Abstract
Pregnancy may be complicated by a rare but life-threatening disease called thrombotic thrombocytopenic purpura (TTP). Most cases of TTP are due to an acquired autoimmune or hereditary (Upshaw-Schulman syndrome [USS]) severe deficiency of a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13). In the present study, we performed a cross-sectional analysis of the national registry of the French Reference Center for Thrombotic Microangiopathies from 2000-2010 to identify all women who were pregnant at their initial TTP presentation. Among 592 adulthood-onset TTP patients with a severe ADAMTS13 deficiency, 42 patients with a pregnancy-onset TTP were included. Surprisingly, the proportion of USS patients (n = 10 of 42 patients [24%]; confidence interval, 13%-39%) with pregnancy-onset TTP was much higher than that in adulthood-onset TTP in general (less than 5%) and was mostly related to a cluster of ADAMTS13 variants. In the present study, subsequent pregnancies in USS patients not given prophylaxis were associated with very high TTP relapse and abortion rates, whereas prophylactic plasmatherapy was beneficial for both the mother and the baby. Pregnancy-onset TTP defines a specific subgroup of patients with a strong genetic background. This study was registered at www.clinicaltrials.gov as number NCT00426686 and at the Health Authority, French Ministry of Health, as number P051064.
Introduction
Approximately 1 in 25 000 to 1 in 100 000 pregnancies worldwide is complicated by very rare but life-threatening (to both mother and baby) diseases called thrombotic microangiopathies (TMAs).1,2 Some TMAs, such as preeclampsia and HELLP (hemolysis elevated liver enzymes low platelet count) syndrome, are pregnancy specific, whereas others, such as hemolytic uremic syndrome and thrombotic thrombocytopenic purpura (TTP), are not.3 TTP is defined by the association of thrombocytopenia, mechanical hemolytic anemia, and multivisceral ischemia occurring by recurrent boots that are often triggered by infections and pregnancy. The incidence of TTP is 4-10 cases/million people/year and the global mortality rate is estimated at 20% in spite of plasmatherapy (PT), which remains the reference treatment.1,2,4,5 The pathophysiology for most cases of TTP was elucidated in 19986,7 : a severe functional deficiency of a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13), the specific VWF-cleaving protease, causes the accumulation of platelet-hyperadhesive, unusually large VWF multimers that lead to the spontaneous formation of microthrombi within the microcirculation. ADAMTS13 deficiency may be due either to autoantibodies to ADAMTS13 (acquired autoimmune TTP) or to recessively inherited mutations of the ADAMTS13 gene (hereditary TTP, also termed Upshaw-Schulman syndrome [USS]).8 In a large majority of cases, TTP is an adulthood-onset disease characterized by a female predominance (approximately 2:1) and more than 95% of cases are the acquired form.
In an obstetrical context, TTP may be particularly challenging because of its difficult differential diagnosis with other TMAs, its dramatic effects on the fetus, and its specific therapeutic management.9 Pregnancy-associated TTP is reported to represent approximately 10%-30% of all adult TTP cases by the North American, European, and Japanese TMA registries.10-16 In addition, based on a literature review,17,18 recent advances in the management of pregnancy-associated TTP resulted from retrospective studies and cohort/case-series studies.10,19-29 However, these studies present 2 major limitations: (1) ADAMTS13 may not be documented and (2) hereditary and acquired TTP are described independently.
Given these facts, a global overview of pregnancy-associated TTP combining well-characterized both acquired and hereditary forms prospectively enrolled is not available so far. In terms of clinical practice however, when pregnancy comes with the first TTP boot, the differential diagnosis between both forms of TTP is a crucial challenge confronting physicians because it may change both short- and long-term management of these patients.10,19 We designed the present study based on a prospective national cohort of pregnancy-onset TTP patients to address the epidemiologic and management issues of the differential diagnosis between the acquired and hereditary forms.
Methods
Patients
Since 2000, all French patients with a presumptive diagnosis of TMA (defined as the presence of Coombs-negative microangiopathic anemia with hemoglobin < 12 g/dL with or without the presence of schistocytes and/or thrombocytopenia < 150 G/L with no comorbidities that could explain these findings) have been enrolled in the registry of the National Reference Center for TMA (Health Ministry National Plan for Rare Diseases). All of these patients have benefited from an ADAMTS13 investigation. A cross-sectional analysis of this registry was performed from January 2000 to December 2010 to identify, among patients with a severe ADAMTS13 deficiency (activity < 10%), the women who presented with their first episode of TTP in an obstetrical context (pregnancy or postpartum). For all patients included, the medical records were analyzed extensively to collect clinical and biologic data. Informed consent was obtained from each patient according to the Declaration of Helsinki and the study was approved by the ethics committee of Hospital Pitié-Salpêtrière and Hospital Saint-Antoine (Paris, France). This study is registered at www.clinicaltrials.gov as number NCT00426686 and at the Health Authority, French Ministry of Health, as number P051064.
Blood collection and laboratory methods
Venous blood was collected at the time of enrollment (TTP boot) before any treatment and during follow-up into a 1:10 final volume of 3.8% sodium citrate; platelet-poor plasma was obtained as described previously.30 Blood was also collected on EDTA for genetic analysis.
VWF Ag measurement.
Plasma VWF:Ag was quantified using the STA-Liatest VWF (Diagnostica Stago) immunoturbidimetric assay. Samples were compared with a normal plasma pool and results are expressed as percentages. The normal range is 50%-150%.
ADAMTS13 activity assay.
In all patients, ADAMTS13 activity was measured using 2 methods: (1) our laboratory-designed ELISA using a full-length VWF substrate and mAbs directed to each part of VWF proteolytic site by ADAMTS13, as described previously,30 and (2) the method of Kokame et al using commercial recombinant FRETS-VWF73 peptide (Peptide Institute) according to the manufacturer's instructions.31 The normal range is 50%-100% using both methods.
ADAMTS13 Ag measurement.
Plasma ADAMTS13 Ag was determined using the IMUBIND ADAMTS13 ELISA kit (American Diagnostica) according to the manufacturer's recommendations. The normal range is 540 ± 190 ng/mL.
ADAMTS13 autoantibodies.
ADAMTS13 autoantibodies were screened using the Technozyme ADAMTS13-INH ELISA commercial kit (Technoclone), which allowed us to titer specific anti-ADAMTS13 IgG. A titer of anti-ADAMTS13 IgG > 12 U/mL was considered positive according to the manufacturer's instructions. In addition, an inhibitor assay was performed as described previously with minor modifications.30 Briefly, patient plasma was heat treated at 56°C for 30 minutes to inactivate any endogenous ADAMTS13 activity and mixed to normal pool plasma (volume-volume ratio, 3:1). The residual ADAMTS13 activity in the mixture was measured after a 30-minute incubation at 37°C using the FRETS-VWF73 assay. The ADAMTS13 inhibitor assay was considered positive if the residual ADAMTS13 activity was lower than 10%.
ADAMTS13 gene sequencing and prediction of the functional effect of mutations.
Genomic DNA was screened for mutations by direct sequencing of the coding sequence of the ADAMTS13 gene (NM_139025.3) using Big Dye Version 3.1 and the ABI3130XL capillary sequencer, and was analyzed using SeqScape Version 2.5 (Applied Biosystems). Sequences of primers and PCR conditions are available upon request. Human Genome Variation Society nomenclature (http://www.hgvs.org/mutnomen/) was used for the sequence variations. All of the novel missense mutations and all single-nucleotide polymorphisms (SNPs) were annotated on PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) and SIFT (http://sift.jcvi.org/) software for the prediction of the functional effect of protein changes (predicted to be deleterious, possibly deleterious, or nondeleterious).
Statistical analysis
Comparison variables are described as means ± SD and qualitative data as proportions with 95% confidence intervals (95% CIs). Comparisons between hereditary and acquired TTP patients were done using χ2 or Fisher exact tests as appropriate. All tests were 2-sided and significance was set at P < .05. Statistical analysis was performed using R Version R.2.13.0 software (R Foundation).
Results
Study population
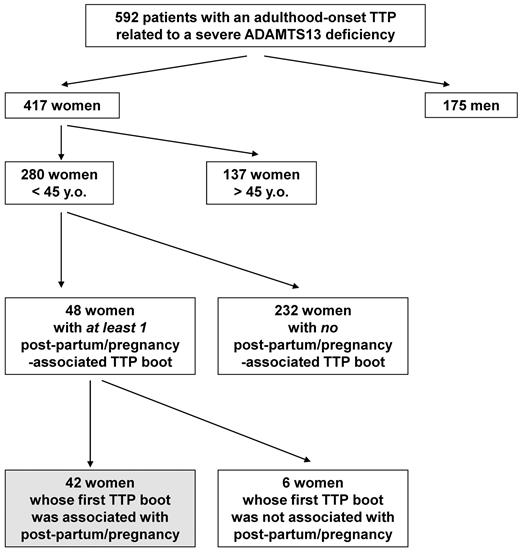
From 2000-2010, 634 consecutive patients with a TMA related to a severe ADAMTS13 functional deficiency were prospectively enrolled in our registry and diagnosed as “TTP with a severe ADAMTS13 deficiency.” Of these, 592 patients had adulthood-onset TTP (first TTP boot after the age of 18 years) and 42 patients had childhood-onset TTP (before 18 years). Pregnancies were observed only in adulthood-onset TTP patients. Among our 592 adulthood-onset TTP patients, 417 were women (sex ratio, 2.3 female:1 male), including 280 women younger than 45 years of age (67%; 95% CI, 62%-79%; Figure 1). In 48 of the latter, 1 or several TTP boots were associated with pregnancy (n = 40) or the postpartum period (n = 8). Six women had already experienced TTP boots before their pregnancy-associated TTP (all of them had been diagnosed previously as having acquired autoimmune TTP). In contrast, the 42 remaining women (15% of women of childbearing age; 95% CI, 11%-20%) experienced their first TTP episode during pregnancy.
Enrollment of the study population. From 2000-2010, 592 consecutive patients with an adulthood-onset TTP related to a severe ADAMTS13 functional deficiency were prospectively enrolled in the French registry for Thrombotic Microangiopathies. A total of 417 patients were women; 280 were less than 45 years of age (67%). In 48 of those latter women, 1 or several TTP boots were associated with pregnancy (n = 40) or the postpartum period (n = 8). Six women had already experienced TTP boots before their pregnancy-associated TTP, whereas pregnancy accounted for the first TTP episode in 42 women.
Enrollment of the study population. From 2000-2010, 592 consecutive patients with an adulthood-onset TTP related to a severe ADAMTS13 functional deficiency were prospectively enrolled in the French registry for Thrombotic Microangiopathies. A total of 417 patients were women; 280 were less than 45 years of age (67%). In 48 of those latter women, 1 or several TTP boots were associated with pregnancy (n = 40) or the postpartum period (n = 8). Six women had already experienced TTP boots before their pregnancy-associated TTP, whereas pregnancy accounted for the first TTP episode in 42 women.
Characteristics of the cohort at presentation and short-term outcome
In our cohort of 42 pregnancy- or postpartum-onset TTP patients, the mean age was 29 years (range, 18-44), the mean hemoglobin level at presentation was 7.3 g/dL (range, 4.4-11), and the mean platelet count at presentation was 31 G/L (range, 4-140). The main ethnic groups were white (75%) and Afro-Caribbean (20%). Fever and neurologic symptoms at admission were present in 25% and 57% of patients, respectively. All patients exhibited a normal renal function. Table 1 summarizes the main biologic and clinical features of our patients during the TTP boot and their outcome. Thirty-two patients were in their first pregnancy, whereas 10 patients had previously experienced 1 or more pregnancies (including 3 patients with fetal loss). TTP occurred during the first trimester of pregnancy in 10 patients, the second trimester in 13 patients, the third trimester in 12 patients, and during the postpartum period in 7 patients. Twenty-nine patients had no other clinical context but pregnancy, whereas 13 patients concomitantly exhibited another clinical condition such as ovarian stimulation (n = 2), autoimmune disease (n = 4), or infection (n = 7). Autoantibodies to ADAMTS13 were positive in 23 patients (9 patients had inhibitory IgG, 7 noninhibitory IgG, and 7 circulating inhibitor without IgG). In contrast, 19 patients had neither anti-ADAMTS13 IgG nor inhibitor. ADAMTS13 Ag was decreased (lower than 300 ng/mL) in 80% of patients and the VWF:Ag levels ranged from 113%-580% and were correlated with the term of pregnancy. All patients except 4 (patients 23, 24, 30, and 31; Table 1) received curative PT consisting of plasma exchange (Table 1). PT was continued until delivery when the fetus was still alive in utero at the time of TTP diagnosis. Other therapies, most often associated with PT, consisted of steroids (n = 17), other immunomodulating agents (cyclophosphamide (n = 1), or rituximab (n = 3) used only after delivery; heparin (n = 1); aspirin (n = 2); or platelet concentrates (n = 2). The maternal survival rate without sequelae was 88% (95% CI, 75%-95%; 37 of 42 patients). Three patients exhibited neurologic or renal sequelae and 2 patients died. The global live-birth rate was 31% (95% CI, 19%-48%; 11 of 35 patients with exclusion of postpartum TTP). Outcome for the babies was strongly related to the term of pregnancy: TTP boots occurring during the first or the second trimester of pregnancy were mostly associated with a miscarriage or an intrauterine fetal death (22 of 23 patients) and TTP boots during the third trimester were mostly associated with good baby outcome (10 of 12 patients; Table 1).
Clinical and biological features of the pregnancy-onset TTP boot in 42 patients
| Patient no. . | Previous pregnancies (fetal loss), n . | Current pregnancy-associated TTP . | |||||
|---|---|---|---|---|---|---|---|
| Term (WG) . | Other associated context . | Anti-ADAMTS13 IgG/inhibitor . | Treatment . | Mother outcome . | Baby outcome . | ||
| 1 | 0 | 1 | Ovarian stimulation (IVF) | +/+ | PT/steroids | Good | Miscarriage |
| 2 | 1 | 1 | Ovarian stimulation (IVF) | +/− | PT/steroids | Good | Miscarriage |
| 3 | 0 | 4 | Thyroitidis | −/− | PT/steroids/antihypertensive drugs | Good | Miscarriage |
| 4 | 1 (1) | 4 | +/− | PT/steroids | Good | Miscarriage | |
| 5* | 0 | 10 | −/− | PT | Good | Miscarriage | |
| 6 | 0 | 11 | Urinary infection | +/+ | PT/steroids | Good | Miscarriage |
| 7 | 0 | 11 | Viral infection | +/− | PT until delivery (38 WG) | Good | Good |
| 8* | 0 | 12 | −/− | PT | Good | Miscarriage | |
| 9 | 0 | 12 | +/+ | PT/steroids/aspirin | Good | Miscarriage | |
| 10 | 0 | 12 | HIV | +/− | PT/Rituximab | Neurologic sequellae | Miscarriage |
| 11 | 1 | 16 | +/+ | PT/steroids | Good | IUFD | |
| 12 | 0 | 20 | +/+ | PT | Good | IUFD | |
| 13 | 0 | 23 | +/+ | PT | Good | IUFD | |
| 14 | 0 | 24 | Influenza infection | −/+ | PT | Good | IUFD |
| 15 | 0 | 24 | Lupus, APLS | +/− | PT/steroids/rituximab | Good | IUFD |
| 16* | 0 | 25 | −/− | PT | Good | Death at day 15 | |
| 17 | 0 | 25 | −/+ | PT | Good | IUFD | |
| 18 | 0 | 25 | −/+ | PT | Good | IUFD | |
| 19 | 0 | 25 | −/+ | PT/steroids | Good | IUFD | |
| 20 | 0 | 25 | −/− | PT/steroids/heparin | Good | IUFD | |
| 21* | 0 | 26 | −/− | PT | Good | IUFD | |
| 22* | 0 | 27 | −/− | PT | Good | IUFD | |
| 23 | 2 | 28 | +/+ | Platelet concentrates | Death | IUFD | |
| 24* | 0 | 30 | −/− | Good | Death at day 40 | ||
| 25 | 0 | 31 | −/− | PT | Good | Good | |
| 26 | 2 (1) | 31 | Thyroitidis | −/− | PT/steroids | Good | Good |
| 27 | 0 | 32 | −/− | PT/steroids | Good | IUFD | |
| 28* | 0 | 33 | +/+ | PT | Good | Good | |
| 29 | 0 | 35 | −/+ | PT/steroids | Good | Good | |
| 30* | 0 | 35 | −/− | Aspirin | Good | Good | |
| 31 | 2 | 36 | Auto-immune thrombocytopenic purpura | −/+ | Platelet concentrates/steroids/IV Ig | Death | Good |
| 32* | 0 | 36 | −/− | PT | Good | Good | |
| 33 | 7 (3) | 36 | −/− | PT | Good | Good | |
| 34 | 0 | 37 | −/− | PT | Good | Good | |
| 35* | 0 | 38 | −/− | PT/steroids | Good | Good | |
| 36 | 1 | PP | +/+ | PT | Good | Good | |
| 37 | 0 | PP | −/− | PT | Good | Good | |
| 38 | 0 | PP | Sepsis | −/− | PT | Good | Good |
| 39 | 4 | PP | +/− | PT/steroids/rituximab/splenectomy | Neurologic sequellae | Good | |
| 40 | 0 | PP | Viral infection | +/− | PT/steroids/cyclophosphamide | Renal sequellae | Good |
| 41 | 0 | PP | −/+ | PT | Good | Good | |
| 42 | 3 | PP | Viral infection | −/− | PT | Good | Good |
| Patient no. . | Previous pregnancies (fetal loss), n . | Current pregnancy-associated TTP . | |||||
|---|---|---|---|---|---|---|---|
| Term (WG) . | Other associated context . | Anti-ADAMTS13 IgG/inhibitor . | Treatment . | Mother outcome . | Baby outcome . | ||
| 1 | 0 | 1 | Ovarian stimulation (IVF) | +/+ | PT/steroids | Good | Miscarriage |
| 2 | 1 | 1 | Ovarian stimulation (IVF) | +/− | PT/steroids | Good | Miscarriage |
| 3 | 0 | 4 | Thyroitidis | −/− | PT/steroids/antihypertensive drugs | Good | Miscarriage |
| 4 | 1 (1) | 4 | +/− | PT/steroids | Good | Miscarriage | |
| 5* | 0 | 10 | −/− | PT | Good | Miscarriage | |
| 6 | 0 | 11 | Urinary infection | +/+ | PT/steroids | Good | Miscarriage |
| 7 | 0 | 11 | Viral infection | +/− | PT until delivery (38 WG) | Good | Good |
| 8* | 0 | 12 | −/− | PT | Good | Miscarriage | |
| 9 | 0 | 12 | +/+ | PT/steroids/aspirin | Good | Miscarriage | |
| 10 | 0 | 12 | HIV | +/− | PT/Rituximab | Neurologic sequellae | Miscarriage |
| 11 | 1 | 16 | +/+ | PT/steroids | Good | IUFD | |
| 12 | 0 | 20 | +/+ | PT | Good | IUFD | |
| 13 | 0 | 23 | +/+ | PT | Good | IUFD | |
| 14 | 0 | 24 | Influenza infection | −/+ | PT | Good | IUFD |
| 15 | 0 | 24 | Lupus, APLS | +/− | PT/steroids/rituximab | Good | IUFD |
| 16* | 0 | 25 | −/− | PT | Good | Death at day 15 | |
| 17 | 0 | 25 | −/+ | PT | Good | IUFD | |
| 18 | 0 | 25 | −/+ | PT | Good | IUFD | |
| 19 | 0 | 25 | −/+ | PT/steroids | Good | IUFD | |
| 20 | 0 | 25 | −/− | PT/steroids/heparin | Good | IUFD | |
| 21* | 0 | 26 | −/− | PT | Good | IUFD | |
| 22* | 0 | 27 | −/− | PT | Good | IUFD | |
| 23 | 2 | 28 | +/+ | Platelet concentrates | Death | IUFD | |
| 24* | 0 | 30 | −/− | Good | Death at day 40 | ||
| 25 | 0 | 31 | −/− | PT | Good | Good | |
| 26 | 2 (1) | 31 | Thyroitidis | −/− | PT/steroids | Good | Good |
| 27 | 0 | 32 | −/− | PT/steroids | Good | IUFD | |
| 28* | 0 | 33 | +/+ | PT | Good | Good | |
| 29 | 0 | 35 | −/+ | PT/steroids | Good | Good | |
| 30* | 0 | 35 | −/− | Aspirin | Good | Good | |
| 31 | 2 | 36 | Auto-immune thrombocytopenic purpura | −/+ | Platelet concentrates/steroids/IV Ig | Death | Good |
| 32* | 0 | 36 | −/− | PT | Good | Good | |
| 33 | 7 (3) | 36 | −/− | PT | Good | Good | |
| 34 | 0 | 37 | −/− | PT | Good | Good | |
| 35* | 0 | 38 | −/− | PT/steroids | Good | Good | |
| 36 | 1 | PP | +/+ | PT | Good | Good | |
| 37 | 0 | PP | −/− | PT | Good | Good | |
| 38 | 0 | PP | Sepsis | −/− | PT | Good | Good |
| 39 | 4 | PP | +/− | PT/steroids/rituximab/splenectomy | Neurologic sequellae | Good | |
| 40 | 0 | PP | Viral infection | +/− | PT/steroids/cyclophosphamide | Renal sequellae | Good |
| 41 | 0 | PP | −/+ | PT | Good | Good | |
| 42 | 3 | PP | Viral infection | −/− | PT | Good | Good |
Patient was diagnosed retrospectively with hereditary TTP (Upshaw-Schulman syndrome).
WG indicates weeks of gestation; IVF, in vitro fertilization; and IUFD, intrauterine fetal death.
Distinction between inherited (USS) and acquired TTP
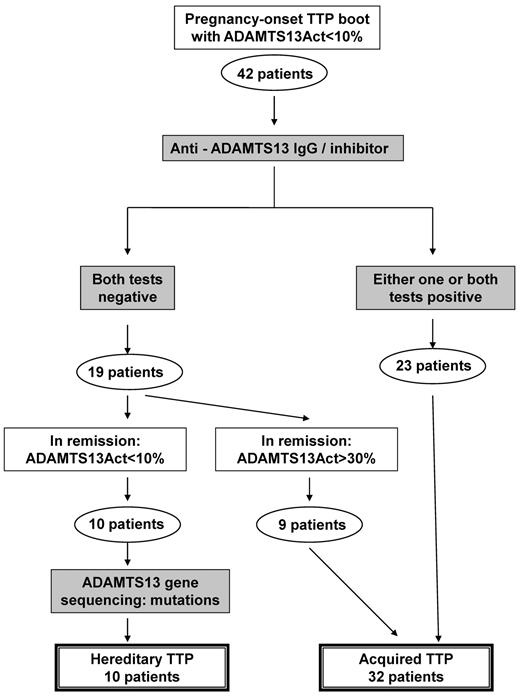
Differential diagnosis between the inherited form (USS) and the acquired form of TTP was performed, mostly retrospectively, using the flow chart presented in Figure 2. By definition, all patients exhibited an ADAMTS13 activity < 10% during the TTP boot. Twenty-three patients had autoantibodies to ADAMTS13 and were diagnosed with acquired TTP at the time of the TTP boot. In contrast, 19 patients had neither anti-ADAMTS13 IgG nor inhibitor during the TTP boot: 9 of them recovered a detectable ADAMTS13 activity (> 30%) in clinical remission and were further considered as acquired TTP. In contrast, 10 patients (including 2 sisters) still exhibited an undetectable ADAMTS13 activity in remission together with the absence of autoantibodies to ADAMTS13. ADAMTS13 gene sequencing confirmed the diagnosis of USS (patients 5, 8, 16, 21, 22, 24, 28, 30, 32, 35; Table 1). Finally, our cohort of 42 obstetrical-onset TTP patients included 10 inherited (USS) forms (24%; 95% CI, 13%-39%) and 32 acquired forms (76%; 95% CI, 61%-87%). All USS patients had undetectable ADAMTS13 Ag levels. In addition, all 32 patients with acquired TTP recovered a detectable ADAMTS13 activity (> 50%) 3 months after the TTP boot.
Flowchart for the differential diagnosis between hereditary and acquired TTP. Differential diagnosis between the inherited form (USS) and the acquired form of TTP was performed using the following flowchart. By definition, all enrolled patients exhibited an ADAMTS13 activity < 10% at inclusion. During the TTP boot, 23 patients had autoantibodies to ADAMTS13 (acquired TTP), whereas 19 patients had neither anti-ADAMTS13 IgG nor inhibitor (no differential diagnosis between inherited and acquired TTP). Among these 19 patients, 9 recovered a detectable ADAMTS13 activity (> 30%) in clinical remission, allowing the retrospective diagnosis of acquired TTP. In contrast, 10 patients still exhibited an ADAMTS13 activity < 10% in remission with no autoantibody against ADAMTS13, and ADAMTS13 gene sequencing confirmed the diagnosis of USS. Our cohort of 42 obstetrical-onset TTP patients included 10 USS and 32 acquired TTP patients.
Flowchart for the differential diagnosis between hereditary and acquired TTP. Differential diagnosis between the inherited form (USS) and the acquired form of TTP was performed using the following flowchart. By definition, all enrolled patients exhibited an ADAMTS13 activity < 10% at inclusion. During the TTP boot, 23 patients had autoantibodies to ADAMTS13 (acquired TTP), whereas 19 patients had neither anti-ADAMTS13 IgG nor inhibitor (no differential diagnosis between inherited and acquired TTP). Among these 19 patients, 9 recovered a detectable ADAMTS13 activity (> 30%) in clinical remission, allowing the retrospective diagnosis of acquired TTP. In contrast, 10 patients still exhibited an ADAMTS13 activity < 10% in remission with no autoantibody against ADAMTS13, and ADAMTS13 gene sequencing confirmed the diagnosis of USS. Our cohort of 42 obstetrical-onset TTP patients included 10 USS and 32 acquired TTP patients.
Comparison between inherited (USS) and acquired TTP
At presentation of the pregnancy-onset TTP, USS and acquired TTP patients (clinical and biologic features) were not significantly different except for nulliparity (100% in USS vs 62.5% in acquired TTP) and the presence of an autoantibody to ADAMTS13 (0% in USS vs 55% in acquired TTP; P < .03). PT (mostly alone) was successfully used in 8 USS patients of 10, whereas, interestingly, 2 USS patients (patients 24 and 30) did not need PT to achieve remission (Table 1). PT (mostly associated with other drugs) was successful in 30 of 32 acquired TTP patients. In our cohort, the clinical short-term outcome was similar between USS and acquired TTP patients, showing a maternal survival rate higher than 90% in both groups and a live-birth rate of 40% and 28% in the USS and acquired TTP groups, respectively.
Genotypic analysis of patients with USS
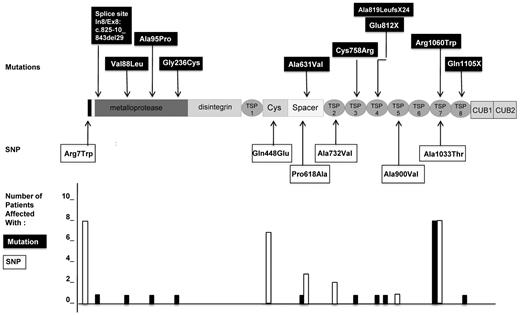
The genotypes of the 10 patients with USS are presented in Table 2. Ten distinct mutations of the ADAMTS13 gene were found, consisting of 1 deletion of 29 bp in the intron7/exon8 junction (previously described in patient 2232 ), 1 deletion of 1 nucleotide resulting in a splice mutation, 2 nonsense mutations, and 6 missense mutations. Five mutations were located in the N-terminal part of ADAMTS13 before or within the spacer domain (In7/Ex8p: c.825-10_843del29, p.Val88Leu, p.Ala95Pro, p.Gly236Cys, and p.Ala631Val) and 5 mutations were located in the C-terminal part of ADAMTS13 (p.Cys758Arg, p.Glu812X, c.2455delG p.Ala819LeufsX24, p.Arg1060Trp, and p.Gln1105X; Figure 3). One patient was a homozygote (patient 8); 7 patients were compound heterozygotes, including 1 patient with 3 mutations (patient 5); and 2 patients were heterozygous for 1 mutation (patients 24 and 30). Interestingly, the mutation p.Arg1060Trp was the most frequent, being found in 8 of 10 patients, and was systematically associated with the p.Arg7Trp and p.Ala1033Thr SNP (Figure 3). Four other SNPs were also found in these patients: p.Gln448Glu, p.Pro618Ala, p.Ala732Val, and p.Ala900Val (Figure 3 and Table 2).
Genetic analysis of ADAMTS13 gene in 10 patients (9 families) with USS
| Patient no. . | ADAMTS13 mutations . | ADAMTS13* polymorphisms . | |||
|---|---|---|---|---|---|
| Intron/exon . | Variant . | Status . | Protein . | ||
| 8 | Exon 7 | c.706G > T | Homozygous | p.Gly236Cys | c.1342C > G p.Gln448Glu |
| 22 | Exon 8 | c.825–10_843del29 | Heterozygous | p. ? | c.1342C > G p.Gln448Glu |
| Exon 19 | c.2272T > C | Heterozygous | p.Cys758Arg | ||
| 5 | Exon 3 | c.283G > C | Heterozygous | p.Ala95Pro | c.19C > T p.Arg7Trp |
| Exon 16 | c.1892C > T | Heterozygous | p.Ala631Val | c.3097G > A p.Ala1033Thr | |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | ||
| 21† | Exon 20 | c.2434G > T | Heterozygous | p.Glu812X | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.3097G > A p.Ala1033Thr | |||||
| 32† | Exon 20 | c.2434G > T | Heterozygous | p.Glu812X | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.3097G > A p.Ala1033Thr | |||||
| 28 | Exon 3 | c.262G > C | Heterozygous | p.Val88Leu | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.1852C > G p.Pro618Ala | |||||
| c.2699 C > T p.Ala900Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
| 16 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| Exon 25 | c.3313C > T | Heterozygous | p.Gln1105X | c.3097G > A p.Ala1033Thr | |
| 35 | Exon 20 | c.2455delG | Heterozygous | p.Ala819LeufsX24 | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.3097G > A p.Ala1033Thr | |
| 24 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| c.1342C > G p.Gln448Glu | |||||
| c.1852C > G p.Pro618Ala | |||||
| c.2195C > T p.Ala732Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
| 30 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| c.1342C > G p.Gln448Glu | |||||
| c.1852C > G p.Pro618Ala | |||||
| c.2195C > T p.Ala732Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
| Patient no. . | ADAMTS13 mutations . | ADAMTS13* polymorphisms . | |||
|---|---|---|---|---|---|
| Intron/exon . | Variant . | Status . | Protein . | ||
| 8 | Exon 7 | c.706G > T | Homozygous | p.Gly236Cys | c.1342C > G p.Gln448Glu |
| 22 | Exon 8 | c.825–10_843del29 | Heterozygous | p. ? | c.1342C > G p.Gln448Glu |
| Exon 19 | c.2272T > C | Heterozygous | p.Cys758Arg | ||
| 5 | Exon 3 | c.283G > C | Heterozygous | p.Ala95Pro | c.19C > T p.Arg7Trp |
| Exon 16 | c.1892C > T | Heterozygous | p.Ala631Val | c.3097G > A p.Ala1033Thr | |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | ||
| 21† | Exon 20 | c.2434G > T | Heterozygous | p.Glu812X | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.3097G > A p.Ala1033Thr | |||||
| 32† | Exon 20 | c.2434G > T | Heterozygous | p.Glu812X | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.3097G > A p.Ala1033Thr | |||||
| 28 | Exon 3 | c.262G > C | Heterozygous | p.Val88Leu | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.1342C > G p.Gln448Glu | |
| c.1852C > G p.Pro618Ala | |||||
| c.2699 C > T p.Ala900Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
| 16 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| Exon 25 | c.3313C > T | Heterozygous | p.Gln1105X | c.3097G > A p.Ala1033Thr | |
| 35 | Exon 20 | c.2455delG | Heterozygous | p.Ala819LeufsX24 | c.19C > T p.Arg7Trp |
| Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.3097G > A p.Ala1033Thr | |
| 24 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| c.1342C > G p.Gln448Glu | |||||
| c.1852C > G p.Pro618Ala | |||||
| c.2195C > T p.Ala732Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
| 30 | Exon 24 | c.3178C > T | Heterozygous | p.Arg1060Trp | c.19C > T p.Arg7Trp |
| c.1342C > G p.Gln448Glu | |||||
| c.1852C > G p.Pro618Ala | |||||
| c.2195C > T p.Ala732Val | |||||
| c.3097G > A p.Ala1033Thr | |||||
In all patients, polymorphisms were found with a heterozygous status except in patient 22 whose single-nucleotide polymorphism was homozygous.
Patients 21 and 32 are sisters.
ADAMTS13 mutations and SNPs in 10 patients with pregnancy-onset USS. Shown are the location and number of affected patients. Ten distinct mutations of ADAMTS13 gene (indicated in black boxes) were found. Five mutations were located in the N-terminal part of ADAMTS13, 4 of them within the metalloprotease domain (In7/Ex8p: c.825-10_843del29, p.Val88Leu, p.Ala95Pro, and p.Gly236Cys) and 1 within the spacer domain (p.Ala631Val). Five mutations were located in the C-terminal part of ADAMTS13, 1 within the third TSP-1 domain (p.Cys758Arg), 2 within the fourth TSP-1 domain (c.2455delG p.Ala819LeufsX24 and p.Glu812X), 1 within the seventh TSP-1 domain (p.Arg1060Trp), and 1 within the 8 TSP-1 domain (p.Gln1105X). Six miscellaneous SNPs of the ADAMTS13 gene (indicated in white boxes) were also found. Three SNPs were located in the N-terminal part of ADAMTS13 within the signal peptide (p.Arg7Trp), the cysteine-rich domain (p.Gln448Glu), or the spacer domain (p.Pro618Ala). Three other SNP were located in the C-terminal part of ADAMTS13 in the second TSP-1 domain (p.Ala732Val), the fifth TSP-1 domain (p.Ala900Val), or the seventh TSP-1 domain (p.Ala1033Thr). The number of patients affected by each mutation (black histograms) and each SNP (white histograms) are presented at the bottom of the figure. Interestingly, the mutation p.Arg1060Trp, the SNP p.Arg7Trp, and the SNP p.Ala1033Thr (systematically associated) were present in 8 of 10 patients and therefore constituted a cluster of ADAMTS13 genetic variants. The 9 other mutations were present only in single patients. The SNP p.Gln448Glu was the second most frequent SNP, present in 7 patients.
ADAMTS13 mutations and SNPs in 10 patients with pregnancy-onset USS. Shown are the location and number of affected patients. Ten distinct mutations of ADAMTS13 gene (indicated in black boxes) were found. Five mutations were located in the N-terminal part of ADAMTS13, 4 of them within the metalloprotease domain (In7/Ex8p: c.825-10_843del29, p.Val88Leu, p.Ala95Pro, and p.Gly236Cys) and 1 within the spacer domain (p.Ala631Val). Five mutations were located in the C-terminal part of ADAMTS13, 1 within the third TSP-1 domain (p.Cys758Arg), 2 within the fourth TSP-1 domain (c.2455delG p.Ala819LeufsX24 and p.Glu812X), 1 within the seventh TSP-1 domain (p.Arg1060Trp), and 1 within the 8 TSP-1 domain (p.Gln1105X). Six miscellaneous SNPs of the ADAMTS13 gene (indicated in white boxes) were also found. Three SNPs were located in the N-terminal part of ADAMTS13 within the signal peptide (p.Arg7Trp), the cysteine-rich domain (p.Gln448Glu), or the spacer domain (p.Pro618Ala). Three other SNP were located in the C-terminal part of ADAMTS13 in the second TSP-1 domain (p.Ala732Val), the fifth TSP-1 domain (p.Ala900Val), or the seventh TSP-1 domain (p.Ala1033Thr). The number of patients affected by each mutation (black histograms) and each SNP (white histograms) are presented at the bottom of the figure. Interestingly, the mutation p.Arg1060Trp, the SNP p.Arg7Trp, and the SNP p.Ala1033Thr (systematically associated) were present in 8 of 10 patients and therefore constituted a cluster of ADAMTS13 genetic variants. The 9 other mutations were present only in single patients. The SNP p.Gln448Glu was the second most frequent SNP, present in 7 patients.
Prediction of the functional effect of protein changes induced by ADAMTS13 mutations and SNPs
None of the ADAMTS13 mutations was present in the dbSNP135 or 1000 Genomes databases or in 200 alleles from 100 controls, enabling us to rule out that the identified variants were common polymorphisms. Except for p.Arg1060Trp, all 9 other mutations were described only in French patients, including 2 previously reported mutations in 1 family32 and 7 novel mutations. The mutations p.Glu812X, p.Gln1105X, and p.Ala819LeufsX24 are certainly deleterious because they induce the destruction of mRNA by nonsense-mediated mRNA decay and thus a secretion defect of ADAMTS13. The mutations p.Val88Leu, p.Gly236Cys, and p.Cys758Arg were predicted to be deleterious using both Polyphen-2 and SIFT software. The prediction of the protein functional effect of mutations p.Ala95Pro and p.Ala631Val, respectively, was discrepant between these software programs.
All SNPs found in our patients have been reported previously in the literature.8 SNP p.Arg7Trp, p.Gln448Glu, and p.Ala900Val were predicted to be nondeleterious using both Polyphen-2 and SIFT, whereas p.Ala732Val was predicted to be deleterious with SIFT only. In contrast, p.Ala618Pro and p.Ala1033Thr were predicted to be deleterious with both programs.
Personal history and familial inquiry of USS patients
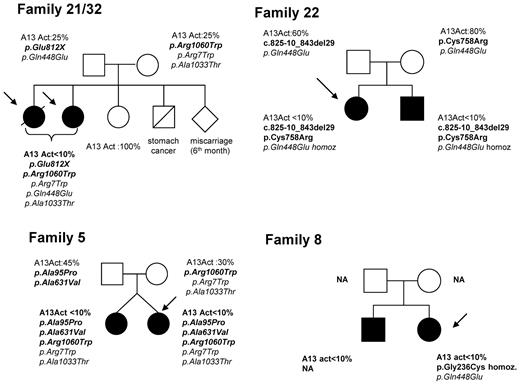
None of our 10 USS patients experienced TTP boot before their first pregnancy. Among our 9 families with USS, 5 had no history of TTP and 4 exhibited a pedigree including affected siblings (Figure 4). However, except for patient 22, the inquiry performed in these latter families did not identify any childhood-onset TTP cases (no neonatal jaundice, no exsanguinous transfusions, no thrombocytopenia, and no hemolytic anemia).
Pedigrees of 4 families of patients with pregnancy-onset hereditary TTP (USS). The siblings of patients 21 and 32 (sisters), who share the same ADAMTS13 genotype, including 2 heterozygous mutations (p.Glu812X and p.Arg1060Trp) and 3 SNPs (p.Arg7Trp, p.Gln448Glu, and p.Ala1033Thr), included 1 miscarriage at 6 months of gestation and 1 unaffected sister. Both parents have a partial ADAMTS13 functional deficiency (ADAMTS13 activity: 25%) and each of them transmitted 1 mutation. Patient 5 has a twin sister who also has a severe ADAMTS13 functional deficiency related to the same ADAMTS13 genotype, including 3 heterozygous mutations (p.Ala95Pro, p.Ala631Val, and p.Arg1060Trp) and 2 polymorphisms (p.Arg7Trp and p.Ala1033Thr). Until now, the twin sister experienced neither pregnancy nor TTP boot. The father has a partial ADAMTS13 functional deficiency (45%) and he transmitted 2 mutations (p.Ala95Pro and p.Ala631Val, likely located on the same allele). The mother also has a partial ADAMTS13 functional deficiency (30%) and she transmitted the third mutation (p.Arg1060Trp). In family of patient 22, both siblings share the same ADAMTS13 phenotype and genotype (2 heterozygous mutations, c.825-10_843del29 and p.Cys758Arg, and 1 homozygous polymorphism, p.Gln448Glu) despite variable clinical expression (childhood-onset TTP in the brother). Both parents exhibit a normal ADAMTS13 activity and each of them transmitted 1 mutation (c.825-10_843del29 for the father and p.Cys758Arg for the mother) and the p.Gln448Glu polymorphism. The brother of patient 8 exhibits an ADAMTS13 activity lower than 10% and a clinical history of recurrent adulthood-onset TTP. His DNA was not available (NA) for genotypic analysis. Neither parent was available for familial inquiry.
Pedigrees of 4 families of patients with pregnancy-onset hereditary TTP (USS). The siblings of patients 21 and 32 (sisters), who share the same ADAMTS13 genotype, including 2 heterozygous mutations (p.Glu812X and p.Arg1060Trp) and 3 SNPs (p.Arg7Trp, p.Gln448Glu, and p.Ala1033Thr), included 1 miscarriage at 6 months of gestation and 1 unaffected sister. Both parents have a partial ADAMTS13 functional deficiency (ADAMTS13 activity: 25%) and each of them transmitted 1 mutation. Patient 5 has a twin sister who also has a severe ADAMTS13 functional deficiency related to the same ADAMTS13 genotype, including 3 heterozygous mutations (p.Ala95Pro, p.Ala631Val, and p.Arg1060Trp) and 2 polymorphisms (p.Arg7Trp and p.Ala1033Thr). Until now, the twin sister experienced neither pregnancy nor TTP boot. The father has a partial ADAMTS13 functional deficiency (45%) and he transmitted 2 mutations (p.Ala95Pro and p.Ala631Val, likely located on the same allele). The mother also has a partial ADAMTS13 functional deficiency (30%) and she transmitted the third mutation (p.Arg1060Trp). In family of patient 22, both siblings share the same ADAMTS13 phenotype and genotype (2 heterozygous mutations, c.825-10_843del29 and p.Cys758Arg, and 1 homozygous polymorphism, p.Gln448Glu) despite variable clinical expression (childhood-onset TTP in the brother). Both parents exhibit a normal ADAMTS13 activity and each of them transmitted 1 mutation (c.825-10_843del29 for the father and p.Cys758Arg for the mother) and the p.Gln448Glu polymorphism. The brother of patient 8 exhibits an ADAMTS13 activity lower than 10% and a clinical history of recurrent adulthood-onset TTP. His DNA was not available (NA) for genotypic analysis. Neither parent was available for familial inquiry.
The pedigree of family 22 was described previously.32 The propositus exhibited a neonatal jaundice requiring exsanguinous transfusion and a moderate fluctuating thrombocytopenia (100 G/L) requiring no treatment. She never experienced any TTP boot before her first pregnancy. In contrast, her brother, who was also born with a jaundice requiring exsanguinous transfusion, exhibited a severe form of childhood-onset TTP requiring monthly prophylactic PT. Both siblings share the same ADAMTS13 phenotype and genotype (2 heterozygous mutations, c.825-10_843del29 and p.Cys758Arg, and 1 homozygous polymorphism, p.Gln448Glu) despite distinct clinical expression. Both parents exhibit a normal ADAMTS13 activity and each of them transmitted one mutation.
In the family of patients 21 and 32 (sisters) whose genotype shows 2 heterozygous mutations (p.Glu812X and p.Arg1060Trp) and 3 polymorphisms (p.Arg7Trp, p.Gln448Glu, and p.Ala1033Thr), 1 miscarriage at 6 months of gestation was seen among the siblings, and another sister is unaffected. Both parents have a partial ADAMTS13 functional deficiency (ADAMTS13 activity, 25%) and each of them transmitted 1 mutation.
In family 5, the propositus has a twin sister who also has a severe ADAMTS13 functional deficiency related to the same ADAMTS13 genotype, including 3 heterozygous mutations (p.Ala95Pro, p.Ala631Val, and p.Arg1060Trp) and 2 polymorphisms (p.Arg7Trp and p.Ala1033Thr). She had never been pregnant before and had never experienced any prior TTP boot. Both parents have a partial ADAMTS13 functional deficiency (father, 45%; mother, 30%). The father transmitted 2 mutations (p.Ala95Pro and p.Ala631Val, likely located on the same allele) and the mother transmitted the third mutation (p.Arg1060Trp).
The brother of the propositus from family 8 exhibits an ADAMTS13 activity lower than 10% and a clinical history of recurrent adult-onset TTP, but his genotypic analysis could not be performed. Neither parent was available for familial inquiry.
In families from propositi 16, 24, 28, 30, and 35, no specific medical antecedent could be observed.
Follow-up and management of later pregnancies in USS patients
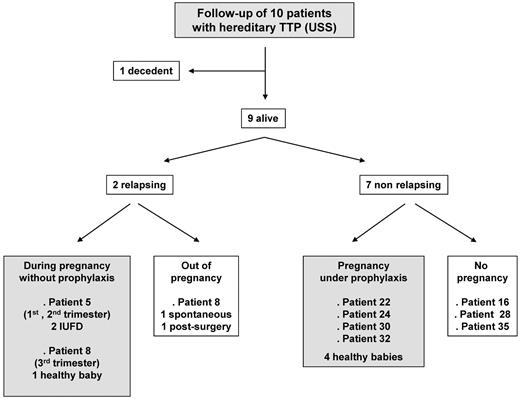
All USS patients (7 pregnancies) benefited from a follow-up (Figure 5). None of them required a prophylactic PT when they were not pregnant. Patient 21 died of a cerebral tumor a few years after her pregnancy. Two patients relapsed either when not pregnant (patient 8) or during subsequent pregnancies led without prophylaxis (patient 5 and 8). Patient 5 experienced 2 other pregnancies, which ended with TTP-induced intrauterine fetal death in the first and second trimesters, respectively. In contrast, the TTP relapse occurred late during the third trimester in patient 8, who benefited from a curative PT (plasma exchange) that resulted in good outcomes for both mother and baby. Seven patients did not relapse, including 4 patients who underwent another pregnancy under prophylaxis (intermittent plasma infusion in 3 patients and aspirin in 1 patient), allowing favorable maternal and baby outcomes.
Follow-up of the 10 USS patients. The 10 USS patients benefited from a follow-up (7 subsequent pregnancies). Patient 21 died of a cerebral tumor during the follow-up. Two patients relapsed either after the pregnancy (patient 8) or during subsequent pregnancies led without prophylaxis (patient 5 and 8). Seven patients did not relapse, including 4 patients who had another pregnancy under prophylaxis, allowing favorable maternal and baby outcomes.
Follow-up of the 10 USS patients. The 10 USS patients benefited from a follow-up (7 subsequent pregnancies). Patient 21 died of a cerebral tumor during the follow-up. Two patients relapsed either after the pregnancy (patient 8) or during subsequent pregnancies led without prophylaxis (patient 5 and 8). Seven patients did not relapse, including 4 patients who had another pregnancy under prophylaxis, allowing favorable maternal and baby outcomes.
Discussion
The present study was focused on pregnancy-onset TTP associated with a severe ADAMTS13 functional deficiency. Our major finding was that the differential diagnosis between hereditary and acquired TTP identified a very high rate of adulthood-onset USS linked to a cluster of genetic variants of the ADAMTS13 gene. Moreover, regardless of the etiology of the TTP, the time of occurrence during pregnancy (30 weeks of gestation threshold) was the major factor related to baby outcome. The results of the present study also emphasize that, for subsequent pregnancies, USS patients require specific management that may dramatically change both the TTP relapse rate and prognosis for the baby.
A strength of our study is that it used an organization as a reference center for TMAs, including a central ADAMTS13 laboratory. Therefore, the 10-year network built with the great majority of the university hospitals in France allowed us to establish a unique national registry, especially for the specific subgroup of patients with TTP related to a severe ADAMTS13 deficiency.33 Of 592 of these adult patients enrolled over 10 years, 48 pregnancy-associated cases of TTP were identified. Considering that France numbers approximately 950 000 pregnancies per year, a TTP related to a severe ADAMTS13 deficiency occurs in at least 1 in 198 000 pregnancies. As expected, this rate is lower than the 1 in 25 000 to 1 in 100 000 range reported previously,1-5 because the latter included either both TTP and hemolytic uremic syndrome or miscellaneous TTP with no systematic documentation of ADAMTS13. In agreement with previous results,1-5,17 our present study shows that pregnancy is an associated condition in 17% (95% CI, 13%-22%) of women of childbearing age with TTP, most of them experiencing their first TTP episode. In addition, TTP occurs most frequently during the second trimester of pregnancy (60%)1-5,17 and, more rarely, during the first trimester26,27 and the postpartum period.17
Surprisingly, the ADAMTS13 genetic analysis performed in the present study emphasizes that the rate of USS is much higher in pregnancy-onset TTP patients (24%; 95% CI, 13%-39%) than in all adulthood-onset TTP patients: less than 5% in previous studies4,5,8,34 and approximately 2% (95% CI, 0.9%-4%) in our own cohort of adulthood-onset TTP (unpublished data). Our 10 pregnancy-onset USS patients exhibited 10 distinct ADAMTS13 mutations, including 3 previously reported mutations32,35 and 7 novel mutations, the deleterious effects of which were supported by genetic software analysis, personal clinical/biologic phenotype, and familial inquiry. In addition, in stark contrast to childhood-onset USS, in which ADAMTS13 mutations are miscellaneous, spread all over the gene, and carried with very heterogeneous combinations,8,34,36 most of our pregnancy-onset USS patients (8 of 10 patients) had in common 1 unique ADAMTS13 mutation: p.Arg1060Trp. This mutation has already been described in 8 European/North American adulthood-onset USS patients, including interestingly, 6 women with pregnancy-onset TTP.22,35,37-39 The p.Arg1060Trp mutation, which affects ADAMTS13 synthesis/secretion,35 appears to be strongly associated with adulthood-onset USS because it has never been found in childhood-onset USS either in previous studies39 or in our own pediatric cohort of 24 families (unpublished data). Interestingly, this mutation seems to be specific to the West because it is not present in the Japanese cohort of adulthood-onset USS patients, including 9 patients with pregnancy-onset USS.19,34 Among our 8 USS patients who were heterozygous for the p.Arg1060Trp mutation, 6 also carried another ADAMTS13 mutation, whereas no other ADAMTS13 mutation could be detected in 2 patients (patients 24 and 30). This isolated heterozygous status for the p.Arg1060Trp mutation has already been reported in 3 USS patients whose first TTP boot was also triggered by pregnancy.22,39 Interestingly, our 2 USS patients heterozygous for the p.Arg1060Trp mutation also carried both the p.Pro618Ala SNP (like the 1 patient described by Camilleri et al39 ) and the p.Ala732Val SNP, the combination of which reduces ADAMTS13 activity significantly.39,40 However, these results do not exclude that another ADAMTS13 mutation undetected by the current genetic analysis (eg, a mutation of the ADAMTS13 gene promoter or an intronic mutation) may be present in both of these patients. Therefore, the diagnosis of USS remains most likely in both of these patients, first, because of their clinical expression and ADAMTS13 phenotype (constant ADAMTS13 severe deficiency with no Ab) and, second, because sequencing of the ADAMTS13 gene revealed one mutation reported previously in association with USS in several studies (p.Arg1060Trp) plus multiple missense SNPs.
The presentation and outcome of our 10 USS patients are consistent with previous data in 15 cases of pregnancy-associated TTP in which the diagnosis of USS was also confirmed by both phenotypic and genotypic analysis of ADAMTS13.19,22,38,39 Pregnancy appears to be a constant trigger of the TTP boot, which usually occurs after 20 weeks of gestation, and maternal outcome is most often good if curative PT is performed; however, in some rare cases, such as our patients 24 and 30, patients are surprisingly able to achieve remission without PT.19 In our USS patients, the stillbirth rate remained very high (60%), with outcome of the baby being closely related to the term of pregnancy during which the TTP boot occurs: best prognosis is strongly associated with the third trimester. In agreement with previous studies,10,19,22 in the USS patients in the present study, subsequent pregnancies led with no prophylaxis were associated with a 100% risk for TTP relapse (3 of 3 pregnancies) and a 66% abortion rate (2 of 3 pregnancies). These observations justified a systematic prophylactic PT (intermittent plasma infusions) for later pregnancies, with an obvious benefit for both the mother and the baby (3 of 3 pregnancies), as described previously.19 The choice of aspirin as a potential prophylactic agent in one patient (patient 30) was empirical and probably linked to the fact that she had already received aspirin and did not need PT to achieve TTP remission during the index pregnancy. So far, the use of antiplatelet agents to prevent TTP relapses during pregnancy remains anecdotal.3,19,20
In the present study, we also identified prospectively 38 cases of pregnancy-associated acquired TTP related to a severe ADAMTS13 deficiency, including 32 patients who underwent an initial TTP boot. Analysis of the literature revealed 17 detailed case reports of pregnancy-associated acquired TTP in which ADAMTS13 was documented.10,19,23,24,28,41-46 Presentation and short-term outcome of our patients were similar to those reported previously, showing a great majority of first pregnancies, a higher prevalence during the second half of pregnancy, the presence of a previously diagnosed autoimmune context (ie, lupus, antiphospholipid antibody syndrome, or acquired TTP) in approximately 25% of cases, and the efficacy of curative PT (however, with a frequent need for additional immune modulators).47 In our patients, the stillbirth rate remains very high (approximately 70%; 95% CI, 52%-81%) and mainly related to the high frequency of TTP before 28 weeks of gestation, whereas episodes occurring during the third trimester of pregnancy were associated with a good baby prognosis.
In conclusion, the results of the present study emphasize that pregnancy-onset TTP defines a specific subgroup of patients among female adulthood-onset TTP patients who are related to a severe ADAMTS13 deficiency. This strong genetic background is based on a cluster of ADAMTS13 mutations and ethnic specificity. The differential diagnosis between hereditary and acquired ADAMTS13 deficiency in pregnancy-onset TTP remains difficult at presentation even though some clinical and biologic criteria (nulliparity, absence of autoimmune diseases, and absence of detectable autoantibodies to ADAMTS13) may be more in favor of USS. In that regard, the familial background is mostly unhelpful because USS is an orphan disease and also because rare cases of familial acquired autoimmune TTP have been reported.48 However, making the differential diagnosis between USS and acquired TTP after remission is of major interest for several reasons. First, it allows us to give specific information to patients in terms of definitive diagnosis (ie, genetic disease vs acquired autoimmune disease) and specific related consequences. Second, it determines a specific and better defined therapeutic management of subsequent pregnancies in USS patients to improve the outcome of both the mother (prevention of relapse) and the baby (prevention of fetal loss and prematurity). Third, it allowed familial inquiry into the asymptomatic nulliparous sisters of patients who experienced a pregnancy-onset USS. Finally, the results of the present study also confirm that any subsequent pregnancy in a TTP patient requires careful biologic and clinical monitoring (ideally as soon as the project of pregnancy) supported by a multidisciplinary team involving obstetricians, hematologists, apheresis specialists, and genetic experts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anita Alemchayan, Emmanuelle Bonelli-Lambling, Sophie Capdenat, Paulette Legendre, Sandrine Malot, Nathalie Marie, Sylvaine Savigny, Christine Thomas, and Sandrine Thouzeau for expert assistance; Dr C. Ternisien (Centre Hospitalier Universitaire de Nantes), Dr V. Guérin (Centre Hospitalier Universitaire de Bordeaux), Dr J.Y. Borg (Centre Hospitalier Universitaire de Rouen), Dr Gobert (Centre Hospitalier Universitaire d'Avignon), Dr B. Bonotte (Centre Hospitalier Universitaire de Dijon), Pr A. Mignon (Centre Hospitalier Universitaire Cochin); and Dr Hélène Agostini and Pr Laurent Becquemont from the Unité de Recherche Clinique Paris Sud.
This work was supported in part by a grant from the Délégation Régionale à la Recherche Clinique, AP-HP (PHRC AOM05012).
Authorship
Contribution: M.M.-C. performed the cross-sectional analysis of the French Registry for Thrombotic Microangiopathies, carried out the phenotypical analysis, interpreted the results, and wrote the manuscript; C.G., P.B., and S.B. performed the genetic analysis and critically reviewed the manuscript; M.W., P.C., and A.V. designed the study, interpreted the results, and critically reviewed the manuscript; L.G., E.A., A.S., Y.D., and E.R. enrolled most of the patients, collected clinical and laboratory information, and critically reviewed the manuscript; and E.A. performed the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Reference Center for the Management of Thrombotic Microangiopathies can be found in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
French Reference Center for Thrombotic Microangiopathies collaborators: Eric Mariotte (Service de Réanimation Médicale, Hôpital Saint Louis, AP-HP, Paris, France); Emmanuel DeMaistre Service d'Hématologie Biologique, CHU de Dijon, Dijon, France); Paquita Nurden (Service d'Hématologie Biologique, CHU de Bordeaux, Bordeaux, France); Francois Provôt (Service de Néphrologie, Centre Hospitalier Régional Universitaire de Lille, Lille, France); Arnaud Hot (Service de Médecine Interne, CHU de Lyon, Lyon, France); Karine Clabault (Service de Réanimation Médicale, CHU de Rouen, Rouen, France); Céline Desconclois (Service d'Hématologie Biologique, Hôpital Antoine Béclère, AP-HP, Clamart, France, and Inserm U770, Université Paris 11, Le Kremlin Bicêtre, France); and Marc Buffet (Département d'Hématologie Clinique, Hôpital Saint Antoine, AP-HP, Université Pierre et Marie Curie [Paris 6], Paris, France).
Correspondence: Pr Agnès Veyradier, MD, PhD, Service d'Hématologie Biologique, Hôpital Antoine Béclère, 157 rue de la Porte-de-Trivaux, 92140 Clamart, France; e-mail: agnes.veyradier@abc.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal