administration. Based on previous data demonstrating that CD8+FoxP3+ Treg undergo conversion from CD8+FoxP3− cells, combinations of donor cells were transplanted as described in the figure legends that resulted in depletion of either CD4+FoxP3+ or CD8+FoxP3+ cells only. A significant increase in survival was noted in the presence of either CD4+FoxP3+ Treg or CD8+FoxP3+ Treg compared with recipients in which all FoxP3+ Treg were depleted (Figure 5A). In the DEREG mice, a rebound population of FoxP3-expressing cells that do not express GFP has been identified, although the suppressive capacity of this population has not been established.33 We thus undertook adoptive transfer experiments to confirm the suppressive function of CD8+FoxP3+ Treg and their ability to alleviate GVHD. In these studies, CD8+FoxP3+Treg were transferred at day 0 with the donor BM in the B6.FoxP3-GFP → BALB/c model to allow for expansion in the lymphopenic environment. Conventional donor T cells were transferred 2 days later.34 GVHD was significantly alleviated in recipients of CD8+FoxP3+ Treg relative to recipients that received no Treg or natural CD4+FoxP3+ Treg (Figure 5B). Natural CD4+FoxP3+ Treg were not effective at suppressing GVHD in these studies because of the low ratio of CD4+FoxP3+ Treg compared with conventional T cells (1:20). Importantly, CD8+FoxP3+ Treg were still detected 14 days after transfer when tracked using luciferase-expressing Treg, indicating sustained FoxP3 expression (Figure 5C). Thus, CD8+FoxP3+ Treg are an important and highly suppressive population generated after BMT.
CD8+FoxP3+ Treg attenuate GVHD mortality after BMT. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM from B6.FoxP3-GFP mice and T cells from both B6.FoxP3-GFP and DEREG mice in the following combinations: (1) WT CD4+ T cells and DEREG CD8+ T cells, (2) DEREG CD4+ T cells and WT CD8+ T cells, or (3) DEREG CD4+ T cells and DEREG CD8+ T cells. *P < .05, B6 (CD8+ WT + CD4+ DEREG) versus B6 (CD8+ DEREG + CD4+ DEREG). **P < .01, B6 (CD8+ DEREG + CD4+ WT) versus B6 (CD8+ DEREG + CD4+ DEREG). Survival data are shown and are combined from 4 duplicate experiments (CD4+FoxP3+ deplete, n = 42; CD8+FoxP3+ deplete, n = 39; CD4+FoxP3+ and CD8+FoxP3+ deplete, n = 39; TCD no depletion, n = 20). (B) BALB/c recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. At day 7 after transplantation, LN and spleens were removed, and CD4+Foxp3+ and CD8+Foxp3+ cells sorted on B6.FoxP3-GFP and CD4 or CD8. Sorted FoxP3+ populations (3.5 × 104) were transplanted with BM from B6.WT donors into lethally irradiated BALB/c recipients. Whole CD3+ T cells (7.5 × 105) were injected 2 days later. *P < .05, BALB/c (+ CD8+FoxP3+) versus BALB/c (+ CD4+FoxP3+). ***P < .001, BALB/c versus BALB/c (+ CD8+FoxP3+). Survival data are shown and are combined from 2 duplicate experiments (no FoxP3+, n = 13; CD8+FoxP3+, n = 17; CD4+FoxP3+, n = 12; no FoxP3+ TCD, n = 6). (C) Recipients were transplanted as in panel B; however, CD8+Foxp3+ were sorted from FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter, labeled as B6.FoxP3-luc+). Recipients were imaged at days 2, 7, and 14 after transfer, with images shown contrasting recipients that did or did not receive Treg.
CD8+FoxP3+ Treg attenuate GVHD mortality after BMT. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM from B6.FoxP3-GFP mice and T cells from both B6.FoxP3-GFP and DEREG mice in the following combinations: (1) WT CD4+ T cells and DEREG CD8+ T cells, (2) DEREG CD4+ T cells and WT CD8+ T cells, or (3) DEREG CD4+ T cells and DEREG CD8+ T cells. *P < .05, B6 (CD8+ WT + CD4+ DEREG) versus B6 (CD8+ DEREG + CD4+ DEREG). **P < .01, B6 (CD8+ DEREG + CD4+ WT) versus B6 (CD8+ DEREG + CD4+ DEREG). Survival data are shown and are combined from 4 duplicate experiments (CD4+FoxP3+ deplete, n = 42; CD8+FoxP3+ deplete, n = 39; CD4+FoxP3+ and CD8+FoxP3+ deplete, n = 39; TCD no depletion, n = 20). (B) BALB/c recipients were lethally irradiated and transplanted with BM and T cells from B6.FoxP3-GFP donors 24 hours later. At day 7 after transplantation, LN and spleens were removed, and CD4+Foxp3+ and CD8+Foxp3+ cells sorted on B6.FoxP3-GFP and CD4 or CD8. Sorted FoxP3+ populations (3.5 × 104) were transplanted with BM from B6.WT donors into lethally irradiated BALB/c recipients. Whole CD3+ T cells (7.5 × 105) were injected 2 days later. *P < .05, BALB/c (+ CD8+FoxP3+) versus BALB/c (+ CD4+FoxP3+). ***P < .001, BALB/c versus BALB/c (+ CD8+FoxP3+). Survival data are shown and are combined from 2 duplicate experiments (no FoxP3+, n = 13; CD8+FoxP3+, n = 17; CD4+FoxP3+, n = 12; no FoxP3+ TCD, n = 6). (C) Recipients were transplanted as in panel B; however, CD8+Foxp3+ were sorted from FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter, labeled as B6.FoxP3-luc+). Recipients were imaged at days 2, 7, and 14 after transfer, with images shown contrasting recipients that did or did not receive Treg.
RAPA and IL-2 Ab complex coadministration massively expands CD8+FoxP3+ Treg after BMT
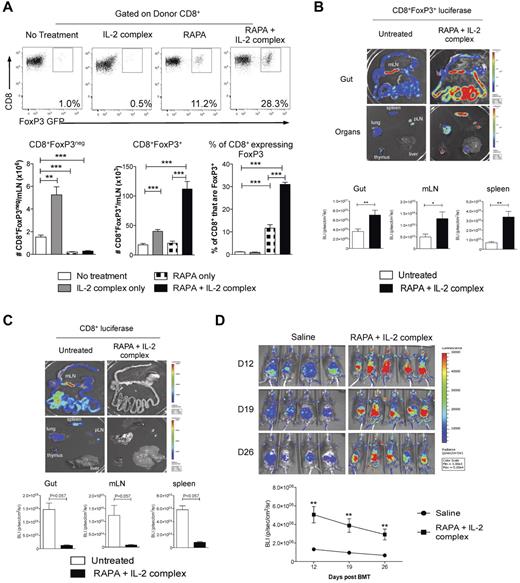
Previous reports have demonstrated that the mTOR inhibitor rapamycin (RAPA) differentially suppresses CD4+FoxP3+ and CD4+FoxP3− T cells after BMT.18 We thus investigated whether FoxP3 expression by CD8+ T cells would also render them relatively resistant to suppression by RAPA. This was indeed the case, with the proportion of CD8+ T cells expressing FoxP3 significantly increasing after RAPA because of the preferential inhibition of CD8+FoxP3− T-cell expansion (Figure 6A). Furthermore, RAPA administration in conjunction with recombinant human IL-2 expands CD4+FoxP3+ Treg after transplantation35 ; however, we found this strategy ineffective at expanding CD8+FoxP3+ Treg (data not shown). We thus used IL-2 antibody complexes because this dramatically increases the biologic activity IL-2 in vivo.36 After treatment with RAPA and IL-2 complexes after transplantation, the absolute numbers of CD8+FoxP3+ Treg were expanded 6- to 7-fold, with the percentage of CD8+ T cells expressing FoxP3 within the mLN increasing 30-fold to 30% (Figure 6A). In contrast, the treatment of recipients with RAPA and IL-2 complexes only increased the percentage of CD4+ T cells expressing FoxP3 4-fold (1.2% ± 0.25% vs 4.2% ± 1.0%, P < .001). The CD8 Treg expansion was confirmed using CD8+ T cells from donors with luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter (FoxP3.LuciDTR-4, labeled as B6.FoxP3-luc+), allowing tracking of CD8+FoxP3+ Treg. At day 7 after transplantation, there was a significant increase in bioluminescence signal after RAPA and IL-2 complex treatment in the GI tract, mLN, and spleen (Figure 6B). Importantly, this occurred in conjunction with a concurrent decrease in the effector CD8+ T-cell expansion in the same organs as assessed by transferring CD8+ T cells with luciferase expressed in all cells (Figure 6C). It is important to note that the bioluminescence signal is approximately 10-fold brighter in the B6.FoxP3-luc+ donors relative to the B6.β-actin-luc+ mice, meaning that luminescence can only be compared when the cells of the same donor origin are transplanted. The long-term expansion of CD8+FoxP3+ Treg was confirmed (Figure 6D) despite administration of only 2 doses of IL-2 Ab complexes (the last on day 4). We confirmed that the RAPA and IL-2 complex expanded CD8+FoxP3+ Treg retained suppressive function (Figure 7A). Finally, we undertook experiments whereby we could expand CD8+FoxP3+ Treg with RAPA and IL-2 complexes and study the physiologic consequence of their specific deletion by DT administration in vivo. Depletion was confirmed by bioluminescence imaging as only the CD8+FoxP3− in the ingoing graft were from B6.FoxP3-luc+ donors (Figure 7B). As shown, GVHD within the GI tract in the animals receiving RAPA and IL-2 complexes was minimal, and specific deletion of the expanded CD8+FoxP3+ Treg resulted in significant GVHD in that organ (Figure 7C). Therefore, the combination of RAPA and IL-2 antibody complex treatment significantly expands the CD8+FoxP3+ regulatory population and provides a potent therapeutic strategy to inhibit GVHD.
CD8+FoxP3+ Treg are expanded after administration of RAPA and IL-2 Ab complexes after BMT. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA daily (1.5 mg/kg intraperitoneally) and/or IL-2 Ab complexes at day 0 and day 4 after transplantation in the combinations shown. At day 7, mLN were removed and analyzed for CD8+FoxP3+ Treg conversion. Representative plots are shown for graphs in the lower panel displaying combined data from 2 duplicate experiments; n = 8 per group. **P < .01. ***P < .005. B6D2F1 recipients were lethally irradiated and received BM and T cells from B6.WT donors combined with sorted CD8+ T cells from either (B) FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter, labeled as B6.FoxP3-luc+; n = 8 per group) or (C) B6.β-actin-luc+ (n = 3 or 4 per group) mice 24 hours later. RAPA and IL-2 Ab complex was administered as in panel A. At day 7, recipients were killed 5 minutes after luciferin injection, and spleen, inguinal LN, liver, thymus, lung, and gut removed and imaged individually. Representative images from 2 duplicate experiments are shown with bioluminescence imaging quantitated and shown as mean ± SEM. *P < .05. **P < .01. (D) Recipients were transplanted as in panel B with only the CD8+ component from B6.FoxP3-luc+ donors and imaged weekly from day 12. Images are shown with graph displaying mean ± SEM. **P < .01, saline versus RAPA + IL-2 Ab complexes at each time point.
CD8+FoxP3+ Treg are expanded after administration of RAPA and IL-2 Ab complexes after BMT. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA daily (1.5 mg/kg intraperitoneally) and/or IL-2 Ab complexes at day 0 and day 4 after transplantation in the combinations shown. At day 7, mLN were removed and analyzed for CD8+FoxP3+ Treg conversion. Representative plots are shown for graphs in the lower panel displaying combined data from 2 duplicate experiments; n = 8 per group. **P < .01. ***P < .005. B6D2F1 recipients were lethally irradiated and received BM and T cells from B6.WT donors combined with sorted CD8+ T cells from either (B) FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter, labeled as B6.FoxP3-luc+; n = 8 per group) or (C) B6.β-actin-luc+ (n = 3 or 4 per group) mice 24 hours later. RAPA and IL-2 Ab complex was administered as in panel A. At day 7, recipients were killed 5 minutes after luciferin injection, and spleen, inguinal LN, liver, thymus, lung, and gut removed and imaged individually. Representative images from 2 duplicate experiments are shown with bioluminescence imaging quantitated and shown as mean ± SEM. *P < .05. **P < .01. (D) Recipients were transplanted as in panel B with only the CD8+ component from B6.FoxP3-luc+ donors and imaged weekly from day 12. Images are shown with graph displaying mean ± SEM. **P < .01, saline versus RAPA + IL-2 Ab complexes at each time point.
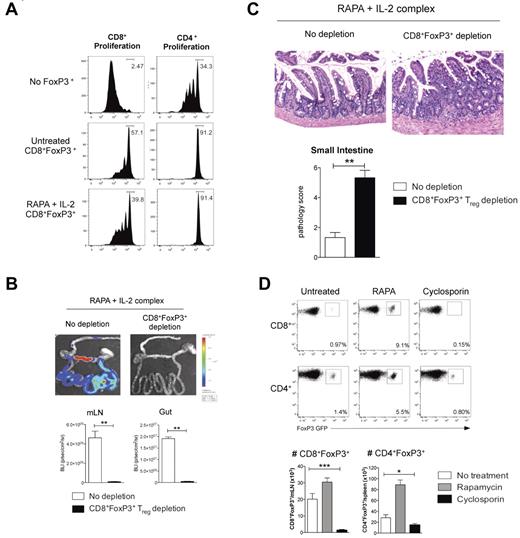
RAPA and IL-2 Ab complex expanded CD8+FoxP3+ Treg are functional and protect from gut GVHD. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA daily (1.5 mg/kg intraperitoneally) and IL-2 Ab complexes at day 0 and day 4 after transplantation. At day 7 after transplantation, LN and spleens were removed, CD8+Foxp3+ cells sorted and cultured with CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. (B) B6D2F1 recipients were lethally irradiated and received BM and 0.5 × 106 sorted CD4+FoxP3− T cells from B6.FoxP3-GFP donors in conjunction with 3 × 106 sorted CD8+FoxP3− cells from FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter). All recipients received RAPA (daily) and IL-2 Ab complexes (day 0 and day 4). DT (160 ng intraperitoneally) or saline was administered daily from day 3 until day 6. Bioluminescent imaging was performed and quantified at day 7; n = 6 per group. **P < .01. (C) Representative images of small intestine taken at day 7 after transplantation (original magnification ×200) and GVHD histopathology scores; n = 6 per group. **P < .01. (D) B6D2F1 recipients were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA or cyclosporine daily. Spleen and mLN were removed at day 7 after transplantation and FoxP3+ populations analyzed. Representative plots are shown of the mLN for CD8+ enumeration or the spleen for CD4+ enumeration. Absolute numbers were quantitated, and graphs represent mean ± SEM; n = 7 or 8 per group. *P < .05. ***P < .005.
RAPA and IL-2 Ab complex expanded CD8+FoxP3+ Treg are functional and protect from gut GVHD. (A) Recipient B6D2F1 mice were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA daily (1.5 mg/kg intraperitoneally) and IL-2 Ab complexes at day 0 and day 4 after transplantation. At day 7 after transplantation, LN and spleens were removed, CD8+Foxp3+ cells sorted and cultured with CFSE-labeled CD45.1+CD3+ T cells, CD45.2+ DCs, and 1 μg/mL CD3 with CFSE dilution quantified in CD45.1+ cells 3 days later. (B) B6D2F1 recipients were lethally irradiated and received BM and 0.5 × 106 sorted CD4+FoxP3− T cells from B6.FoxP3-GFP donors in conjunction with 3 × 106 sorted CD8+FoxP3− cells from FoxP3.LuciDTR-4 donors (luciferase, GFP, and the DT receptor-driven of the FoxP3 promoter). All recipients received RAPA (daily) and IL-2 Ab complexes (day 0 and day 4). DT (160 ng intraperitoneally) or saline was administered daily from day 3 until day 6. Bioluminescent imaging was performed and quantified at day 7; n = 6 per group. **P < .01. (C) Representative images of small intestine taken at day 7 after transplantation (original magnification ×200) and GVHD histopathology scores; n = 6 per group. **P < .01. (D) B6D2F1 recipients were lethally irradiated and 24 hours later transplanted with BM and T cells from B6.FoxP3-GFP mice. Recipients received RAPA or cyclosporine daily. Spleen and mLN were removed at day 7 after transplantation and FoxP3+ populations analyzed. Representative plots are shown of the mLN for CD8+ enumeration or the spleen for CD4+ enumeration. Absolute numbers were quantitated, and graphs represent mean ± SEM; n = 7 or 8 per group. *P < .05. ***P < .005.
Lastly, an obvious and important question is whether conversion of CD8+FoxP3+ Treg occurs in clinical patients after BMT. We examined this in the peripheral blood of patients in the first 6 months after BMT but disappointingly could not find convincing evidence of CD8+FoxP3+ Treg (data not shown). All these patients were receiving standard GVHD prophylaxis with cyclosporine or tacrolimus. We thus investigated conversion of CD8+FoxP3+ Treg in our murine systems in the context of cyclosporine administration after BMT. Surprisingly, although a distinct population of CD4+FoxP3+ Treg remained in cyclosporine-treated recipients, CD8+FoxP3+ Treg were absent (Figure 7D). Thus, CD8+FoxP3+ Treg appear exquisitely sensitive to inhibition by cyclosporine, and the presence of this cell population will need to be examined in patients receiving calcineurin-free (and ideally RAPA-based) immune suppression early after BMT.
Discussion
In the current study, we identify and characterize a highly suppressive CD8+FoxP3+ regulatory T-cell population that is generated by a process of conversion after allogeneic BMT. This represents the first time that CD8+FoxP3+ cells have been induced after physiologic uptake and presentation of endogenous antigen, although similar populations have been identified using artificial antigen presentation in transgenic models, strong adjuvants, or modified CD3 antibody.37-39 CD8+FoxP3+ Treg occur in negligible numbers in naive mice, which makes isolation and subsequent characterization of viable cells impossible.9
Naturally occurring CD8 regulatory T-cell populations have been identified on the basis of surface markers and have been shown to be regulatory in several autoimmune diseases, including systemic lupus erythematosus,40 experimental autoimmune encephalitis,41,42 and myasthenia gravis43 and to induce tolerance after solid organ transplantation.44 However, these cells have not been demonstrated to express FoxP3 in vivo and are sometimes present without having suppressive capacity. Furthermore, they have a somewhat controversial history, and interstudy comparisons are difficult because of the use of varied cell surface markers or properties (CD28−,44,45 CD122+,46 CD103+47 and Qa-1 restriction45,48,49 ) for their identification.
Given that CD8+FoxP3+ Treg are highly suppressive, it is surprising that they express lower levels of CTLA/4, GARP, and FoxP3, as these molecules contribute to CD4+FoxP3+ Treg function.50-53 Thus, these molecules appear less important in mediating CD8+FoxP3+ Treg suppression, suggesting differing pathways of effector function. The other phenotypic differences between CD8+FoxP3+ Treg and both posttransplantation and naive CD4+FoxP3+ Treg were CD62L, α4β7, CD28, and GITR. The high expression of α4β7 on CD8+FoxP3+ Treg might be anticipated given that α4β7 is involved in migration to the GI tract. Similarly, reduced expression of CD62L is consistent with the fact that these cells are undergoing conversion from actively proliferating CD8+FoxP3− T cells. The lower CD28 and GITR expression may be advantageous for maintenance of suppressive function, as it has been shown that TCR stimulation and CD28 ligation can break anergy in CD4 Treg,54 whereas signaling of CD4+CD25+ cells through GITR also breaks tolerance.55
The use of CD4 Treg to suppress deleterious immune responses responsible for GVHD has been extensively investigated over the previous decade. This initially occurred in preclinical models2,5 and has now progressed to clinical trials involving CD4 Treg sorted straight from the donor56 or alternatively ex vivo expanded CD4 Treg.57 These studies have yielded promising results with reduced incidence of acute GVHD and no effects on relapse or infection. In this study, we have clearly identified a regulatory T-cell population that is greater than 8 times more suppressive than the natural CD4 Treg used in these trials. Because clinical GVHD is classically CD8+ T cell and MHC class I dependent, the ability of these CD8+FoxP3+ Treg to suppress CD8+ responses far more effectively than naive CD4+FoxP3+ Treg would be expected to correlate with enhanced suppression of GVHD in vivo after adoptive transfer.
Despite the increased suppressive capacity of these cells, in vitro or in vivo methods will still probably be necessary to further expand CD8+FoxP3+ Treg. Although CD8+FoxP3+ cells have been expanded in vitro in an artificial system using ovalbumin as antigen, in the presence of IL-2, TGF-β and retinoic acid, results indicated that, in contrast to our results, these cells were not as suppressive as CD4+Foxp3+ Treg.58 A probable reason for this is that the in vitro systems used to generate and expand these cells are not optimal. In support of this, we have expanded these cells in vitro under similar conditions and found that they lose suppressive capacity (data not shown). Clearly, expansion protocols that are optimal for CD4+Foxp3+ Treg expansion are not effective for CD8+FoxP3+ Treg, and the specific requirements for expansion of these cells in vitro needs further investigation.
When we investigated what was required for CD8+FoxP3+ Treg conversion in vivo, the process of BMT itself was not sufficient, as demonstrated by lack of conversion after syngeneic transplantation. Thus, because of the requirement for alloreactivity, it is not surprising that recipient DCs significantly contribute to their conversion. It has previously been demonstrated in models of colitis that specialized DCs, which express CD103 and produce TGF-β and retinoic acid, are involved in peripheral conversion of CD4 Treg.59 Because the mLN is the dominant site of conversion of CD8+FoxP3+ Treg, it is probable that this DC subset is involved at this site. In conjunction with adoptive transfer of Treg populations to alleviate GVHD, using immune suppressant drugs that do not inhibit Treg expansion is also advantageous. Therefore, the ability of RAPA to inhibit CD8+FoxP3− expansion while allowing conversion of FoxP3− to FoxP3+ CD8 T cells after BMT provides further rationale for its use in the clinic. When IL-2 antibody complexes were administered in conjunction with RAPA, CD8+FoxP3+ Treg were not only maintained but significantly increased after BMT. This in vivo manipulation of CD8+FoxP3+ would appear preferable to in vitro expansion, as it does not require the expensive and time-intensive processes required for the latter. Therefore, it is likely that, in conjunction with known mechanisms via which RAPA alleviates GVHD, the maintenance of FoxP3 expression in CD8+FoxP3+ Treg also contributes. The addition of IL-2 Ab complexes to RAPA would be expected to further improve survival because of the massive and preferential expansion of this CD8+FoxP3+ regulatory population.
In conclusion, we have identified a new regulatory population, provided a therapeutic approach to their expansion, and demonstrated their functional importance in defining transplant outcome. Importantly, this population appears as important for protection from GVHD as the well-studied CD4+FoxP3+ population. Given the potent MHC class I dominant suppressive function, CD8+FoxP3+ cells represent a new regulatory population highly suited to manipulation for the control of deleterious alloimmune responses after transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
G.R.H. is a National Health and Medical Research Council Australia Fellow and Queensland Health Senior Clinical Research Fellow. K.A.M. is a National Health and Medical Research Council Clinical Training Fellow. K.P.A.M. is a Cancer Council Queensland Senior Research Fellow. R.J.R. is an LFQ PhD Scholarship Recipient.
This work was supported by the Cancer Council Queensland and the National Health and Medical Research Council Australia.
Authorship
Contribution: R.J.R. performed and designed experiments and wrote the manuscript; K.E.L., R.D.K., Y.A.W., N.C.R., S.D.O., A.V., K.A.A., B.E.T., K.A.M., and M.K. performed experiments; G.J.H. provided FoxP3.LuciDTR mice; T.S. provided DEREG mice; A.D.C. performed histologic analysis; C.R.E. provided essential reagents; and G.R.H. and K.P.A.M. designed studies and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kelli P. A. MacDonald, Antigen Presentation and Immunoregulation Laboratory, 300 Herston Road, Herston, QLD, 4006, Australia; e-mail: kelli.macdonald@qimr.edu.au.
References
Author notes
G.R.H. and K.P.A.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal