Abstract
Carfilzomib is a selective proteasome inhibitor that binds irreversibly to its target. In phase 1 studies, carfilzomib elicited promising responses and an acceptable toxicity profile in patients with relapsed and/or refractory multiple myeloma (R/R MM). In the present phase 2, multicenter, open-label study, 129 bortezomib-naive patients with R/R MM (median of 2 prior therapies) were separated into Cohort 1, scheduled to receive intravenous carfilzomib 20 mg/m2 for all treatment cycles, and Cohort 2, scheduled to receive 20 mg/m2 for cycle 1 and then 27 mg/m2 for all subsequent cycles. The primary end point was an overall response rate (≥ partial response) of 42.4% in Cohort 1 and 52.2% in Cohort 2. The clinical benefit response (overall response rate + minimal response) was 59.3% and 64.2% in Cohorts 1 and 2, respectively. Median duration of response was 13.1 months and not reached, and median time to progression was 8.3 months and not reached, respectively. The most common treatment-emergent adverse events were fatigue (62.0%) and nausea (48.8%). Single-agent carfilzomib elicited a low incidence of peripheral neuropathy—17.1% overall (1 grade 3; no grade 4)—in these pretreated bortezomib-naive patients. The results of the present study support the use of carfilzomib in R/R MM patients. This trial is registered at www.clinicaltrials.gov as NCT00530816.
Introduction
The American Cancer Society estimates that there will be approximately 21 700 new cases of multiple myeloma (MM) causing 10 710 deaths in the United States in 2012.1 There have been considerable advances in the treatment of MM over the last few years because of the introduction of the immunomodulatory agents thalidomide and its analog lenalidomide and approval of the proteasome inhibitor bortezomib.2 These agents have led to significant improvements in 5-year survival rates, from 26% in the 1970s to 37% in the period 1999-2005.3 However, almost all patients with MM will eventually relapse, and new treatment options are urgently needed, particularly for patients who relapse after more than one therapy.4
The clinical activity of bortezomib in hematologic malignancies, including MM and mantle cell lymphoma, has resulted in further interest in the proteasome as a therapeutic target in a broad range of cancers. The ubiquitin-proteasome pathway is a key regulator of the production and destruction of cellular proteins and mediates cell proliferation and survival, especially in malignant cells.5 Intracellular proteins targeted for degradation by the proteasome are first ubiquitinated via the ubiquitin conjugation system, and are then cleaved within the proteasome by 1 or more of 3 threonine protease activities: a chymotrypsin-like activity, a trypsin-like activity, or a caspase-like activity.5,6 Bortezomib acts predominantly to inhibit the chymotrypsin-like activity of the proteasome and achieves 70%-84% inhibition at the doses used in clinical practice.7
There are several limitations to the use of bortezomib, including development of resistance8 and, more importantly, dose-limiting peripheral neuropathy (PN).9 It is hoped that the next generation of proteasome inhibitors may be able to overcome some of these limitations. One such compound is carfilzomib, formerly PR-171, a potent, highly selective, and irreversible epoxyketone proteasome inhibitor that targets the chymotrypsin-like activity of the 20S proteasome.10,11 In preclinical studies, carfilzomib demonstrated potent proteasome inhibition in several tumor cell lines with minimal off-target activity,10,12 as well as in vitro activity in bortezomib-resistant cell lines, alone or in combination with other agents.13,14 Inhibition of nonproteasomal targets by bortezomib may play a role in the clinical adverse effects observed with this drug.15 In early clinical studies, carfilzomib has shown promising activity in patients with relapsed/refractory hematologic malignancies, including patients with MM. Responses have been rapid in onset and toxicities have been manageable (M.A., S. Trudel, R. R. Furman, P.J.R., O. A. O'Connor, R. L. Comenzo, A.F.W., L.A.K., C. J. Molineaux, A. Goy, A phase 1 single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res; manuscript submitted, 2012).16
Based on these data, 2 parallel phase 2 studies, PX-171-003 and PX-171-004, were designed to evaluate carfilzomib in patients with relapsed and/or refractory (R/R) MM. PX-171-003 evaluated patients who had R/R MM after at least 2 prior lines of therapy and who had been exposed to bortezomib and an immunomodulatory therapy, and PX-171-004 evaluated patients who had R/R MM with 1-3 prior lines of therapy. PX-171-003, the results of which have been submitted to the US Food & Drug Administration for regulatory review and accelerated approval of carfilzomib, involved a larger population of relapsed and refractory patients (N = 266).17 PX-171-004 was a smaller study originally designed to investigate the impact of carfilzomib treatment in relationship to bortezomib treatment in less heavily pretreated (1-3 prior regimens) patients. An amendment to PX-171-004 provided an opportunity to evaluate carfilzomib separately in patients who were bortezomib naive, a specific population not represented in PX-171-003, the results of which are provided here.
Methods
Patients
Patients 18 years of age or older with measurable R/R MM were eligible to participate in the study. Measurable disease was defined as one or both of the following: serum M-protein ≥ 1 g/dL or urine M-protein ≥ 200 mg/24 hours; before an early amendment to the protocol, 7 patients were permitted to enter the study with disease measurable by serum free light chain (sFLC) only. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, had been responsive to prior therapy, and had R/R progressive disease after 1-3 prior therapies for MM. Refractory disease was defined as ≤ 25% response or progression either during therapy or within 60 days after completion of therapy. Induction therapy, stem cell transplantation, and planned posttransplantation maintenance were considered as 1 regimen. Other inclusion criteria included: adequate hepatic function, total WBC count ≥ 2000 mm3, absolute neutrophil count ≥ 1000/mm3, platelet count ≥ 50 000/mm3, hemoglobin ≥ 8.0 g/dL, creatinine clearance ≥ 30 mL/min, and serum creatinine ≤ 2 mg/dL.
Patients were excluded from this analysis of the PX-171-004 study if they had been treated previously with any proteasome inhibitor (including carfilzomib; to reduce confounding factors, data from patients previously treated with bortezomib were analyzed separately and are reported separately [R.V., D.S., S.J., An open-label, single-arm, phase-2 study of carfilzomib in patients with relapsed multiple myeloma who have been previously treated with bortezomib: final results. Br J Haematol; manuscript submitted, April 2012]). Additional exclusion criteria included glucocorticoid therapy (prednisone > 10 mg/d or equivalent) or chemotherapy with an approved or investigative anticancer agent (including steroid therapy) within 3 weeks before the first carfilzomib dose; radiation therapy within 4 weeks or localized radiation therapy within 1 week before the first carfilzomib dose; immunotherapy within 4 weeks; or participation in an investigational therapeutic study within 3 weeks, or within 5 drug half-lives (t1/2; whichever time was greater). In addition, patients were excluded if they had cardiovascular disease (eg, congestive heart failure, symptomatic ischemia, or myocardial infarction); had active infection requiring systemic antibiotics, antivirals, or antifungals within 2 weeks before first dose; were known to be seropositive for human immunodeficiency virus or had active hepatitis; or had significant neuropathy (grade 3 or 4, or grade 2 with pain) at the time of study initiation.
Study design and treatment
This was a phase 2 multicenter, open-label, single-arm study (PX-171-004, NCT00530816). Written informed consent was obtained from all enrolled patients in accordance with the Declaration of Helsinki, and the study was approved by the institutional review board of each participating center.
Carfilzomib was supplied as a lyophilized powder in single-use vials and reconstituted with an appropriate volume of sterile water for injection to yield a solution containing 2 mg/mL of carfilzomib. The study was originally designed to evaluate the efficacy and safety of carfilzomib at 20 mg/m2 for all treatment cycles, administered as an IV infusion (2-10 minutes) on days 1, 2, 8, 9, 15, and 16 of every 28-day cycle for a maximum of 12 cycles. However, based on encouraging safety and efficacy data for carfilzomib at 27 mg/m2 in the overlapping phase 1 study (PX-171-002; NCT00150462), the decision was made to increase the carfilzomib dose in this trial to 27 mg/m2 after completion of cycle 1 with the goal of improving response rates.
After a protocol amendment, a new cohort was created to examine bortezomib-naive patients separately. At the same time, safety concerns had been satisfied to enable testing of an escalated dosing schedule based on the hypothesis that the dose-dependent proteasome inhibition seen in preclinical studies10,14 would be associated with better efficacy. For the analysis presented herein, patients with prior proteasome inhibitor treatment were excluded and, based on the timing of the escalation of the carfilzomib dose and the date of informed consent, the enrolled patients were separated into Cohort 1 (n = 59), scheduled to receive 20 mg/m2 intravenously for all treatment cycles, and Cohort 2 (n = 70), scheduled to receive 20 mg/m2 intravenously for cycle 1 and escalated to 27 mg/m2 for all subsequent cycles for 6 days of a 28-day cycle for up to 12 cycles (Figure 1). Patients who completed the study (ie, 12 cycles of carfilzomib treatment) were eligible to enroll in a phase 2 extension study (PX-171-010, NCT00884312).
In phase 1 studies of carfilzomib, fever, chills, shortness of breath, and/or rigors were observed in a small subset of patients in cycle 1 and occasionally in cycle 2. To ameliorate these symptoms, dexamethasone 4 mg orally or intravenously was administered before each dose of carfilzomib (20 mg/m2) in cycle 1 and before the first dose of the carfilzomib dose-escalation cycle (27 mg/m2). If any of these treatment-related effects were observed after any dose of carfilzomib after dexamethasone had been discontinued, oral or intravenous dexamethasone 4 mg was permitted to be readministered before subsequent doses of carfilzomib.
Assessments
Patients were evaluated for disease response by both an independent review committee and study investigators according to the International Myeloma Working Group (IMWG) Uniform Response Criteria for MM assessment parameters,18 modified to include minimal response (MR) per European Blood and Marrow Transplantation Group (EBMT) response criteria,19 on day 15 of cycle 1, day 1 of cycles 2 through 12, and at the end of the study. The primary efficacy end point was overall response rate (ORR), defined as the number of patients whose best response to single-agent carfilzomib after 6 cycles was stringent complete response (sCR), complete response (CR), very good partial response (VGPR), or partial response (PR) based on serum and/or urine M-protein levels (referred to as the response-evaluable subset population, which excluded patients with disease measurable by sFLC only).
Secondary efficacy end points were analyzed for the response-evaluable population (including patients with disease measurable by sFLC only) and included ORR defined as the number of subjects with sCR, CR, VGPR, or PR by serum and/or urine M-protein or sFLC assay; clinical benefit response (CBR) rate (ORR + MR) assessed throughout the treatment period; duration of response (DOR); time to disease progression (TTP); progression-free survival (PFS); and overall survival (OS). DOR was defined as the time from first evidence of PR or better (ie, first observation of PR before confirmation) to disease progression or death due to any cause. TTP was defined as the time from study entry (first dose of carfilzomib) to disease progression. PFS was defined as the time from start of treatment to disease progression or death due to any cause, irrespective of disease status. OS was defined as the time from the first dose of carfilzomib to the date of death.
An exploratory posthoc analysis using patient baseline characteristics including ECOG performance status, International Staging System (ISS) stage,20 cytogenetic/FISH prognostic markers of high-risk disease per mSMART criteria [del17p, t(4;14), t(14;16), del13 by karyotype and hypodiploidy; 2007 criteria used per protocol],21,22 and serum β2-microglobulin concentration was also conducted to determine whether ORR was affected by baseline characteristics.
Safety assessments included adverse events (AEs), laboratory assessments, and vital signs. AEs were assessed by incidence, severity, and duration, including all AEs, serious AEs (SAEs), and those AEs considered by the investigators to be related to carfilzomib administration. Medical Dictionary for Regulatory Activities (MedDRA) terminology was used to code AEs, and AEs were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (Version 3.0).23
A detailed neuropathy history was taken at baseline. Neurologic assessments included neurophysical examination and a PN-related quality-of-life survey, the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT-GOG/Ntx Version 4.0) at baseline, day 1 of cycles 3, 5, 7, 9, and 11, and at the end of study assessments.
Statistical analyses
The response-evaluable population comprised all evaluable patients who completed at least 1 dose of treatment with carfilzomib, had at least 1 postbaseline response assessment, and had disease that was measurable by protein electrophoresis and/or sFLC assay. Although disease measurable by sFLC is not specified by IMWG criteria, patients with disease measurable only by sFLC were permitted early in the study before amendments to the protocol, but were not to exceed approximately 10% of the population. The ORR was determined for each cohort based on a 2-sided 95% confidence interval (CI) for patients whose responses were classified as sCR, CR, VGPR, or PR. This approach was also used for all other response end points. Distributions of PFS, OS, TTP, and DOR were summarized for each cohort using the Kaplan-Meier method. Median time-to-event was estimated for each end point, as well as event-free rates at certain time points. Median follow-up time was estimated by reverse Kaplan-Meier method.
All patients who received at least 1 dose of carfilzomib were considered evaluable for the safety analysis. AEs were summarized by the number and percentage of patients who experienced the event, by system organ class, by preferred term, and by relationship to treatment. AEs were also summarized by severity and by relationship to carfilzomib. The results of laboratory assessments were evaluated similarly.
After protocol amendment and separation of dosing cohorts, the target sample size was revised to 115 patients to include approximately 65 patients in Cohort 1 (bortezomib-naive 20 mg/m2) and 50 patients in Cohort 2 (bortezomib-naive 20/27 mg/m2) to detect differences based on both the 9-month PFS and the ORR in patients enrolled under the final amendment. This sample size with at least 50 evaluable patients in Cohort 2 (bortezomib-naive 20/27 mg/m2) provided a 1-sample exact binomial test with a 1-sided significance level of 2.5% and 80% power to test the null hypothesis that the true ORR was 40% versus the alternative hypothesis that the ORR was 61% (based on monotherapy bortezomib literature controls24 ); this sample size also provided 93% power to detect a 16% absolute increase in the true 9-month PFS rate (ie, from 40% based on literature controls24 to 56%) using a 1-sample exact binomial test with a 1-sided significance level of 2.5%.
Results
Patient population
A total of 129 bortezomib-naive patients from 18 centers were enrolled in the study between September 2007 and November 2010: 59 in Cohort 1 (carfilzomib 20 mg/m2) and 70 in Cohort 2 (carfilzomib 20/27 mg/m2). This number exceeded the target enrollment of 115 patients because it included patients who had already signed consent forms when the target enrollment was reached. All 129 patients received at least 1 dose of carfilzomib and so were evaluable for safety. Three patients from Cohort 2 were not evaluable for efficacy because they had missing baseline or postbaseline disease assessments; therefore, the response-evaluable population comprised 59 patients in Cohort 1 and 67 patients in Cohort 2.
Enrolled patients had a median age of 65 years (range, 38-85), 76.0% were white, and 58.9% were male. The median time from diagnosis was 3.6 years (range, 0.7-24.4), 59.7% of patients had ECOG performance status of 1 or 2, 72.9% had ISS stage I or II disease, and 79.8% had normal or favorable cytogenetics or FISH prognostic markers at baseline (Table 1). Whereas most demographic and disease characteristics were similar between the 2 dosing cohorts, there were a few notable differences, including a larger number of patients in Cohort 1 with IgA-isotype disease (32.2% vs 20.0%) and larger numbers of patients in Cohort 2 who were male (62.9% vs 54.2%) and who had IgG-isotype disease (77.1% vs 54.2%), ECOG performance status of 1 or 2 (67.1% vs 50.8%), and ISS stage III disease (22.9% vs 10.2%). Patients in both cohorts had received a median of 2 prior therapies (range, 1-4; Table 2). The majority of patients (96.9%) had received a corticosteroid. Most patients (92.2%) had received immunomodulatory agents (including 58.9% who had received lenalidomide), and 72.9% of patients had received prior stem cell transplantations. Three patients in Cohort 2 had received prior bortezomib for less than 1 cycle and at cumulative doses that were considered subtherapeutic.
Patient demographics and baseline characteristics
| Characteristic . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Median age, y (range) | 65 (38-82) | 66 (45-85) | 65 (38-85) |
| Male, n (%) | 32 (54.2) | 44 (62.9) | 76 (58.9) |
| Ethnicity, n (%) | |||
| White | 46 (78.0) | 52 (74.3) | 98 (76.0) |
| African American | 6 (10.2) | 12 (17.1) | 18 (14.0) |
| Asian/Pacific Islander | 2 (3.4) | 3 (4.3) | 5 (3.9) |
| Hispanic | 4 (6.8) | 2 (2.9) | 6 (4.7) |
| Other | 1 (1.7) | 1 (1.4) | 2 (1.6) |
| Median time from diagnosis, y (range) | 3.5 (0.7-24.4) | 3.6 (0.7-12.2) | 3.6 (0.7-24.4) |
| Ig subtype, n (%) | |||
| IgG | 32 (54.2) | 54 (77.1) | 86 (66.7) |
| IgA | 19 (32.2) | 14 (20.0) | 33 (25.6) |
| Not reported/missing | 8 (13.6) | 2 (2.9) | 10 (7.8) |
| Light-chain subtype, n (%) | |||
| Kappa | 44 (74.6) | 50 (71.4) | 94 (72.9) |
| Lambda | 15 (25.4) | 20 (28.6) | 35 (27.1) |
| ECOG performance status, n (%) | |||
| 0 | 29 (49.2) | 23 (32.9) | 52 (40.3) |
| 1 or 2 | 30 (50.8) | 47 (67.1) | 77 (59.7) |
| ISS stage, n (%) | |||
| I or II | 41 (69.5) | 53 (75.7) | 94 (72.9) |
| III | 6 (10.2) | 16 (22.9) | 22 (17.1) |
| Not reported/missing | 12 (20.3) | 1 (1.4) | 13 (10.1) |
| Baseline evaluations, n (%) | |||
| Neuropathy history* | 42 (71.2) | 48 (68.6) | 90 (69.8) |
| CrCl < 50 mL/min | 8 (13.6) | 12 (17.1) | 20 (15.5) |
| Cytogenetic/FISH prognostic markers, n (%) | |||
| Normal/favorable | 46 (78.0) | 57 (81.4) | 103 (79.8) |
| Poor | 9 (15.3) | 10 (14.3) | 19 (14.7) |
| Unknown or not done | 4 (6.8) | 3 (4.3) | 7 (5.4) |
| Characteristic . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Median age, y (range) | 65 (38-82) | 66 (45-85) | 65 (38-85) |
| Male, n (%) | 32 (54.2) | 44 (62.9) | 76 (58.9) |
| Ethnicity, n (%) | |||
| White | 46 (78.0) | 52 (74.3) | 98 (76.0) |
| African American | 6 (10.2) | 12 (17.1) | 18 (14.0) |
| Asian/Pacific Islander | 2 (3.4) | 3 (4.3) | 5 (3.9) |
| Hispanic | 4 (6.8) | 2 (2.9) | 6 (4.7) |
| Other | 1 (1.7) | 1 (1.4) | 2 (1.6) |
| Median time from diagnosis, y (range) | 3.5 (0.7-24.4) | 3.6 (0.7-12.2) | 3.6 (0.7-24.4) |
| Ig subtype, n (%) | |||
| IgG | 32 (54.2) | 54 (77.1) | 86 (66.7) |
| IgA | 19 (32.2) | 14 (20.0) | 33 (25.6) |
| Not reported/missing | 8 (13.6) | 2 (2.9) | 10 (7.8) |
| Light-chain subtype, n (%) | |||
| Kappa | 44 (74.6) | 50 (71.4) | 94 (72.9) |
| Lambda | 15 (25.4) | 20 (28.6) | 35 (27.1) |
| ECOG performance status, n (%) | |||
| 0 | 29 (49.2) | 23 (32.9) | 52 (40.3) |
| 1 or 2 | 30 (50.8) | 47 (67.1) | 77 (59.7) |
| ISS stage, n (%) | |||
| I or II | 41 (69.5) | 53 (75.7) | 94 (72.9) |
| III | 6 (10.2) | 16 (22.9) | 22 (17.1) |
| Not reported/missing | 12 (20.3) | 1 (1.4) | 13 (10.1) |
| Baseline evaluations, n (%) | |||
| Neuropathy history* | 42 (71.2) | 48 (68.6) | 90 (69.8) |
| CrCl < 50 mL/min | 8 (13.6) | 12 (17.1) | 20 (15.5) |
| Cytogenetic/FISH prognostic markers, n (%) | |||
| Normal/favorable | 46 (78.0) | 57 (81.4) | 103 (79.8) |
| Poor | 9 (15.3) | 10 (14.3) | 19 (14.7) |
| Unknown or not done | 4 (6.8) | 3 (4.3) | 7 (5.4) |
CrCl indicates creatinine clearance.
Baseline PN: Cohort 1, 29 (49.2); Cohort 2, 39 (55.7); Total, 68 (52.7).
Prior therapies
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Median no. of prior regimens (range) | 2 (1-4) | 2 (1-4) | 2 (1-4) |
| Prior therapy, n (%) | |||
| Bortezomib | 0 (0) | 3 (4.3)* | 3 (2.3) |
| Thalidomide | 39 (66.1) | 37 (52.9) | 76 (58.9) |
| Lenalidomide | 27 (45.8) | 49 (70.0) | 76 (58.9) |
| Thalidomide or lenalidomide | 55 (93.2) | 64 (91.4) | 119 (92.2) |
| Corticosteroid | 58 (98.3) | 67 (95.7) | 125 (96.9) |
| Alkylating agent | 49 (83.1) | 56 (80.0) | 105 (81.4) |
| Anthracycline | 13 (22.0) | 17 (24.3) | 30 (23.3) |
| Stem cell transplant | 47 (79.7) | 47 (67.1) | 94 (72.9) |
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Median no. of prior regimens (range) | 2 (1-4) | 2 (1-4) | 2 (1-4) |
| Prior therapy, n (%) | |||
| Bortezomib | 0 (0) | 3 (4.3)* | 3 (2.3) |
| Thalidomide | 39 (66.1) | 37 (52.9) | 76 (58.9) |
| Lenalidomide | 27 (45.8) | 49 (70.0) | 76 (58.9) |
| Thalidomide or lenalidomide | 55 (93.2) | 64 (91.4) | 119 (92.2) |
| Corticosteroid | 58 (98.3) | 67 (95.7) | 125 (96.9) |
| Alkylating agent | 49 (83.1) | 56 (80.0) | 105 (81.4) |
| Anthracycline | 13 (22.0) | 17 (24.3) | 30 (23.3) |
| Stem cell transplant | 47 (79.7) | 47 (67.1) | 94 (72.9) |
Bortezomib exposure not considered therapeutic.
Efficacy
In this bortezomib-naive patient population who had received a median of 2 prior therapies, single-agent carfilzomib had an ORR of 42.4% and a CBR rate of 59.3% in Cohort 1, and an ORR of 52.2% and a CBR rate of 64.2% in Cohort 2 (Table 3). The response was robust, with an increase from 42.4% to 52.2% with the higher dose; however, the lower bound of the 95% CI for ORR in the 20/27 mg/m2 dose group was 39.7%, which did not exceed the 40% chosen based on historical controls. Two patients (3.4%) in Cohort 1 and 1 patient (1.5%) in Cohort 2 achieved a CR, and 8 patients (13.6%) in Cohort 1 and 18 patients (26.9%) in Cohort 2 achieved a VGPR. In the response-evaluable subset population, in which patients with disease measured by sFLC only were excluded, the ORR was 47.1% (39.6% in Cohort 1 and 53.0% in Cohort 2).
Response rates after 6 cycles of treatment in response-evaluable population
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 67) . | Total (N = 126) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR | 2 (3.4) | 1 (1.5) | 3 (2.4) |
| VGPR | 8 (13.6) | 18 (26.9) | 26 (20.6) |
| PR | 15 (25.4) | 16 (23.9) | 31 (24.6) |
| MR | 10 (16.9) | 8 (11.9) | 18 (14.3) |
| SD | 13 (22.0) | 10 (14.9) | 23 (18.3) |
| PD | 7 (11.9) | 11 (16.4) | 18 (14.3) |
| ORR (CR + VGPR + PR), n (%) | 25 (42.4) | 35 (52.2) | 60 (47.6) |
| 95% CI* | 29.6-55.9 | 39.7-64.6 | 38.7-56.7 |
| CBR (ORR + MR), n (%) | 35 (59.3) | 43 (64.2) | 78 (61.9) |
| 95% CI* | 45.7-71.9 | 51.5-75.5 | 52.8-70.4 |
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 67) . | Total (N = 126) . |
|---|---|---|---|
| Best response, n (%) | |||
| CR | 2 (3.4) | 1 (1.5) | 3 (2.4) |
| VGPR | 8 (13.6) | 18 (26.9) | 26 (20.6) |
| PR | 15 (25.4) | 16 (23.9) | 31 (24.6) |
| MR | 10 (16.9) | 8 (11.9) | 18 (14.3) |
| SD | 13 (22.0) | 10 (14.9) | 23 (18.3) |
| PD | 7 (11.9) | 11 (16.4) | 18 (14.3) |
| ORR (CR + VGPR + PR), n (%) | 25 (42.4) | 35 (52.2) | 60 (47.6) |
| 95% CI* | 29.6-55.9 | 39.7-64.6 | 38.7-56.7 |
| CBR (ORR + MR), n (%) | 35 (59.3) | 43 (64.2) | 78 (61.9) |
| 95% CI* | 45.7-71.9 | 51.5-75.5 | 52.8-70.4 |
PD indicates progressive disease; and SD, stable disease.
Exact 95% CI for ORR and CBR rate.
An exploratory analysis for which ORR was stratified by cohort and by patient baseline characteristics is shown in Table 4. The numbers of patients in each subset precluded any firm conclusions to be drawn. A higher ORR was seen in patients < 65 years of age and in those with an ISS stage of I. The ORR for patients who had received thalidomide previously was 56.0% compared with 35.3% for those who did not receive thalidomide. In addition, ORR was 52.2% in patients who had discontinued thalidomide previously because of neuropathy. ORR for patients who received lenalidomide previously was 41.9% compared with 55.8% for those who did not, and there was not much difference in ORR with an increased number of prior regimens. In addition, although the study population was small, a 36.8% response rate was seen in patients with poor cytogenetic prognostic markers.
ORR stratified by cohort and by baseline characteristics
| . | n . | ORR % (95% CI) . |
|---|---|---|
| Carfilzomib cohort | ||
| Overall (N = 126) | 47.6 (38.7-56.7) | |
| Cohort 1 (20 mg/m2 only) | 59 | 42.4 (29.6-55.9) |
| Cohort 2 (20/27 mg/m2) | 67 | 52.2 (39.7-64.6) |
| Baseline characteristics | ||
| Age, y | ||
| < 65 | 58 | 58.6 (44.9-71.4) |
| ≥ 65 | 68 | 38.2 (26.7-50.8) |
| ECOG performance status | ||
| 0 | 51 | 52.9 (38.5-67.1) |
| ≥ 1 | 75 | 44.0 (32.5-55.9) |
| ISS stage | ||
| I | 52 | 57.7 (43.2-71.3) |
| II | 41 | 34.1 (20.1-50.6) |
| III | 20 | 45.0 (23.1-68.5) |
| Baseline neuropathy | ||
| Grade 0 | 59 | 39.0 (26.5-52.6) |
| ≥ grade 1 | 66 | 56.1 (43.3-68.3) |
| Cytogenetic/FISH prognostic markers | ||
| Normal/favorable | 100 | 51.0 (40.8-61.1) |
| Poor | 19 | 36.8 (16.3-61.6) |
| Serum β2-microglobulin, mg/L | ||
| < 5.5 | 93 | 47.3 (36.9-57.9) |
| ≥ 5.5 | 20 | 45.0 (23.1-68.5) |
| Received prior lenalidomide | ||
| Yes | 74 | 41.9 (30.5-53.9) |
| No | 52 | 55.8 (41.3-69.5) |
| Received prior thalidomide | ||
| Yes | 75 | 56.0 (44.1-67.5) |
| No | 51 | 35.3 (22.4-49.9) |
| . | n . | ORR % (95% CI) . |
|---|---|---|
| Carfilzomib cohort | ||
| Overall (N = 126) | 47.6 (38.7-56.7) | |
| Cohort 1 (20 mg/m2 only) | 59 | 42.4 (29.6-55.9) |
| Cohort 2 (20/27 mg/m2) | 67 | 52.2 (39.7-64.6) |
| Baseline characteristics | ||
| Age, y | ||
| < 65 | 58 | 58.6 (44.9-71.4) |
| ≥ 65 | 68 | 38.2 (26.7-50.8) |
| ECOG performance status | ||
| 0 | 51 | 52.9 (38.5-67.1) |
| ≥ 1 | 75 | 44.0 (32.5-55.9) |
| ISS stage | ||
| I | 52 | 57.7 (43.2-71.3) |
| II | 41 | 34.1 (20.1-50.6) |
| III | 20 | 45.0 (23.1-68.5) |
| Baseline neuropathy | ||
| Grade 0 | 59 | 39.0 (26.5-52.6) |
| ≥ grade 1 | 66 | 56.1 (43.3-68.3) |
| Cytogenetic/FISH prognostic markers | ||
| Normal/favorable | 100 | 51.0 (40.8-61.1) |
| Poor | 19 | 36.8 (16.3-61.6) |
| Serum β2-microglobulin, mg/L | ||
| < 5.5 | 93 | 47.3 (36.9-57.9) |
| ≥ 5.5 | 20 | 45.0 (23.1-68.5) |
| Received prior lenalidomide | ||
| Yes | 74 | 41.9 (30.5-53.9) |
| No | 52 | 55.8 (41.3-69.5) |
| Received prior thalidomide | ||
| Yes | 75 | 56.0 (44.1-67.5) |
| No | 51 | 35.3 (22.4-49.9) |
The table is based on a response-evaluable population.
For Cohort 1, the median DOR (calculated for patients with ≥ PR) was 13.1 months and median duration of CBR (calculated for patients with a ≥ MR) was 11.5 months (Table 5). Neither DOR nor duration of CBR has been reached for Cohort 2. Median TTP was 8.3 months in Cohort 1 and had not been reached for Cohort 2, but median time to follow-up was 11.5 months. Median time to response and median time to CBR was 1.0 and 0.5 months for Cohort 1 and 1.9 and 0.5 months for Cohort 2, respectively.
DOR, TTP, and time to response
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 67) . | Total (N = 126) . |
|---|---|---|---|
| DOR, mo* | |||
| N | 25 | 35 | 60 |
| Median, mo (95% CI) | 13.1 (7.2-NR) | NR (NR-NR) | NR (13.1-NR) |
| Duration of CBR, mo | |||
| N | 35 | 43 | 78 |
| Median, (95% CI) | 11.5 (6.2-NR) | NR (NR-NR) | NR (11.5-NR) |
| TTP, mo | |||
| N | 59 | 67 | 126 |
| Median (95% CI) | 8.3 (6.0-12.3) | NR (11.3-NR) | 12.0 (8.2-NR) |
| Time to response, mo | |||
| N | 25 | 35 | 60 |
| Median, (min, max) | 1.0 (0.5, 3.7) | 1.9 (0.5, 3.7) | 1.7 (0.5-3.7) |
| Time to CBR, mo | |||
| N | 35 | 43 | 78 |
| Median, (min, max) | 0.5 (0.5, 6.5) | 0.5 (0.5, 5.9) | 0.5 (0.5-6.5) |
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 67) . | Total (N = 126) . |
|---|---|---|---|
| DOR, mo* | |||
| N | 25 | 35 | 60 |
| Median, mo (95% CI) | 13.1 (7.2-NR) | NR (NR-NR) | NR (13.1-NR) |
| Duration of CBR, mo | |||
| N | 35 | 43 | 78 |
| Median, (95% CI) | 11.5 (6.2-NR) | NR (NR-NR) | NR (11.5-NR) |
| TTP, mo | |||
| N | 59 | 67 | 126 |
| Median (95% CI) | 8.3 (6.0-12.3) | NR (11.3-NR) | 12.0 (8.2-NR) |
| Time to response, mo | |||
| N | 25 | 35 | 60 |
| Median, (min, max) | 1.0 (0.5, 3.7) | 1.9 (0.5, 3.7) | 1.7 (0.5-3.7) |
| Time to CBR, mo | |||
| N | 35 | 43 | 78 |
| Median, (min, max) | 0.5 (0.5, 6.5) | 0.5 (0.5, 5.9) | 0.5 (0.5-6.5) |
The table is based on a response-evaluable population.
CBR indicates clinical benefit response; DOR, duration of response; NR, not reached; and TTP, time to progression.
Calculated for ORR.
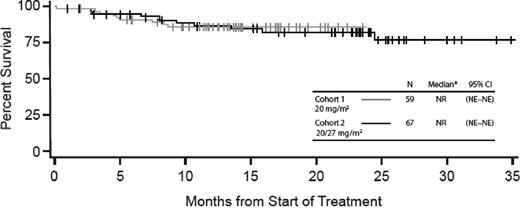
Median PFS was 8.2 months (95% CI, 6.0-12.3) for Cohort 1 and is not yet calculable for Cohort 2 because of an insufficient number of evaluable events; median time to follow-up was 11.5 months (Figure 2). In addition, 9-month PFS was 44.8% for Cohort 1 and 62.3% for Cohort 2. Combining the 2 dose groups, the 9-month PFS rate was 54.3% (95% CI, 44.3-63.2%). Because the lower bound for the 95% CI exceeded the 40% chosen based on historical controls,24 the null hypothesis is rejected. Median OS was not evaluable for either cohort because of an insufficient number of evaluable events, but median time to follow-up was 23.2 months for Cohort 1 and 13.8 months for Cohort 2 as of December 2011 (Figure 3).
PFS for Cohorts 1 and 2. NE indicates not estimable; and NR, not reached
OS for Cohorts 1 and 2. NE indicates not estimable; and NR, not reached. *Median follow-up is 23.2 months for Cohort 1 and 13.8 months for Cohort 2.
OS for Cohorts 1 and 2. NE indicates not estimable; and NR, not reached. *Median follow-up is 23.2 months for Cohort 1 and 13.8 months for Cohort 2.
Safety
All 129 enrolled patients were evaluable for safety, and all reported at least 1 treatment-emergent AE. The most common AEs of any grade regardless of relationship to study drug were fatigue (62.0%), nausea (48.8%), anemia (41.9%), dyspnea (38.8%), cough (34.1%), and pyrexia (34.1%; Table 6). The most common AEs of at least grade 3 in severity regardless of study drug relationship were primarily hematologic in nature and included lymphopenia (16.3%), anemia (14.7%), neutropenia (13.2%), thrombocytopenia (13.2%), and pneumonia (12.4%; Table 7).
Treatment-emergent AEs of all grades reported in ≥ 20% (safety population)
| Treatment-emergent AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 27 (45.8) | 27 (38.6) | 54 (41.9) |
| Neutropenia | 18 (30.5) | 21 (30.0) | 39 (30.2) |
| Thrombocytopenia | 20 (33.9) | 19 (27.1) | 39 (30.2) |
| Lymphopenia | 20 (33.9) | 13 (18.6) | 33 (25.6) |
| Leukopenia | 12 (20.3) | 4 (5.7) | 16 (12.4) |
| Nonhematologic | |||
| Fatigue | 42 (71.2) | 38 (54.3) | 80 (62.0) |
| Nausea | 32 (54.2) | 31 (44.3) | 63 (48.8) |
| Dyspnea | 29 (49.2) | 21 (30.0) | 50 (38.8) |
| Cough | 23 (39.0) | 21 (30.0) | 44 (34.1) |
| Pyrexia | 21 (35.6) | 23 (32.9) | 44 (34.1) |
| Headache | 19 (32.2) | 23 (32.9) | 42 (32.6) |
| Diarrhea | 21 (35.6) | 19 (27.1) | 40 (31.0) |
| Upper respiratory infection | 20 (33.9) | 20 (28.6) | 40 (31.0) |
| Peripheral edema | 17 (28.8) | 18 (25.7) | 35 (27.1) |
| Chills | 15 (25.4) | 14 (20.0) | 29 (22.5) |
| Insomnia | 11 (18.6) | 18 (25.7) | 29 (22.5) |
| Vomiting | 15 (25.4) | 14 (20.0) | 29 (22.5) |
| Constipation | 15 (25.4) | 10 (14.3) | 25 (19.4) |
| Increased serum creatinine | 14 (23.7) | 10 (14.3) | 24 (18.6) |
| Muscle spasms | 6 (10.2) | 18 (25.7) | 24 (18.6) |
| Arthralgia | 15 (25.4) | 7 (10.0) | 22 (17.1) |
| Back pain | 8 (13.6) | 14 (20.0) | 22 (17.1) |
| Dizziness | 12 (20.3) | 10 (14.3) | 22 (17.1) |
| Treatment-emergent AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 27 (45.8) | 27 (38.6) | 54 (41.9) |
| Neutropenia | 18 (30.5) | 21 (30.0) | 39 (30.2) |
| Thrombocytopenia | 20 (33.9) | 19 (27.1) | 39 (30.2) |
| Lymphopenia | 20 (33.9) | 13 (18.6) | 33 (25.6) |
| Leukopenia | 12 (20.3) | 4 (5.7) | 16 (12.4) |
| Nonhematologic | |||
| Fatigue | 42 (71.2) | 38 (54.3) | 80 (62.0) |
| Nausea | 32 (54.2) | 31 (44.3) | 63 (48.8) |
| Dyspnea | 29 (49.2) | 21 (30.0) | 50 (38.8) |
| Cough | 23 (39.0) | 21 (30.0) | 44 (34.1) |
| Pyrexia | 21 (35.6) | 23 (32.9) | 44 (34.1) |
| Headache | 19 (32.2) | 23 (32.9) | 42 (32.6) |
| Diarrhea | 21 (35.6) | 19 (27.1) | 40 (31.0) |
| Upper respiratory infection | 20 (33.9) | 20 (28.6) | 40 (31.0) |
| Peripheral edema | 17 (28.8) | 18 (25.7) | 35 (27.1) |
| Chills | 15 (25.4) | 14 (20.0) | 29 (22.5) |
| Insomnia | 11 (18.6) | 18 (25.7) | 29 (22.5) |
| Vomiting | 15 (25.4) | 14 (20.0) | 29 (22.5) |
| Constipation | 15 (25.4) | 10 (14.3) | 25 (19.4) |
| Increased serum creatinine | 14 (23.7) | 10 (14.3) | 24 (18.6) |
| Muscle spasms | 6 (10.2) | 18 (25.7) | 24 (18.6) |
| Arthralgia | 15 (25.4) | 7 (10.0) | 22 (17.1) |
| Back pain | 8 (13.6) | 14 (20.0) | 22 (17.1) |
| Dizziness | 12 (20.3) | 10 (14.3) | 22 (17.1) |
Treatment-emergent AEs ≥ grade 3 in ≥ 5% (safety population)
| Treatment-emergent AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Lymphopenia | 8 (13.6) | 13 (18.6) | 21 (16.3) |
| Anemia | 7 (11.9) | 12 (17.1) | 19 (14.7) |
| Neutropenia | 7 (11.9) | 10 (14.3) | 17 (13.2) |
| Thrombocytopenia | 9 (15.3) | 8 (11.4) | 17 (13.2) |
| Leukopenia | 4 (6.8) | 1 (1.4) | 5 (3.9) |
| Nonhematologic | |||
| Pneumonia | 8 (13.6) | 8 (11.4) | 16 (12.4) |
| Fatigue | 7 (11.9) | 1 (1.4) | 8 (6.2) |
| Dyspnea | 3 (5.1) | 4 (5.7) | 7 (5.4) |
| Acute renal failure | 2 (3.4) | 4 (5.7) | 6 (4.7) |
| Congestive heart failure | 2 (3.4) | 4 (5.7) | 6 (4.7) |
| Serum phosphorus decrease | 1 (1.7) | 5 (7.1) | 6 (4.7) |
| Hyperglycemia | 4 (6.8) | 2 (2.9) | 6 (4.7) |
| Hypophosphatemia | 1 (1.7) | 5 (7.1) | 6 (4.7) |
| Hypokalemia | 1 (1.7) | 4 (5.7) | 5 (3.9) |
| Hyponatremia | 0 (0.0) | 5 (7.1) | 5 (3.9) |
| Serum sodium decreased | 4 (6.8) | 0 (0.0) | 4 (3.1) |
| AST increase | 3 (5.1) | 0 (0.0) | 3 (2.3) |
| Treatment-emergent AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Lymphopenia | 8 (13.6) | 13 (18.6) | 21 (16.3) |
| Anemia | 7 (11.9) | 12 (17.1) | 19 (14.7) |
| Neutropenia | 7 (11.9) | 10 (14.3) | 17 (13.2) |
| Thrombocytopenia | 9 (15.3) | 8 (11.4) | 17 (13.2) |
| Leukopenia | 4 (6.8) | 1 (1.4) | 5 (3.9) |
| Nonhematologic | |||
| Pneumonia | 8 (13.6) | 8 (11.4) | 16 (12.4) |
| Fatigue | 7 (11.9) | 1 (1.4) | 8 (6.2) |
| Dyspnea | 3 (5.1) | 4 (5.7) | 7 (5.4) |
| Acute renal failure | 2 (3.4) | 4 (5.7) | 6 (4.7) |
| Congestive heart failure | 2 (3.4) | 4 (5.7) | 6 (4.7) |
| Serum phosphorus decrease | 1 (1.7) | 5 (7.1) | 6 (4.7) |
| Hyperglycemia | 4 (6.8) | 2 (2.9) | 6 (4.7) |
| Hypophosphatemia | 1 (1.7) | 5 (7.1) | 6 (4.7) |
| Hypokalemia | 1 (1.7) | 4 (5.7) | 5 (3.9) |
| Hyponatremia | 0 (0.0) | 5 (7.1) | 5 (3.9) |
| Serum sodium decreased | 4 (6.8) | 0 (0.0) | 4 (3.1) |
| AST increase | 3 (5.1) | 0 (0.0) | 3 (2.3) |
AST indicates aspartate aminotransferase.
The majority of patients (119 patients, 92.2%) experienced at least 1 treatment-related AE. The most common treatment-related AEs were fatigue (45.0%), nausea (41.9%), anemia (31.0%), dyspnea (27.9%), thrombocytopenia (23.3%), and neutropenia (22.5%; Table 8).
Treatment-related AEs of all grades reported in ≥ 20% (safety population)
| Treatment-related AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 21 (35.6) | 19 (27.1) | 40 (31.0) |
| Thrombocytopenia | 14 (23.7) | 16 (22.9) | 30 (23.3) |
| Neutropenia | 11 (18.6) | 18 (25.7) | 29 (22.5) |
| Lymphopenia | 17 (28.8) | 11 (15.7) | 28 (21.7) |
| Nonhematologic | |||
| Fatigue | 31 (52.5) | 27 (38.6) | 58 (45.0) |
| Nausea | 27 (45.8) | 27 (38.6) | 54 (41.9) |
| Dyspnea | 21 (35.6) | 15 (21.4) | 36 (27.9) |
| Pyrexia | 12 (20.3) | 12 (17.1) | 24 (18.6) |
| Diarrhea | 14 (23.7) | 9 (12.9) | 23 (17.8) |
| Headache | 7 (11.9) | 14 (20.0) | 21 (16.3) |
| Peripheral edema | 12 (20.3) | 5 (7.1) | 17 (13.2) |
| Constipation | 12 (20.3) | 4 (5.7) | 16 (12.4) |
| Cough | 12 (20.3) | 3 (4.3) | 15 (11.6) |
| Treatment-related AE, n (%) . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| Hematologic | |||
| Anemia | 21 (35.6) | 19 (27.1) | 40 (31.0) |
| Thrombocytopenia | 14 (23.7) | 16 (22.9) | 30 (23.3) |
| Neutropenia | 11 (18.6) | 18 (25.7) | 29 (22.5) |
| Lymphopenia | 17 (28.8) | 11 (15.7) | 28 (21.7) |
| Nonhematologic | |||
| Fatigue | 31 (52.5) | 27 (38.6) | 58 (45.0) |
| Nausea | 27 (45.8) | 27 (38.6) | 54 (41.9) |
| Dyspnea | 21 (35.6) | 15 (21.4) | 36 (27.9) |
| Pyrexia | 12 (20.3) | 12 (17.1) | 24 (18.6) |
| Diarrhea | 14 (23.7) | 9 (12.9) | 23 (17.8) |
| Headache | 7 (11.9) | 14 (20.0) | 21 (16.3) |
| Peripheral edema | 12 (20.3) | 5 (7.1) | 17 (13.2) |
| Constipation | 12 (20.3) | 4 (5.7) | 16 (12.4) |
| Cough | 12 (20.3) | 3 (4.3) | 15 (11.6) |
SAEs occurred in 18 patients (30.5%) in Cohort 1 and in 27 patients (38.6%) in Cohort 2. The most common SAE was pneumonia, which occurred in 6 patients (10.2%) in Cohort 1 and 6 patients (8.6%) in Cohort 2 and was the only SAE that was observed in ≥ 5% of patients.
Ninety patients (69.8%) in the overall population had a history of neuropathy at baseline, and 68 patients (52.7%) entered the study with grade 1 or 2 PN. Treatment-emergent PN occurred infrequently (17.1% overall; 15.3% in Cohort 1 and 18.6% in Cohort 2) and did not limit treatment. Only 1 patient (in Cohort 1) experienced grade 3 PN, and there were no discontinuations of treatment because of PN. The median score on the 5 subscales (Physical Well-Being, Social and Family Well-Being, Emotional Well-Being, Functional Well-Being, and Additional Concerns/Neurotoxicity subscale) from the FACT-GOG quality-of-life questionnaire showed no worsening from baseline to end of study (data not shown).
Patients received a median of 7 cycles of carfilzomib (range, 1-21; Table 9). The median relative dose intensity for the combined dose group was 94.0%. Forty-six patients (35.7%) completed 12 cycles of study drug, including 17 patients (28.8%) in Cohort 1 and 29 patients (41.4%) in Cohort 2. Thirty-eight patients continued beyond 12 cycles and received study drug as part of an extension study (PX-171-010). Most patients who discontinued treatment before the completion of 12 cycles did so because of progressive disease: 24 patients (40.7%) in Cohort 1 and 24 patients (34.3%) in Cohort 2. Twenty patients (15.5%) discontinued treatment as a result of treatment-emergent AEs: 13 (22%) in Cohort 1 and 7 (10.0%) in Cohort 2. Dose reduction because of an AE occurred in only 15 patients (11.6%; n = 10 in Cohort 1 and n = 5 in Cohort 2). Approximately half of the patients in each cohort missed at least 1 dose of carfilzomib (38.8% because of an AE). Similarly, approximately half of the patients in each cohort had at least 1 dose delay (28.7% because of an AE). There were 4 deaths on study or within 30 days of study completion: 1 (1.7%) in Cohort 1 and 3 (4.3%) in Cohort 2. The primary cause of death was multiorgan failure for the patient in Cohort 1 (probably related to carfilzomib), and SAE (2 patients, cardiac disorder possibly related to carfilzomib and acute renal failure unlikely related) and disease progression (1 patient) in Cohort 2.
Study drug administration
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| No. of cycles started, n (%) | |||
| Median (range) | 7.0 (1-21) | 6.5 (1-13) | 7.0 (1-21) |
| 1-3 | 17 (28.8) | 21 (30.0) | 38 (29.5) |
| 4-6 | 12 (20.3) | 14 (20.0) | 26 (20.2) |
| 7-9 | 7 (11.9) | 4 (5.7) | 11 (8.5) |
| ≥ 10 | 23 (39.0) | 31 (44.3) | 54 (41.9) |
| Completed 12 cycles, n (%) | 17 (28.8) | 29 (41.4) | 46 (35.7) |
| Mean dose, mg/m2 | |||
| Mean (SD) | 20.4 (1.9) | 24.5 (2.4) | 22.6 (3.0) |
| Median (range) | 20.0 (16.0-25.2) | 25.7 (17.3-26.6) | 22.5 (16.0-26.6) |
| Relative dose intensity, % | |||
| Median (range) | 91.8 (36.5-100.0) | 95.1 (50.0-100.0) | 94.0 (36.5-100.0) |
| . | Cohort 1, 20 mg/m2 (n = 59) . | Cohort 2, 20/27 mg/m2 (n = 70) . | Total (N = 129) . |
|---|---|---|---|
| No. of cycles started, n (%) | |||
| Median (range) | 7.0 (1-21) | 6.5 (1-13) | 7.0 (1-21) |
| 1-3 | 17 (28.8) | 21 (30.0) | 38 (29.5) |
| 4-6 | 12 (20.3) | 14 (20.0) | 26 (20.2) |
| 7-9 | 7 (11.9) | 4 (5.7) | 11 (8.5) |
| ≥ 10 | 23 (39.0) | 31 (44.3) | 54 (41.9) |
| Completed 12 cycles, n (%) | 17 (28.8) | 29 (41.4) | 46 (35.7) |
| Mean dose, mg/m2 | |||
| Mean (SD) | 20.4 (1.9) | 24.5 (2.4) | 22.6 (3.0) |
| Median (range) | 20.0 (16.0-25.2) | 25.7 (17.3-26.6) | 22.5 (16.0-26.6) |
| Relative dose intensity, % | |||
| Median (range) | 91.8 (36.5-100.0) | 95.1 (50.0-100.0) | 94.0 (36.5-100.0) |
Discussion
The primary objective of this phase 2 study was to determine the ORR to single-agent carfilzomib after 6 cycles in adult patients with MM who were naive to bortezomib therapy but had relapsed or were refractory to 1-3 prior MM therapies. The ORR of 47.6% in the 126 response-evaluable patients demonstrated single-agent activity for carfilzomib in this patient population who had received a median of 2 prior therapies, including lenalidomide, thalidomide, and stem cell transplantation. Additional response of MR is shown in the CBR of 61.9%. The responses to single-agent carfilzomib appeared to be durable, with a TTP of at least 8.3 months and a DOR of at least 13.1 months (for Cohort 1). Nine-month follow-up of PFS revealed that 62.3% of patients in Cohort 2 had not progressed. Bortezomib is currently the only proteasome inhibitor approved for the treatment of MM, with reported monotherapy response rates in patients with R/R MM of 38%24 and 41%.25 Studies of monotherapy bortezomib had a TTP of 6.2 months24 and 6.5 months25 and a DOR of 8 months24 and 7 months.25 However, there were several differences between the bortezomib monotherapy studies and the carfilzomib study presented here. IMWG criteria were used in the present study and EBMT criteria in the monotherapy bortezomib studies. Another important difference between the studies was the increased availability of thalidomide and lenalidomide in the period after the conduct of the monotherapy bortezomib studies; the population enrolled in the carfilzomib trial consisted largely of patients who had been treated with either 1 or both of the immunomodulatory agents. However, comparison of independent phase 2 studies is fraught with risks and one should exercise extreme caution in the interpretation of these data.
In the present study, significant responses were achieved in patients with more advanced disease and unfavorable prognostic markers, as demonstrated by stratification of ORR by patient baseline disease characteristics. The small numbers of patients in the subgroups preclude further statistical analysis to draw firm conclusions. Differences detected between the subgroups, including an increased response rate in patients who had received prior thalidomide and in patients with baseline PN may possibly merit future investigation in new or ongoing trials.
The incidence and severity of AEs—both treatment emergent and treatment related—were similar between the 2 cohorts and were generally grade 1 or 2 in severity. The most common treatment-emergent AEs (including those of grade 3 or higher, mainly hematologic in nature) and treatment-related AEs (ie, fatigue, nausea, anemia, and dyspnea) were similar to results observed in other studies of single-agent carfilzomib in patients with R/R MM using comparable dosage regimens.26,27
In patients with MM, PN occurs secondary to the disease, but it is also a known AE associated with the use of thalidomide and bortezomib.9 Consequently, particular attention was paid to the incidence of treatment-emergent PN in this study, as has been done in other carfilzomib studies. A prior statistical analysis of a subpopulation of patients (n = 108) from across phase 2 trials of carfilzomib in patients with R/R MM showed a statistically significant improvement in neuropathic symptoms in patients with ≥ PR.28 In the present study, there were low rates of treatment-emergent grade 1/2 PN. More than two-thirds of patients had a history of PN at baseline, but only 17% of patients experienced any PN and only 1% severe PN during the study, and it did not limit treatment. Patients with baseline PN (grade 1 or 2) had a higher ORR compared with patients with no baseline PN. In comparison, historically, PN has been reported in up to 70% of patients with MM receiving thalidomide and in approximately 37% of patients receiving bortezomib (range, 14%-52%), and it is a common cause of treatment discontinuation or dose reduction.9 Recently, overall PN was reported in 38% of patients receiving subcutaneous bortezomib and 53% of patients receiving intravenous bortezomib, and ≥ grade 3 PN was reported in 6% and 16% of patients, respectively.29 In the carfilzomib study reported herein, carfilzomib was effective even in patients who discontinued thalidomide treatment because of PN. Therefore, PN does not appear to be clinically limiting with extended carfilzomib treatment; patients from the present study have continued to receive carfilzomib as part of an extension study, and to date there has been no evidence of cumulative neurotoxicities in these patients.30
The present study was not designed or powered to make comparisons between the 2 carfilzomib dose cohorts with respect to efficacy or safety. The safety profiles were similar in the 2 cohorts, and the ORR increased from 42.4% to 52.2% at the higher dose. This adds credence to the hypothesis that dose-dependent proteasome inhibition seen in preclinical studies10,14 may be correlated with better efficacy. Preliminary observations of a dose-response relationship have been detected in other carfilzomib phase 2 studies. In particular, 3 phase 1 dose-escalation studies (M.A., S. Trudel, R. R. Furman, P.J.R., O. A. O'Connor, R. L. Comenzo, A.F.W., L.A.K., C. J. Molineaux, A. Goy, A phase 1 single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma, Clin Cancer Res; manuscript submitted, 2012)31,32 show increased response at higher doses (including up to 56 mg/m2), and a multivariate modeling analysis of two phase 2 studies (PX-171-003 and PX-171-004)33 shows evidence of a dose-response relationship.
Overall, the response data for single-agent carfilzomib are very promising and are noteworthy as one of the highest to date for a single agent in this pretreated patient population. Perhaps more importantly, the substantial percentage (35.7%) of patients who received carfilzomib treatment for at least 12 months without significant toxicities supports its prolonged administration, its use in patients with significant comorbidities, and its potential use in combination with other agents. An extension study (PX-171-010; NCT00884312) is demonstrating long-term tolerability to carfilzomib. Along with patients who have completed other carfilzomib studies, 38 patients from the study described herein have enrolled in the extension study, and 14 of those patients are currently still undergoing carfilzomib treatment. In addition, two phase 3 studies evaluating carfilzomib are currently in progress.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients and their families who contributed to this study; the staff from the participating study sites, including Chaim Shustik, MD (Royal Victoria Hospital Division of Hematology) and Kevin Doner, MD (Texas Oncology Cancer Center) and all of the participating research nurses and data coordinators; Sunhee Kwon Ro, PhD (Onyx Pharmaceuticals Inc) for statistical support; Leanne M. McCulloch, PharmD (Onyx Pharmaceuticals Inc) for safety analysis support; and Thomas Renau, PhD (Onyx Pharmaceuticals Inc) for critical review of the manuscript. Medical writing and editorial assistance was provided by Brian Szente, PhD, and Melissa Kirk, PhD (Fishawack Communications) and was funded by Onyx Pharmaceuticals Inc.
This study was supported by Onyx Pharmaceuticals Inc, and the Multiple Myeloma Research Consortium.
Authorship
Contribution: R.V. designed and performed the research, contributed vital new reagents or analytical tools, and analyzed the data; M.W., J.L.K., A.J.J., F.J.R., D.H.V., and S.M.W. performed the research and analyzed the data; S.L., A.K.S., S.J., R.Z.O., and D.S.S. designed and performed the research and analyzed the data; V.K., K.T.M., N.J.B., N.Y.G., A.B., J.V.M., P.L., P.R., and M.S. performed the research; M.A. designed and performed the research; and L.A.K and A.F.W. designed the research, contributed vital new reagents or analytical tools, and analyzed the data. The investigators and representatives from Onyx Pharmaceuticals designed the study. The data were collected and analyzed by medical and statistical representatives from Onyx Pharmaceuticals in conjunction with the investigators. All authors had access to the primary data and participated in writing and editing this manuscript. All participating institutions received support from Onyx Pharmaceuticals for the conduct of the study.
Conflict-of-interest disclosure: R.V. receives consultancy and research funding from Onyx Pharmaceuticals. M.W. receives research funding from Onyx Pharmaceuticals. S.L. receives consultancy funding from Millennium, Celgene, Novartis, Bristol-Myers Squibb, Onyx Pharmaceuticals, and Merck. J.L.K. receives consultancy funding from Novartis, Onyx, Celgene, and Millennium; honoraria from Novartis, Onyx, Celgene, and Millennium; and research funding from Celgene, Merck, Novartis, and Onyx. A.J.J. receives consultancy funding from Ortho Biotech, Celgene, Millennium, Onyx Pharmaceuticals, Bristol-Myers Squibb, and Exelixis; honoraria from Ortho Biotech, Celgene, Millennium, Bristol-Myers Squibb, and Exelixis; is on the speaker's bureau for Ortho Biotech, Celgene, and Millennium; is on the board of directors for Millennium, Onyx Pharmaceuticals, and Bristol-Myers Squibb; and is on the advisory committees for Onyx Pharmaceuticals and Bristol-Myers Squibb. A.K.S. receives consultancy and research funding from Celgene, Millennium, Novartis, Bristol-Myers Squibb, and Onyx Pharmaceuticals. V.K. receives honoraria from Celgene, Janssen Ortho, and Roche. S.J. receives honoraria from Millennium, Celgene, Onyx Pharmaceuticals, and Merck and is on the board of directors or advisory committees for Ortho Biotech, Imedex, Medicom World Wide, Optum Health Education, and the PER Group. M.A. receives consultancy funding from Millennium and Novartis; is on the board of directors or advisory committees for Millennium and Novartis; and receives research funding from Millennium and Allergan. N.J.B. receives honoraria from and is on the speaker's bureau for Celgene. N.Y.G. receives research funding from Millennium. J.V.M. is on the speaker's bureaus for Celgene and Millennium. M.S. receives honoraria from Celgene and Ortho-Biotech (Johnson & Johnson). D.H.V. is on the board of directors and advisory committee for Celgene and the speaker's bureaus for Celgene and Millennium. R.Z.O. receives honoraria from and is on the board of directors or advisory committee for Onyx Pharmaceuticals. L.A.K. receives consultancy funding from VLST Biotech, Threshold, and Onyx Pharmaceuticals. A.F.W. is employed by and has equity ownership in Onyx Pharmaceuticals. D.S.S. receives consultancy funding and honoraria from and is on the board of directors or advisory committees for Millennium and Celgene. The remaining authors declare no competing financial interests.
The current affiliation for A.J.J. is Professor of Medicine, Director, Myeloma Program Section of Hematology/Oncology, University of Chicago Medical Center, Chicago, IL.
Correspondence: Ravi Vij, MD, Associate Professor of Medicine, Washington University School of Medicine, Section of Stem Cell Transplant and Leukemia, Division of Medical Oncology, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: rvij@im.wustl.edu.