Abstract
ALK1 is a type I receptor of the TGF-β family that is involved in angiogenesis. Circulating BMP9 was identified as a specific ligand for ALK1 inducing vascular quiescence. In this work, we found that blocking BMP9 with a neutralizing antibody in newborn mice significantly increased retinal vascular density. Surprisingly, Bmp9-KO mice did not show any defect in retinal vascularization. However, injection of the extracellular domain of ALK1 impaired retinal vascularization in Bmp9-KO mice, implicating another ligand for ALK1. Interestingly, we detected a high level of circulating BMP10 in WT and Bmp9-KO pups. Further, we found that injection of a neutralizing anti-BMP10 antibody to Bmp9-KO pups reduced retinal vascular expansion and increased vascular density, whereas injection of this antibody to WT pups did not affect the retinal vasculature. These data suggested that BMP9 and BMP10 are important in postnatal vascular remodeling of the retina and that BMP10 can substitute for BMP9. In vitro stimulation of endothelial cells by BMP9 and BMP10 increased the expression of genes involved in the Notch signaling pathway (Jagged1, Dll4, Hey1, Hey2, Hes1) and decreased apelin expression, suggesting a possible cross-talk between these pathways and the BMP pathway.
Introduction
Activin receptor-like kinase 1 (ALK1) is a type I receptor of the TGF-β receptor family that is specifically expressed by endothelial cells.1 Mutations in ACVRL1 (the gene encoding ALK1) and in ENG (the gene encoding its co-receptor endoglin) cause Rendu-Osler disease, also known as HHT (hereditary hemorrhagic telangiectasia).2 HHT is a rare genetic vascular disease characterized by numerous epistaxes, cutaneous telangiectasias and arteriovenous malformations (AVM) in the lungs, liver and central nervous system. ALK1 inactivation in mice leads to embryonic lethality at E11 because of major angiogenesis defects.3,4 These data clearly demonstrate that ALK1 is an important player in angiogenesis, but its molecular role is still not completely clear.
The retina of newborn mice is avascular, and development of retinal blood vessels progressively occurs during the first week after birth to form a highly organized vascular network composed of arteries, veins and capillaries.5 Retinal vascularization in newborn mice is therefore a very interesting model to study physiologic angiogenesis. The roles of endoglin and ALK1 in the vascularization of the retina have been recently demonstrated.6,7 Using endoglin-inducible KO in endothelial cells (Eng-iKOe), it was shown that absence of endoglin delayed remodeling of the capillary plexus, increased endothelial proliferation and induced localized AVMs in retinas.6 It was also published that injection of the extracellular domain of ALK1 (ALK1ecd) strongly affected retinal vascularization further supporting the importance of ALK1 and its ligands in retinal angiogenesis.7
In 2007, we identified bone morphogenetic protein 9 (BMP9) and BMP10 as specific ligands for ALK1.8 BMP9 was shown to be present in adult blood of rodents and humans and to circulate in both an active and an inactive form.9,10 On the other hand, BMP10 has been shown to be mainly expressed in the embryo and to be involved in heart development.11 We further showed that addition of serum to endothelial cells induced a phospho-Smad1/5 response that could be completely inhibited by the addition of a neutralizing anti-BMP9 antibody, supporting a major role for BMP9 in adult angiogenesis, while BMP10 function would mainly be restricted to embryogenesis.9,10 Therefore many studies have focused on the role of BMP9 on angiogenesis. The in vitro effects of BMP9 on endothelial cell migration and proliferation are still under debate, as some groups have found an inhibition,8,12 while another group, using endothelial cells from a different tissue origin, has described an induction.13 BMP9 was also shown to inhibit ex vivo endothelial sprouting from metatarsals12 and to inhibit FGF-2 induced angiogenesis in vivo in the mouse angiogenesis model of subcutaneously implanted sponges,10 while it increased angiogenesis in a Matrigel plug assay and in a xenograft model of human pancreatic cancer.13 Taken together these data demonstrate that BMP9 is involved in angiogenesis, although its precise cellular functions are still under debate. All of these prior studies have addressed the role of BMP9 by supplementing BMP9 in vitro or in vivo. To date, nobody has addressed the effect of blocking BMP9 in vivo on angiogenesis.
To address this issue, we investigated the role of endogenous BMP9 on retinal angiogenesis using anti-BMP9 antibodies and Bmp9-KO mice. This study allowed us to demonstrate that BMP9 and BMP10 control retinal postnatal vascularization, one being able to rescue the absence of the other. Injection of neutralizing BMP10 antibodies to Bmp9-KO mice induced a higher vessel density and multilayered retinal vascularization suggesting a possible role of these 2 factors in vascular remodeling. We also found that BMP9 and BMP10 significantly regulated expression of components of the Notch and apelin signaling pathways, both of which are known as important regulators of retinal vascularization.
Methods
Mice
All animal studies were approved by the institutional guidelines and those formulated by the European Community for the Use of Experimental Animals. OF1 mice were bought from Charles River Laboratories. Bmp9-KO null mice were obtained by homologous recombination in R1 embryonic stem cells such that exon 2 encoding the entire mature C-terminal region was replaced by a neomycin resistance cassette. Heterozygous offspring of chimeras were mated out 9 generations to C57BL6/J (S.-J.L. and T.A.Z., Arteriovenous malformations and skeletal abnormalities in mice deficient for Gdf2/Bmp9, manuscript in preparation).
Inhalation anesthesia with isoflurane was performed before blood puncture. Blood was collected into EDTA-coated tubes (BD Biosciences) from OF1 and C57BL/6 wild-type (WT) and Bmp9-KO mouse embryos and pups, and plasma was frozen until BRE activity determination or BMP9 and BMP10 measurements.
Recombinant mouse ALK1 extracellular domain (ALK1ecd, 770-MA; R&D Systems) and anti–human BMP9 monoclonal antibody (MAB3209; R&D Systems) were prepared at 2 μg/μL. Anti–human BMP10 monoclonal antibody (MAB2926; R&D Systems) was prepared at 6 μg/μL. Injections were done intraperitoneally in mice at postnatal day 1 (P1) and P3 (5 mg/kg for ALK-1ecd and anti-BMP9, 15 mg/kg for anti-BMP10) and the mice were killed at P5 or P6. BrdU (B-5002; Sigma-Aldrich) was freshly diluted in PBS and injected intraperitoneally in P5 mice at 50 mg/kg. Two hours later, the mice were killed.
Retinal whole mount staining and imaging
Eyes were collected between P5 and P10 and fixed 2 hours at room temperature (RT) in 4% (wt/vol) paraformaldehyde (PFA) in PBS and then washed in PBS. Retina isolation was performed as described.14 Retinas were fixed 30 minutes in 4% PFA at RT and then saturated and permeabilized overnight in 1% BSA, 0.1% Triton X-100 in PBS at 4°C. Retinas were then incubated for 1 hour at RT with biotinylated Isolectin B4 (iB4, 1/20, L2140; Sigma-Aldrich) and/or rabbit anti-NG2 antibody (homemade, 1/200) diluted in wash buffer (1% BSA, 0.5% Tween in PBS). Retinas were washed 5 × 20 minutes with wash buffer at RT. Retinas were then incubated for 1 hour at RT in washing buffer with Streptavidin AlexaFluor 488 conjugate (S11223; Invitrogen) diluted at 5 μg/mL for iB4 staining and donkey anti-rabbit Cy3 antibody (711-165-152; Jackson ImmunoResearch Laboratories) diluted at 1:200 for NG2 detection. Retinas were washed 5 × 20 minutes at RT with wash buffer. For BrdU labeling, retinas were incubated in HCl 6N, 0.1% Triton X-100 for 15 minutes at RT after iB4 labeling. Retinas were then washed in 0.1M Tris-HCl pH8 (2 × 10 minutes) and in PBS (2 × 10 minutes). After saturation in 1% BSA, 1% Triton X-100 for 1 hour at room temperature, retinas were incubated overnight at 4°C with mouse anti-BrdU monoclonal antibody (347580; BD Bioscience) diluted in wash buffer (1/50). Retinas were then washed 5 × 20 minutes in wash buffer and incubated with goat anti–mouse Cy3 antibody (115-165-166; Jackson ImmunoResearch Laboratories) for 1 hour at RT. Retinas were then washed 5 × 20 minutes in wash buffer. Mounting of stained retinas was performed as described.14 Observations were made using confocal microscopy (Leica; TCS SP2). The number of branchpoints was counted manually per 200 × 200 μm visual field from ×63 micrographs; an average of 12 fields per region, front or capillary plexus were taken for each retina as previously described.15 Each point at which 3 capillary segments met was counted as 1 junction; intersections of 4 capillary segments were counted as 2 junctions, and so on.
Cell lines
NIH-3T3 fibroblasts cells were maintained in Dulbecco modified Eagle medium, 4.5 g/L glucose (Invitrogen) supplemented with 10% fetal bovine serum (Biowest). Human pulmonary arterial endothelial cells (HPAEc; PromoCell) were maintained in endothelial growth medium (PromoCell) supplemented with 2% fetal bovine serum (FBS; PromoCell).
Quantitative RT-PCR
HPAEc were stimulated for the indicated times with recombinant human BMP9 (0.5 ng/mL) or recombinant human BMP10 (0.5 ng/mL) from R&D Systems. At the times indicated, cells were lysed and RNAs were extracted using the NucleoSpin RNAII kit (Macherey-Nagel). Total RNAs from the liver of Bmp9-KO and WT mice were extracted using TRIzol reagent (Invitrogen). cDNAs were generated from 1 μg total RNA by reverse transcription using the iScript system (Bio-Rad) according to the manufacturer recommendations. Real-time PCR was performed using Bio-Rad CFX96 apparatus and qPCR Master Mix (Promega). Specific primers used are listed in Table 1.
Primer list used for RT-qPCR
| Gene . | Mouse primers . | Human primers . |
|---|---|---|
| Dll4 | ACTGGGAGAAGAAAGTGGACAGGT AGTTCACAGTAGGTGCCCGTGAAT | |
| Jagged1 | AGTGGTCTTTCAGGTGTGAGCAGT TTGTGAGCCTAATCCCTGCCAGAA | |
| Apelin | GAATCTGCGGCTCTGCGTGC TCGGGAAGCGGCATCAGGGA | |
| Hey1 | AGCTAGAAAAAGCCGAGATCCTGCA CCCGAAATCCCAAACTCCGATAGTC | |
| Hes1 | GCTGCTACCCCAGCCAGTGTC TTCTCCAGCTTGGAATGCCGC | |
| Hey2 | ACCATCGACGTGGGGAGCGA ATCCGATCCCGACGCCTTTTC | |
| HPRT | CACAGGACTAGAACACCTGC GCTGGTGAAAAGGACCTCT | TTGAGCACACAGAGGGCTACAATG ATGGACAGGACTGAACGTCTTGCT |
| BMP9 | GAAGAGGAGCACCGGAGCCA AGGGTTGGCACCCCCATGTCA |
| Gene . | Mouse primers . | Human primers . |
|---|---|---|
| Dll4 | ACTGGGAGAAGAAAGTGGACAGGT AGTTCACAGTAGGTGCCCGTGAAT | |
| Jagged1 | AGTGGTCTTTCAGGTGTGAGCAGT TTGTGAGCCTAATCCCTGCCAGAA | |
| Apelin | GAATCTGCGGCTCTGCGTGC TCGGGAAGCGGCATCAGGGA | |
| Hey1 | AGCTAGAAAAAGCCGAGATCCTGCA CCCGAAATCCCAAACTCCGATAGTC | |
| Hes1 | GCTGCTACCCCAGCCAGTGTC TTCTCCAGCTTGGAATGCCGC | |
| Hey2 | ACCATCGACGTGGGGAGCGA ATCCGATCCCGACGCCTTTTC | |
| HPRT | CACAGGACTAGAACACCTGC GCTGGTGAAAAGGACCTCT | TTGAGCACACAGAGGGCTACAATG ATGGACAGGACTGAACGTCTTGCT |
| BMP9 | GAAGAGGAGCACCGGAGCCA AGGGTTGGCACCCCCATGTCA |
Reporter gene constructs
The reporter plasmid pGL3(BRE)2-luc encoding firefly luciferase downstream of a BMP response element was kindly provided by Dr P. ten Dijke16 (Leiden University Medical Center, Leiden, The Netherlands). The ALK1 plasmid cloned in pcDNA3 was kindly provided by Dr C. H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). The pRL-TKluc plasmid encoding renilla luciferase downstream of the thymidine kinase promoter was purchased from Promega.
DNA transfection and dual luciferase activity assay
NIH-3T3 cells were transfected in Opti-MEM (Invitrogen) using lipofectamine (Invitrogen) with 0.2 μg pGL3(BRE)2-luc, 0.02 μg of pRL-TKluc, and 0.005 μg of ALK1 plasmid per well. Four hours after transfection, cells were treated for 15 hours with 0.5% of the indicated mouse plasma or recombinant BMP9 (5-100 pg/mL; R&D Systems). Firefly and renilla luciferase activities were measured sequentially with the Dual Luciferase Reporter Assay (Promega).
BMP10 and BMP9 ELISAs
Mouse plasma BMP10 (1/20 dilution) was measured using a commercially available ELISA (Uscn Life Science Inc). Mouse plasma BMP9 (1/2 dilution) was measured as previously described.9
Statistical analyses
Mann-Whitney tests were used to assess the statistical differences between measurements (Prism 4, GraphPad Software). Error bars show SEM. Differences were considered statistically significant for P values of .05 or less.
Results
Anti-BMP9 treatment increases vascular density of the retina of WT mice
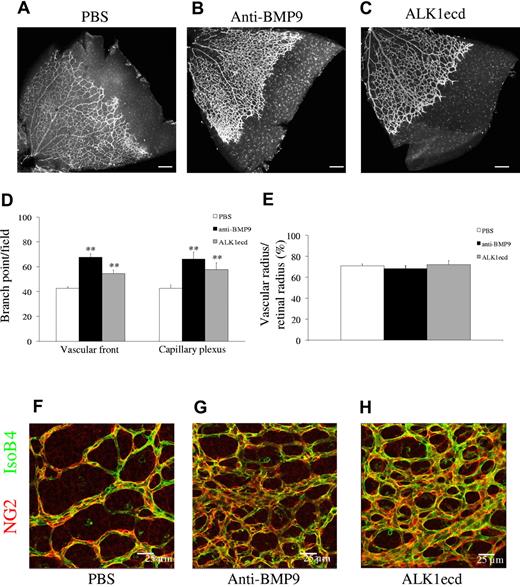
It was previously described that injection of ALK1ecd to newborn pups increased postnatal retinal vascular density.7 This indicated that the ALK1 pathway controls postnatal angiogenesis. However, in this prior study, the nature of the ligand(s) blocked by the addition of ALK1ecd was not characterized. We have previously shown that BMP9 binds to ALK1 with strong affinity (EC50 = 2pM)8 and that BMP9 circulates in a biologically active form in human and mouse blood and is present at higher levels around birth than during adulthood (6 ng/mL in newborn vs 2 ng/mL in adult mice).9,10 We therefore asked whether circulating BMP9 triggered the biologic effects blocked by ALK1ecd. Analysis of mouse retinas at postnatal day 6 (P6) after a systemic treatment of pups (OF1 background) with a monoclonal anti-BMP9 antibody (5 mg/kg, at P1 and P3) revealed vascular patterning defects, with vessels forming a hyperbranched plexus (Figure 1A-B). We quantified the number of branching points both at the vascular front and at the capillary plexus and found that anti-BMP9 treatment significantly increased vascular branching (Figure 1D). We observed a similar effect with ALK1ecd treatment (5 mg/kg; Figure 1C-D). On the other hand, we did not observe any differences on radial vascular expansion (Figure 1E). The coverage of the vessels by pericytes, as assessed by immunostaining of the proteoglycan NG2, was not modified by treatment with either anti-BMP9 or ALK1ecd (Figure 1F-H). Similar results on the vascularization of the retina were observed in mice from another genetic background (C57Bl6/J, data not shown). To confirm that treatment with anti-BMP9 or ALK1ecd completely abolished plasma BMP9 activity, we measured active circulating BMP9 levels in these mice using a BMP responsive (BRE) reporter assay. Specifically, 0.5% of plasma from P5 pups was added to 3T3 cells transfected with the BRE-reporter plasmid together with a plasmid encoding ALK1. Under these conditions, we have previously described that only BMP9 is detected.10 We found that treatment with anti-BMP9 or ALK1ecd strongly decreased BMP9 circulating activity (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Anti-BMP9 treatment increases vascularization of the retina. (A-C) Visualization of blood vessels by isolectin B4 (iB4) staining of OF1 retinas at P6 after treatment with BMP9-blocking antibodies (5 mg/kg) or recombinant ALK1ecd (5 mg/kg) injected at P1 and P3. (D) Number of branching points at the capillary plexus and at the front of migration. (E) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage. (F-H) Pericyte coverage visualized by NG2 immunostaining of OF1 retinas at P6. Panels A through H show data from 2 litters containing 10 PBS treated pups, 9 anti-BMP9 treated pups and 5 ALK1ecd treated pups. Error bars show SEM. **P < .01 versus PBS group. (A-C) Scale bar = 200 μm.
Anti-BMP9 treatment increases vascularization of the retina. (A-C) Visualization of blood vessels by isolectin B4 (iB4) staining of OF1 retinas at P6 after treatment with BMP9-blocking antibodies (5 mg/kg) or recombinant ALK1ecd (5 mg/kg) injected at P1 and P3. (D) Number of branching points at the capillary plexus and at the front of migration. (E) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage. (F-H) Pericyte coverage visualized by NG2 immunostaining of OF1 retinas at P6. Panels A through H show data from 2 litters containing 10 PBS treated pups, 9 anti-BMP9 treated pups and 5 ALK1ecd treated pups. Error bars show SEM. **P < .01 versus PBS group. (A-C) Scale bar = 200 μm.
Bmp9-KO mice present no detectable defects in retinal vascularization
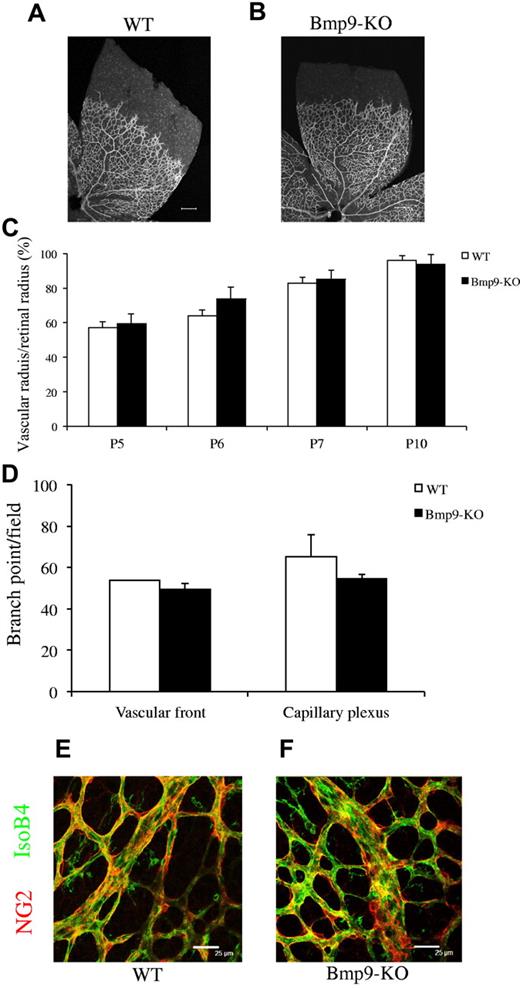
Next, we wondered whether Bmp9-KO mice would exhibit defects in retinal vascularization. Bmp9-KO adult mice are viable and fertile, and pups are grossly normal. A full characterization of the adult mice will be described elsewhere (T.A.Z., S.-J.L., Arteriovenous malformations and skeletal abnormalities in mice deficient for Gdf2/Bmp9, manuscript in preparation). In these mice, no BMP9 mRNA was detected by RT-PCR in liver (supplemental Figure 2A). Furthermore, no circulating BMP9 in Bmp9-KO plasma was detected either using a BMP9 ELISA (supplemental Figure 2B) or by measuring the BRE activity (supplemental Figure 2C). Pups were killed between P5 and P10, and no obvious defects in the organization of the retinal vascular network could be observed in Bmp9-KO versus wild-type (WT) mice (Figure 2A-B). The veins and the arteries were well-differentiated and organized. The radial expansion of the vascular network measured at different postnatal days (P5-P10) was not significantly different between the 2 groups (Figure 2C). The numbers of branching points in the retinal vasculature were not significantly different between WT and Bmp9-KO mice (Figure 2D). We did not observe any differences in vessel coverage by pericytes, using NG2 labeling, between WT and Bmp9-KO mice at P6 (Figure 2E-F). These data indicate that interfering with BMP9 through the use of blocking antibodies versus through genetic targeting generate different phenotypes.
Bmp9-KO mice have a normal vascularized retina. (A-B) Visualization of blood vessels by isolectin B4 (iB4) staining of WT and Bmp9-KO retinas at P6. (C) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage at P5 (n = 6), P6 (n = 7), P7 (n = 9), and P10 (n = 4) from 2 litters. (D) Number of branching points at the capillary plexus and at the front of migration of P6 pups (n = 3 in each group). (E-F) Pericyte coverage was visualized by NG2 immunostaining. Error bars show SEM. (A-B) Scale bar = 200 μm.
Bmp9-KO mice have a normal vascularized retina. (A-B) Visualization of blood vessels by isolectin B4 (iB4) staining of WT and Bmp9-KO retinas at P6. (C) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage at P5 (n = 6), P6 (n = 7), P7 (n = 9), and P10 (n = 4) from 2 litters. (D) Number of branching points at the capillary plexus and at the front of migration of P6 pups (n = 3 in each group). (E-F) Pericyte coverage was visualized by NG2 immunostaining. Error bars show SEM. (A-B) Scale bar = 200 μm.
ALK1ecd treatment of Bmp9-KO mice strongly modifies retinal vascularization
A possible explanation that could account for the discrepancy between the absence of phenotype in Bmp9-KO mice and the increase of vascular branching in response to an acute blockade of BMP9 with anti-BMP9 antibody is that either the anti-BMP9 antibody cross reacts with another BMP or that Bmp9-KO mouse might have compensated for the chronic loss of BMP9. To test the first hypothesis, we added anti-BMP9 antibodies to Bmp9-KO mice and found no significant differences with PBS-treated pups (data not shown), thereby excluding a possible cross-reaction of the anti-BMP9 antibody with another BMP. We next tested for the presence of another ALK1 ligand by injecting ALK1ecd into Bmp9-KO mice. We observed that ALK1ecd treatment strongly affected retinal vascularization of Bmp9-KO pups (Figure 3A-B). The vascularization was denser, and the radial expansion of the vascular network was slightly decreased (Figure 3C). Higher power views showed that the plexus in the retinas of ALK1ecd treated mice consisted of capillaries that were larger in diameter (as visualized along the z-axis), more highly interconnected and multilayered (Figure 3D-G). To determine whether this was attributable to an increased endothelial cell number, treated retinas were stained for BrdU. In control retinas at P5, veins and peripheral capillaries contained proliferating cells, whereas arteries were almost exclusively composed of nonproliferating cells (supplemental Figure 3A-B). In contrast, in ALK1ecd treated pups, most of the vascular plexus was composed of proliferating cells (supplemental Figure 3C-D). Taken together, these data support the hypothesis of functional rescue in Bmp9-KO mice by another ALK1 ligand.
ALK1ecd treatment in Bmp9-KO mice increases vascularization of the retina. (A-B) Visualization of blood vessels by isolectin B4 (iB4) staining of retinas at P5 from Bmp9-KO mice treated or not with ALK1ecd (5 mg/kg) injected at P1 and P3. (C) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage. (D-E) Higher magnifications of the retinas show that ALK1ecd treatment in Bmp9-KO mice lead to larger and multilayered blood vessels (see also z scan in panels F and G). Panels A through G show data from 2 litters containing 8 PBS treated Bmp9-KO mice and 7 ALK1ecd treated Bmp9-KO mice. Error bars show SEM. (A-B) Scale bar = 200 μm. (D-E) Scale bar = 25 μm.
ALK1ecd treatment in Bmp9-KO mice increases vascularization of the retina. (A-B) Visualization of blood vessels by isolectin B4 (iB4) staining of retinas at P5 from Bmp9-KO mice treated or not with ALK1ecd (5 mg/kg) injected at P1 and P3. (C) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage. (D-E) Higher magnifications of the retinas show that ALK1ecd treatment in Bmp9-KO mice lead to larger and multilayered blood vessels (see also z scan in panels F and G). Panels A through G show data from 2 litters containing 8 PBS treated Bmp9-KO mice and 7 ALK1ecd treated Bmp9-KO mice. Error bars show SEM. (A-B) Scale bar = 200 μm. (D-E) Scale bar = 25 μm.
Anti-BMP10 treatment disrupts retinal vascularization in Bmp9-KO mice
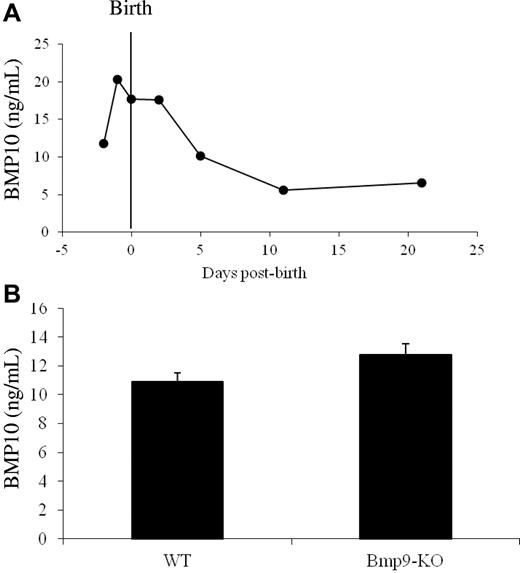
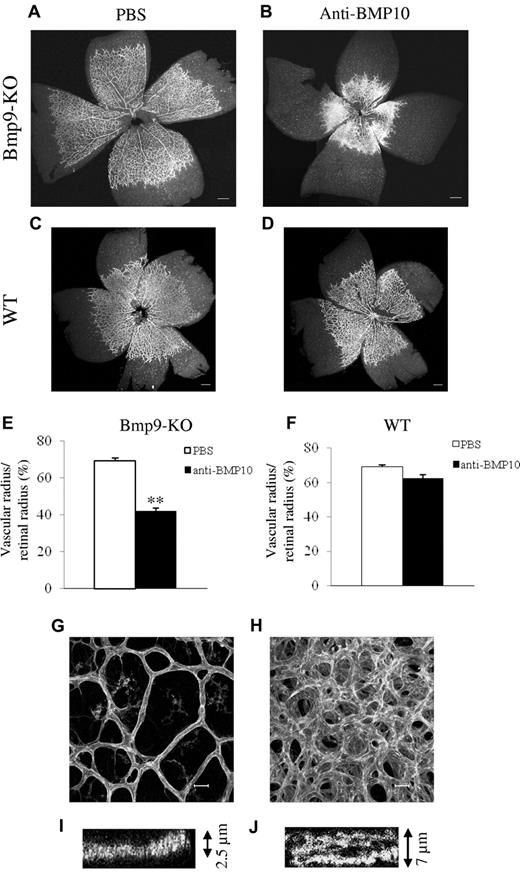
We previously showed that BMP9 and BMP10 bind with a similarly strong affinity to ALK1.8 Therefore, we hypothesized that BMP10 is the ligand blocked by ALK1ecd in Bmp9-KO mice. To test this hypothesis, we first checked whether we could detect circulating BMP10. Using a murine BMP10 ELISA, we found high levels of BMP10 from embryonic day E17.5 until postnatal day P5 (10-20 ng/mL) in WT plasma, which then decreased progressively with age (Figure 4A). We next measured circulating BMP10 levels in WT and Bmp9-KO P5 pups and found no statistically significant differences between Bmp9-KO pups and WT pups (12 vs 10 ng/mL, respectively; Figure 4B). This circulating BMP10 was unable to activate the BRE reporter gene using 0.5% plasma demonstrating that BMP10 circulates under an inactive form (supplemental Figure 2C). This result is in accordance with our previous work showing that the circulating BRE-inducing activity is solely because of active BMP9.10 This result demonstrated the absence of a circulating compensating BRE-inducing activity in Bmp9-KO pups. Still, we tested whether BMP10 could play a role in postnatal angiogenesis using an anti-BMP10 neutralizing antibody. We used a higher quantity of anti-BMP10 antibody (15 mg/mL) than the quantity used for the anti-BMP9 antibody (5 mg/mL), as the anti-BMP10 antibody was less effective in vitro than the anti-BMP9 antibody (data not shown). Systemic injection of anti-BMP10 antibodies into Bmp9-KO pups at P1 and P3 significantly decreased the overall growth of the pups and pulmonary hemorrhages were observed (data not shown). Some of their vessels also appeared to be dilated (supplemental Figure 4). Examining vascularization of their retina, we found that anti-BMP10 treatment in Bmp9-KO pups resulted in a dramatic increase in vascular density and a strong decrease in vascular radial expansion (Figure 5A-B,E). In contrast, anti-BMP10 antibody injection in WT pups had no significant effects on the pups and did not alter retinal vascularization of the retina (Figure 5C-D,F). The addition of anti-BMP9 and anti-BMP10 antibodies to WT pups induced a similar effect on retinal expansion and density as compared with addition of anti-BMP10 antibody in Bmp9-KO pups (data not shown). Higher power views showed that the vascular network in the retinas of Bmp9-KO pups treated with anti-BMP10 antibodies was formed of capillaries that were hyperfused, larger in diameter and that presented several layers (as visualized along the z-axis, Figure 5G-J). Normally, the vascular network extends directionally in a narrow plane defined by the astrocytic network secreting VEGF-A17 as observed in Figure 5G. In contrast, in Bmp9-KO pups treated with anti-BMP10, we clearly see the presence of several layers of vessels (Figure 5H). This is particularly well illustrated when looking at serial confocal slices where at least 2 layers can be detected (supplemental Figure 5A-C). In some areas, the vessels coalesced to form a plexus as already observed with ALK1ecd-treated mice, although the effect was stronger with anti-BMP10 antibodies (Figures 3B and 5B). We did not observe a significant effect of anti-BMP10 antibody treatment on the number of tip cells at the periphery of the vascularized retina in Bmp9-KO mice (data not shown). Taken together, these data therefore support a cooperative role for BMP9 and BMP10 in postnatal angiogenesis of the retina.
BMP10 expression in WT and Bmp9-KO mice plasma. (A-B) BMP10 levels were measured from pooled diluted plasma (1/20) taken from WT OF1 mice at the indicated developmental stages (A) and from WT (n = 9) and Bmp9-KO (n = 14) P5 pups (B) with a murine BMP10 ELISA. Error bars show SEM.
BMP10 expression in WT and Bmp9-KO mice plasma. (A-B) BMP10 levels were measured from pooled diluted plasma (1/20) taken from WT OF1 mice at the indicated developmental stages (A) and from WT (n = 9) and Bmp9-KO (n = 14) P5 pups (B) with a murine BMP10 ELISA. Error bars show SEM.
Anti-BMP10 treatment disrupts vascularization of the retina of Bmp9-KO mice. Visualization of blood vessels by isolectin B4 (iB4) staining of retinas at P5 from Bmp9-KO (A-B) or WT (C-D) mice treated with PBS or anti-BMP10 antibody (15 mg/kg) injected at P1 and P3. (E-F) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage in Bmp9-KO mice (E) and in WT mice (F). (G-H) Higher magnifications of the retinas show that anti-BMP10 treatment in Bmp9-KO mice lead to larger and multilayered blood vessels (see also z scan in panels I and J). Panels A, B, E, G, H, I, and J show data from 4 litters containing 12 PBS-treated Bmp9-KO pups and 7 anti-BMP10 treated pups. Panels C, D, and F show data from 2 litters containing 6 PBS treated WT pups and 6 anti-BMP10 treated pups. Error bars show SEM. **P < .01 versus PBS group. (A-D) Scale bar = 200 μm. (G-H) Scale bar = 20 μm.
Anti-BMP10 treatment disrupts vascularization of the retina of Bmp9-KO mice. Visualization of blood vessels by isolectin B4 (iB4) staining of retinas at P5 from Bmp9-KO (A-B) or WT (C-D) mice treated with PBS or anti-BMP10 antibody (15 mg/kg) injected at P1 and P3. (E-F) Bar graphs show the relative distance covered by the vascular plexus calculated as the ratio of the vascular radius over the retinal radius in percentage in Bmp9-KO mice (E) and in WT mice (F). (G-H) Higher magnifications of the retinas show that anti-BMP10 treatment in Bmp9-KO mice lead to larger and multilayered blood vessels (see also z scan in panels I and J). Panels A, B, E, G, H, I, and J show data from 4 litters containing 12 PBS-treated Bmp9-KO pups and 7 anti-BMP10 treated pups. Panels C, D, and F show data from 2 litters containing 6 PBS treated WT pups and 6 anti-BMP10 treated pups. Error bars show SEM. **P < .01 versus PBS group. (A-D) Scale bar = 200 μm. (G-H) Scale bar = 20 μm.
In vitro stimulation of endothelial cells with BMP9 and BMP10 regulates the Notch signaling pathway and apelin expression
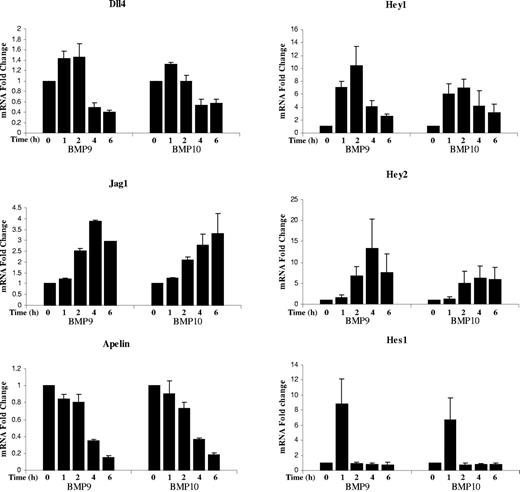
The phenotype resulting from blockade of BMP9 and BMP10 partially phenocopied the hyperplasic vascular pattern obtained from inhibition of Dll4/Notch signaling and apelin inactivation.18,19 We therefore analyzed whether these genes were targets of BMP9 and BMP10 in endothelial cells. Human pulmonary arterial endothelial cells (HPAEc) were treated with BMP9 or BMP10 (0.5 ng/mL), and RNA was extracted at different time points (1 to 6 hours) for quantitation by RT-qPCR. We found that BMP9 and BMP10 transiently increased expression of the Notch ligands Dll4 and Jagged1 (Figure 6). It is interesting to note that Dll4 mRNA levels were increased during the first 2 hours of stimulation but then reduced to lower than its basal level at 4 and 6 hours after stimulation. This was not the case for Jagged1. BMP9 and BMP10 stimulation also strongly and transiently increased Hey1, Hey2 and Hes1 mRNA levels (Figure 6). On the other hand, mRNA apelin expression was strongly reduced in response to BMP9 and BMP10 treatment (Figure 6). Taken together, these data show that BMP9 and BMP10 modulate genes of the Notch and apelin signaling pathways suggesting a possible cooperation between these pathways.
BMP9 and BMP10 regulated genes in endothelial cells. mRNA fold changes in Dll4, Jag1, Hey1, Hey 2, Hes1, and apelin mRNA levels in hPAEcs stimulated with 0.5 ng/mL BMP9 or BMP10 at different time points (1, 2, 4, and 6 hours). Expression of HPRT was used as the normalization control. Data pooled from 3 individual experiments, each containing 2 replicates. Error bars show SEM.
BMP9 and BMP10 regulated genes in endothelial cells. mRNA fold changes in Dll4, Jag1, Hey1, Hey 2, Hes1, and apelin mRNA levels in hPAEcs stimulated with 0.5 ng/mL BMP9 or BMP10 at different time points (1, 2, 4, and 6 hours). Expression of HPRT was used as the normalization control. Data pooled from 3 individual experiments, each containing 2 replicates. Error bars show SEM.
Discussion
The goal of this study was to analyze the role of BMP9 and other possible ligands for ALK1 in angiogenesis using the model of postnatal retinal vascularization. The present work shows that BMP9, and also BMP10, are involved in retinal angiogenesis and that they can, at least partially, functionally substitute for each other. These data indicate that the ALK1 neutralizing treatments currently tested in preclinical studies for cancer treatment20 will affect both BMP9 and BMP10 functions.
Using a neutralizing anti-BMP9 antibody, we demonstrated that BMP9 blockade induces an increase in retinal vascular density, supporting a role for BMP9 in the remodeling of the retinal vascularization. However, Bmp9-KO pups had no obvious defect in the vascularization. A similar discrepancy in retinal vascularization between VEGFR-3 antibodies and genetic targeting has been previously reported.21 One possible explanation for this discrepancy would be a possible cross-reaction of the anti-BMP9 antibody with another BMP. However, injection of anti-BMP9 antibodies to Bmp9-KO pups did not affect retinal vascularization (data not shown). This demonstrates that acute blockade might not be similar to permanent blockade and could indicate a possible adaptation to the loss of BMP9 in Bmp9-KO mice through expression of another ALK1 ligand. Indeed, injection of ALK1ecd in Bmp9-KO pups induced a strong modification of retinal vascularization confirming the presence of another ALK1 ligand. Using an anti-BMP10 antibody, we demonstrated that BMP10 can substitute for BMP9. However, no significant difference in circulating BMP10 levels or BRE activity could be observed between WT and Bmp9-KO pups. Further work will be necessary to understand the reason of the absence of phenotype in Bmp9-KO pups.
Our data demonstrate a new and unexpected function for BMP10 in postnatal angiogenesis. Indeed, up to now, BMP10 was mainly known to play a critical role in heart formation.11 Still, BMP10 has also been described to be present in human and murine adult sera using a proteomic approach.22 However, we previously demonstrated that plasmatic BRE-inducing activity via ALK1 is only attributable to BMP9,10 which, in contrast to BMP10, was shown to be active when bound to its prodomain.23,24 Our present work further supports these biochemical data. Indeed, we confirm the presence of circulating BMP10 in the plasma of mouse neonates and could determine, for the first time, that it circulates at a concentration of around 10 ng/mL. These levels are elevated in mouse embryos just before birth and during early postnatal life, when there is active retinal angiogenesis occurring, and then steadily decrease as blood vessels reach maturity. This profile of expression is extremely similar to the one we observed for circulating BMP9.9 However, this circulating BMP10 is not able to activate the BRE promoter via ALK1, as we observed no BRE-inducing activity in Bmp9-KO plasma. Still, adding anti-BMP10 antibodies to Bmp9-KO mice had a dramatic effect on postnatal vascularization suggesting that BMP10 must be locally activated. BMP1 has been recently demonstrated to activate BMP10.24 We detected BMP1 mRNA in the retina, suggesting that a local activation of BMP10 could occur. However, no changes in its mRNA level could be observed between WT pups and Bmp9-KO pups (data not shown). Another possibility is that circulating BMP10, while being unable to activate the BRE activity, could still be able to regulate retinal vascularization via a Smad-independent mechanism. Indeed, we have previously shown that the constitutively active form of ALK1 inhibited endothelial cell migration in a Smad-independent manner involving MAP kinase signaling (JNK and ERK).25 Further, Eng-iKOe retinas exhibited delayed remodeling of the capillary plexus while their phospho-Smad1/5 levels were not affected as compared with WT pups.26
Anti-BMP10 treatment had no significant effect on the vascularization of WT retinas, indicating that the presence of BMP9 is sufficient to maintain retinal vascularization. This was not the case with anti-BMP9 treatment, which can on its own already affect retinal vascularization. It is tempting to relate this differential effect to the differences in latency between BMP9 and BMP10. BMP9 being available in the circulation in a biologically active form is efficient as is, while BMP10 circulating in an inactive form would probably require local rate-limiting proteolytic activation to be efficient. It is also interesting to note that the inhibitory effect of ALK1ecd injection had a lower impact on retinal vascularization than anti-BMP10 antibody treatment in Bmp9-KO mice. This can either be because of the fact that ALK1ecd blocks BMP9 and BMP10 active forms, while anti-BMP10 may interact with BMP10 active and inactive forms, or to the fact that ALK1ecd half-life is shorter than that of the neutralizing anti-BMP10 antibody, thereby leading to a less severe phenotype. Taken together, these data suggest that BMP9 is important for retinal vascularization, and that at least in absence of BMP9, BMP10 can take over.
Injection of BMP10 antibodies into Bmp9-KO mice also affected the overall diameters of vessels (supplemental Figure 4). These data would suggest that BMP9 and BMP10 are involved in the control of vessel diameter. This is in accordance to what has been observed in Alk1-conditional deletion in endothelial cells27 and in inducible Alk1-conditional deletion in adult mice.28 Arterio-venous malformations have been described in HHT patients and in retinas of conditional endothelial ENG deletion.6 We therefore looked for their presence in Bmp9-KO retinas treated with anti-BMP10 antibodies but could not detect any. Hemorrhages are also often observed in many organs in HHT patients. Interestingly, pulmonary hemorrhages could be observed in Bmp9-KO pups treated with anti-BMP10 antibodies. These results could suggest that the ligands BMP9 and BMP10 are relevant for HHT pathogenesis.
We focused our analysis on the retina of Bmp9-KO pups treated with anti-BMP10 antibodies, which presented the strongest phenotype with a significantly reduced vascular radial expansion and increased vascular density. The vessels were larger and hyperfused, and higher magnification and projection along the z-axis clearly demonstrated the presence of several layers of vessels, while controls had a single layer of vessels. The vascularization of the retina occurs through sprouting angiogenesis, which involves the selection of a leading tip cell triggered by a gradient of VEGF-A. In response to VEGF-A, tip cells become enriched in Dll4 and instruct adjacent endothelial cells to become stalk cells via Dll4/Notch1-mediated lateral inhibition.17,18 The tip and stalk cell phenotypes are remarkably transient and exchangeable as endothelial cells dynamically shuffle position along the angiogenic sprouts, and compete for the tip cell position.29 Multilayered blood vessels have been previously described in mice overexpressing the diffusible VEGF-A-120 isoform, demonstrating the need of a proper VEGF-A gradient for guidance of endothelial cells.17 This multilayered phenotype was also observed in Eng-iKOe.26 The phenotype observed here could therefore suggest a defect in tip cell versus stalk cell status, but also in oriented migration and/or polarity. We have previously described a role for ALK1 in orientated migration which could partially explain what is observed here.30 Looking at retinal vascularization from P2 to P5, we found that anti-BMP10 treatment of Bmp9-KO mice inhibited the vascular remodeling of the vessels as soon as P3. In WT pups, the vessels grow steadily and get thinner as they extend. In Bmp9-KO pups treated with anti-BMP10 antibody, the vessels stopped expanding at P3, failed to thin down and formed multilayered vessels (data not shown). The hyperdensity could also be attributed to the ability of BMP9 and BMP10 to act as quiescence factors via binding to ALK1.10 Therefore, absence of these 2 factors could induce over-activation of angiogenesis.
Bmp9-KO pups treated with anti-BMP10 antibodies presented a retinal phenotype partially resembling those of Dll4+/− mice or mice treated with the γ-secretase inhibitor (DAPT), with an increase in branching and a decrease in radial expansion.15,18,31,32 Jagged1 and Hey1 have been previously described as direct BMP9 target genes in endothelial cells33 suggesting a possible interconnection of the Notch and BMP pathways. We therefore analyzed whether some of these genes were targets of BMP9 and BMP10 in endothelial cells. We found that BMP9 and BMP10 affected the Notch signaling pathway in a transient manner, increasing the level of Dll4 (× 1.5-fold for BMP9) at early time points, while decreasing Dll4 lower than its basal level after 4 hours (× 0.4-fold for BMP9). Jagged1 expression was increased in response to BMP9 and BMP10, in accordance with a previous work.33 We further confirmed Hey1 as a target regulated by BMP9 but also by BMP10 and identified Hey2 and Hes1, as new targets. The kinetics of these transcription factors were slightly different, Hes1 was increased at 1 hour and returned to its basal level, Hey1 peaked at 2 hours, while Hey2 peaked at 4 hours. These transient regulations can be linked to the remarkably transient and dynamic changes of endothelial cells from tip to stalk cells.29 The rapid induction of some of these genes (eg, Hes1) is in favor of a direct effect of BMP9/10 on their expression rather than through a feedback of the Notch signaling pathway. Altogether these results indicate that some of the previously described Notch target genes are also BMP9/BMP10 target genes in endothelial cells. While submitting this manuscript, another study confirmed that BMP9 regulated the expression of Notch target genes.34 This work further supports the interdependence of the Notch pathway and the BMP9/BMP10 pathways. Cross-signaling between the Notch and BMP/TGFß/Smad pathways has been documented in various cell types among which endothelial cells. In endothelial cells, Smad1 and Smad5 form on receptor activation a complex with the Notch intracellular domain (NICD) to potentiate Hey1 expression.35 A more recent study shows that endothelium-specific inactivation of Smad1/5 impairs Notch signaling, and affects Hes1, Hey1, and Jagged1 expressions in mouse embryos and endothelial cells resulting in deficient vessel organization.36
Apelin is the endogenous ligand for the receptor APJ and is expressed in many cells, including endothelial cells. Apelin was shown to stimulate the proliferation and migration of endothelial cells and to be involved in the regulation of blood vessel diameter during angiogenesis.37,38 Apelin has also been recently shown to be involved in vascularization of the retina.19,39 Its deletion decreased radial endothelial expansion of the retina, but its effect on vascular density was reported to be different in the 2 published studies. We found that apelin expression was strongly decreased by either BMP9 or BMP10 treatment in endothelial cells. These data therefore suggest that apelin could be one of the target genes leading to the inhibition of radial vascular expansion and the increase in blood vessel diameter.
In conclusion, we demonstrate here that BMP9, but also BMP10, are important regulators of postnatal angiogenesis. This work proposes a new role for BMP10 in angiogenesis. Furthermore, it supports an important cross-talk between the BMP9/10, the Notch and the apelin pathways during postnatal angiogenesis. This interplay reveals a mechanism whereby endothelial cells make continuous dosage dependent decisions on whether to carry out effective expansion of the vascular network or become quiescent contributing to the maturation of the blood vessels.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Inserm, CEA (Commissariat à l'Energie Atomique et aux Energies Alternatives), UJF (Université Joseph Fourier-Grenoble 1), and Ligue nationale contre le Cancer and ARC (Association pour la Recherche sur le Cancer). N.R. was supported by the CEA and the ARC. S.L. was supported by the CEA. D.C. was supported by the Fondation Lefoulon-Delalande. Work in S.-J.L.'s laboratory was supported by National Institutes of Health grant R01HD35887/AR060636.
National Institutes of Health
Authorship
Contribution: N.R., S.L., M.S., D.C., M.B., and C.M. performed research; T.Z. and S.-J.L. generated the Bmp9-KO mice; N.R., S.B., and J.J.F. designed research; and S.B., N.R., J.J.F., and D.C. wrote the paper.
Conflict-of-interest disclosure: Under licensing agreements between Johns Hopkins University (JHU) and Acceleron Pharma Inc and between JHU and Novartis Inc, S.-J.L. and T.A.Z. are entitled to receive a share of licensing fees received by JHU for the use of Bmp9 knockout mice. Except for these licensing fees, which are fixed in their total amounts, JHU is not entitled to any additional future payments from Acceleron or Novartis as a result of these agreements. The remaining authors declare no competing financial interests.
Correspondence: Sabine Bailly, U1036 Inserm, 17 rue des Martyrs, 38054 Grenoble, France; e-mail: sbailly@cea.fr.
References
Author notes
D.C. and S.L. are co–second authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal