Abstract
MicroRNAs (miRNAs) inhibit HIV-1 expression by either modulating host innate immunity or by directly interfering with viral mRNAs. We evaluated the expression of 377 miRNAs in CD4+ T cells from HIV-1 élite long-term nonprogressors (éLTNPs), naive patients, and multiply exposed uninfected (MEU) patients, and we observed that the éLTNP patients clustered with naive patients, whereas all MEU subjects grouped together. The discriminatory power of miRNAs showed that 21 miRNAs significantly differentiated éLTNP from MEU patients and 23 miRNAs distinguished naive from MEU patients, whereas only 1 miRNA (miR-155) discriminated éLTNP from naive patients. We proposed that miRNA expression may discriminate between HIV-1–infected and –exposed but negative patients. Analysis of miRNAs expression after exposure of healthy CD4+ T cells to gp120 in vitro confirmed our hypothesis that a miRNA profile could be the result not only of a productive infection but also of the exposure to HIV-1 products that leave a signature in immune cells. The comparison of normalized Dicer and Drosha expression in ex vivo and in vitro condition revealed that these enzymes did not affect the change of miRNA profiles, supporting the existence of a Dicer-independent biogenesis pathway.
Introduction
MicroRNAs (miRNAs) are small, single-strand noncoding RNAs that repress gene expression by inhibiting translation and inducing mRNA degradation.1,2 miRNAs may regulate up to 92% of the human genes.3 There is evidence that miRNAs can modulate host innate immunity against viruses. Specifically, miRNAs inhibit HIV-1 gene expression by decreasing P300/CBP-associated factor expression and interfering with histone acetylation, thereby promoting HIV-1 latency.4 miRNAs also may participate in repressing HIV-1 gene expression by directly targeting HIV-1 mRNAs. Five cellular miRNAs recognize the 3′ end of HIV-1 mRNAs and are up-regulated in resting, but not activated, CD4+ T cells.5 Two independent groups showed that HIV-1 Nef gene contains a miR-29a–targeted site that interferes with the replication of the virus.6,7 Other miRNAs were involved in the different monocyte or macrophage susceptibility to the HIV infection,8,9 and it has been proposed that miRNAs either could directly influence viral RNA sequences or could affect cellular factors involved in HIV replication.10 However, cellular miRNA expression also may favor HIV-1 infection.11
Assessment of the extent to which the altered profiles of miRNA expression influences viral replication and latency, as well as the efficiency of host defences, may be useful for understanding the basis of the HIV-1–related alterations in cellular physiology and immunologic control.
To this end, we enrolled 3 patient groups. One group consisted of 7 subjects who were classified as élite control long-term nonprogressors (éLTNPs) with a documented history of at least 12 years of infection and an undetectable viremia. A second study group was HIV-1–positive subjects, who were antiretroviral therapy naive. A third group was multiply exposed to HIV-1, but uninfected (MEU). None of the subjects had coinfections such as HBV, HCV, or human T-lymphotropic viruses type I that could influence miRNA expression. The CD4+ T-lymphocyte population was purified from each patient class and analyzed for the expression of 377 miRNAs. This study allowed us to investigate the existence of a miRNAs signature able to discriminate among different stages of HIV-1 infection. Furthermore, interesting and unforeseen results were obtained by evaluating the modulation of miRNA expression after HIV-1 antigen exposure.
Patients and methods
Purification of CD4+ T cells
Blood samples were obtained from 17 HIV-1–infected (14 males and 3 females; aged 34-57 years), 7 HIV-1–exposed uninfected (4 males and 3 females; aged 36-57 years) patients, and 6 healthy donors.
HIV-1–positive subjects were classified as éLTNP and naive on the basis of the time of infection, HIV-1 RNA viral load, and CD4+ T count. éLTNP subjects had a documented history of infection of a mean 15 years (range, 10-18 years) and showed a mean CD4+ T count of 932 cells/mm3 (range, 792-1209 cells/mm3) and undetectable viremia in the absence of therapy. Naive patients, who reported HIV-1 infection of a mean value of 5 years duration had a mean CD4+ T count of 325 cells/mm3 (range, 228-390 cells/mm3) and a mean viremia of 169 795 copies/mL (range, 48 930-447 000 copies/mL) in the absence of therapy. The inclusion criterion of MEU patients was a history of penetrative sexual intercourse without condom at least twice per week and for at least 2 years with no other known risk factors during that period or afterward. HIV-1 infection was excluded by absence of anti–HIV-1 antibodies and viral DNA (data not shown). Seronegativity was confirmed 6 months after enrollment. After enrollment, counselling was offered and most couples switched to safe-sex practices. There was no occurrence of a specific genotype that was known to restrict HIV-1 susceptibility in any of the patient groups, specifically, CCR5Δ32 allele, CCR5 and CCR2 polymorphisms, CCL3L copy number, or HLA class II alleles.
All of the recruited patients gave informed consent, according to the Italian laws and the Declaration of Helsinki. The study was approved by the institutional ethics committees of all participating institutions. The CD4+ T cells were negatively selected from the whole blood by using CD4+ T cell isolation kit II (Milteny Biotec). The purity of the CD4+ T-cell population was always more than 95%, as assessed by cytofluorimetric analysis (data not shown). Cells were stored in RNAlater at −80°C or directly processed for miRNA extraction by the mirVana miRNA isolation kit (Ambion).
Flow cytometry
Directly conjugated monoclonal antibodies recognizing surface antigens (eBioscience, R&D Systems, and Serotec) were pretitrated with the appropriate buffer before use. Cells were stained in PBS with the LIVE/DEAD Red Fixable Dead Cell Stain kit (Invitrogen) to eliminate dead cells from the analysis, and with different antibodies for surface antigens; incubated for 20 minutes at room temperature; and washed with PBS containing 5% FCS and 5mM EDTA. Samples were fixed in PBS added with 1% paraformaldehyde, put at 4°C, and immediately analyzed. A 16-parameter CyFlow ML flow cytometer (Partec), equipped with a 488 nm blue laser, a 635 nm red laser, and a 405 nm violet laser was used in this study. A minimum sample size of 50 000 cells was acquired in list mode and analyzed using FlowJo 6.3 (TreeStar) and FloMax (Partec) software. Single staining and fluorescence minus one controls were performed periodically for all antibody panels to set proper compensation and define positive signals. Simplified Presentation of Incredibly Complex Evaluation, version 2.9 software (provided by Dr Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) was used to graphically depict and analyze polychromatic flow cytometry data.12
Proviral HIV DNA load quantification
To estimate HIV-1 proviral DNA load in purified CD4+ T cells, an assay based on real-time PCR (intracellular HIV DNA quantitative (q)PCR kit; GeneMoRe) was performed. The test uses 2 parallel reactions that quantify HIV-1 proviral DNA and nDNA for any given sample, to normalize the number proviral DNA copies to the number of starting cells.
miRNA array analysis
miRNA expression was determined by applying the Megaplex Pools protocol (Applied Biosystems). In total, 100 ng of RNA from CD4+ T cells was reverse transcribed using the TaqMan MicroRNA reverse transcription kit in combination with the Megaplex RT Primers Pool A that permits analysis of 377 human miRNAs of greatest interest to the research community (functionally defined or highly expressed) and an endogenous control. The following run protocol was used: 40 cycles at 16°C for 2 minutes, 42°C for 1 minute and 50°C for 1 second followed by 1 step at 85°C for 5 minutes. Before loading the TaqMan MicroRNA array, a preamplification of the miRNA cDNA target (2.5 μL) was performed by PCR using TaqMan PreAmp Master Mix kit and Megaplex PreAmp Primers Pool A (Applied Biosystems). The following run protocol was applied: denaturation for 10 minutes at 95°C, 1 step at 55°C for 2 minutes followed by 2 minutes at 72°C, and 12 cycles (95°C for 15 seconds, 60°C for 4 minutes). According to manufacturer's instructions, the preamplified cDNA product was loaded onto the array for PCR amplification by using TaqMan MicroRNA Array A and TaqMan Universal PCR Master Mix, No AmpErase UNG. The following real-time PCR protocol was used: 50°C for 2 minutes, 94.5°C for 10 minutes, and 40 cycles at 97°C for 30 seconds and 59.7°C for 1 minute. All reactions (reverse transcription, preamplification, and real-time PCR) were performed on a 7900HT Fast Real-Time PCR system (Applied Biosystems).
The results were analyzed using Relative Quantification Manager 1.2 software (Applied Biosystems), based on the comparative Ct method (ΔΔCt). Amplification signal was checked on each sample by SDS Version 2.3 software (Applied Biosystems). Undetermined raw Ct values were set to 40. The RNA extracted from the pool of healthy donors was used as calibrator, whereas the small noncoding MammU6 RNA was tested as housekeeping gene. The expression of endogenous control was relatively stable in all groups. Contamination was excluded by the analysis of a negative control. Each sample was retested at least once on a separate microfluidic card. Microarray data can be viewed at the Gene Expression Omnibus under accession GSE32231.
Target screening
A computational procedure using miRECORDS (http://miRecords.umn.edu/miRecords) and miRBase Targets 5 (http://www.mirbase.org) resources was performed.
Gene functional analysis
To each target gene, functional annotations were provided by DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov) on the basis of gene ontology (GO) resources. The significance of gene-term enrichment was examined using a modified Fisher exact test (Expression Analysis Systematic Explorer score). The GO term selection was obtained using a significant P value less than .05 as a statistical threshold. The selected GO term associated genes were clustered by Tool of Grouping Gene Ontology Annotation (TAGGO) 1.0 at http://bioacademy.gr/bioinformatics/projects/GOmir that divides GO in 3 ontology aspects: molecular function, cellular component, and biologic process. The cellular component aspect addresses the questions of gene function, where the active form can be found, and the biologic process aspect clarifies the biologic objective of a gene product. To avoid a generic classification of terms, a normalized information content threshold was fixed for any aspect. Any general term below 4% was classified as “no entry” category.
Data analysis
Hierarchical clustering of the samples was done by Cluster 2.11 software (http://rana.lbl.gov/EisenSoftware.htm) using average linkage and Pearson correlation.
For comparison of miRNAs' transcript expression between patients groups, 2-way ANOVA followed by Bonferroni posthoc test was applied. All statistical analysis was performed using Prism 5 software (GraphPad Software; differences between groups were considered significant at *P < .05, **P < .01, and ***P < .001).
Exposure of healthy CD4+ T lymphocytes to gp120 in presence or absence of anti-gp120 mAbs
The cells were negatively selected by column exclusion from PBMCs. Purity of CD4+ T cells (96% or more) was determined by flow cytometric analysis. CD4+ T cells (1.5 × 106/mL) and HeLa-LAI env-transformed cells (1.5 × 106/mL) were cocultured in a transwell system up to 6 hours after pretreated or not HeLa-LAI cells with anti-gp120 (mAbs IgG1 b12, 2F5, 2G12; NIH AIDS reagent) at 1 μg/mL for 1 hour at 37°C. Similarly, CD4+ T cells (1.5 × 106/mL) were cultured for 6 hours by recombinant gp120 at 0.1 μg/mL (rHIV-1 IIIB gp120 CHO; NIH AIDS reagent) in the absence or in the presence of the same anti-gp120 mAbs. Untreated cultures of healthy CD4+ T lymphocytes were used as control. Cultures were harvested, and expression of miRNAs was determined by TaqMan miRNA qRT-PCR.
Analysis of DICER and DROSHA mRNA levels by real-time PCR
Total RNA from CD4+ T cells was purified using RNaqueous-4 PCR kit (Ambion). Reverse transcription was performed by MessageSensor RT kit (Ambion). The following primers and labeled probes were used for DROSHA cDNA: forward, 5′-gctctgtccgtatcgatcaact; reverse, 5′-aagtggacgataatcggaaaagt; and probe, 5′-FAM-atcgtgaacagttcaaccccgatgtg-BHQ1. For DICER cDNA, primers were forward, 5′-ggccccaatcctggactta; reverse, 5′-aagccgctccaggttaaatc; and probe, 5′-FAM-ctttgactctgtcaaacgctagtga-BHQ1. Primers for 18s rRNA cDNA were forward, 5′-tcgaacgtctgccctatcaa; reverse, primer 5′-cccgtggtcaccatggt; and probe, 5′-Texas-Red-tttcgatggtagtcgccgtgcc-BHQ-3. The PCR conditions were 95°C for 5 minutes, followed by 45 cycles of 95°C for 15 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. The runs were performed on the Chromo4 continuous fluorescence detector (MJ Research). Results were analyzed by using sequence detector software, and relative fold differences were determined applying the comparative Ct method (ΔΔCt). The RNA extracted from purified healthy CD4+ T cells (in ex vivo condition or cultured) was used as calibrator. 18s rRNA was tested as housekeeping gene.
Results
éLTNP had a lower amount of activated T lymphocytes, less activated regulatory T cells, more T-cell receptor rearrangement excision circle (TREC+) cells, and less HIV DNA than naive patients
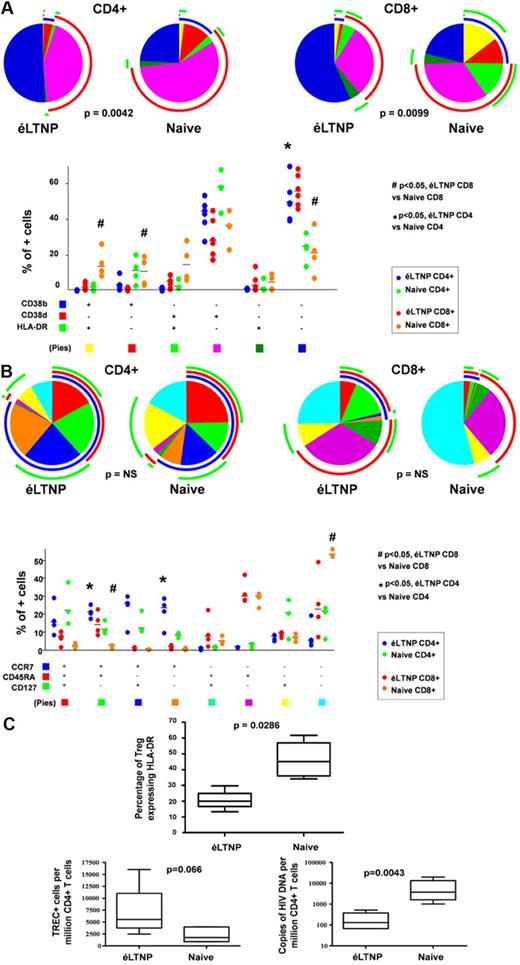
Before exploration of the miRNA profile in patient CD4+ cells, we determined their phenotypic analysis, HIV-1-DNA load and signal joint (sj)TREC determination.10 As expected, éLTNP had fewer peripheral activated T lymphocytes in comparison with those of naive patients (Figure 1A). Moreover, éLTNP had fewer regulatory T cells that expressed an activation marker such as HLA-DR, a higher number of sjTREC+ cells, and a lower amount of intracellular HIV-1 DNA within CD4+ T lymphocytes than naive patients (Figure 1B). No significant differences were observed between éLTNP and naive patients by analysis of the differentiation marker CD127 expression in either virgin or memory T cells (Figure 1C).
Viro-immunologic features in éLTNP and naive patients. (A) éLTNP patients had fewer peripheral activated T lymphocytes (triple negative cells) in comparison with those of naive patients, either among CD4+ or CD8+ T lymphocytes. Pie charts (top) and bars (referred to median values) among dots (bottom) show the qualitative composition of activated CD4+ or CD8+ T cells in éLTNP patients (blue and red dots, respectively) and in naive patients (green and orange dots, respectively). Each pie graph represents the mean proportion of the different populations identified by anti-CD38 (b indicates bright, ie, cells with high amounts of CD38 on the cell surface; d, dim, cells with low amounts of CD38) and anti–HLA-DR mAbs as indicated in the legend under the histograms. Arcs designed outside the pies represent the fraction of total cells expressing a particular marker, irrespective of the positive or negative expression of other markers (blue, CD38 bright; red, CD38 dim; green, HLA-DR). (B) No relevant differences were observed in analysis of differentiation markers (ie, virgin or memory T cells whether or not expressing CD127). Pie charts (top) and bars (referred to median values) among dots (bottom) show the differentiation of CD4+ or CD8+ T cells in éLTNP patients (blue and red dots, respectively) and in naive patients (green and orange dots, respectively). Each pie graph represents the mean proportion of the different populations identified by anti-CCR7, anti-CD45RA, and anti-CD127 mAbs as indicated in the legend under the histogram. Arcs designed outside the pies represent the fraction of total cells expressing a particular marker, irrespective of the positive or negative expression of other markers (blue, CCR7; red, CD45RA; green, CD127). (C) éLTNP patients had fewer regulatory T cells expressing HLA-DR, higher numbers of sjTREC+ cells, and less intracellular HIV-1 DNA within CD4+ T lymphocytes than naive patients. Boxes limit the first and third quartile, the line in the box represents the median value; whiskers indicate minimum and maximum.
Viro-immunologic features in éLTNP and naive patients. (A) éLTNP patients had fewer peripheral activated T lymphocytes (triple negative cells) in comparison with those of naive patients, either among CD4+ or CD8+ T lymphocytes. Pie charts (top) and bars (referred to median values) among dots (bottom) show the qualitative composition of activated CD4+ or CD8+ T cells in éLTNP patients (blue and red dots, respectively) and in naive patients (green and orange dots, respectively). Each pie graph represents the mean proportion of the different populations identified by anti-CD38 (b indicates bright, ie, cells with high amounts of CD38 on the cell surface; d, dim, cells with low amounts of CD38) and anti–HLA-DR mAbs as indicated in the legend under the histograms. Arcs designed outside the pies represent the fraction of total cells expressing a particular marker, irrespective of the positive or negative expression of other markers (blue, CD38 bright; red, CD38 dim; green, HLA-DR). (B) No relevant differences were observed in analysis of differentiation markers (ie, virgin or memory T cells whether or not expressing CD127). Pie charts (top) and bars (referred to median values) among dots (bottom) show the differentiation of CD4+ or CD8+ T cells in éLTNP patients (blue and red dots, respectively) and in naive patients (green and orange dots, respectively). Each pie graph represents the mean proportion of the different populations identified by anti-CCR7, anti-CD45RA, and anti-CD127 mAbs as indicated in the legend under the histogram. Arcs designed outside the pies represent the fraction of total cells expressing a particular marker, irrespective of the positive or negative expression of other markers (blue, CCR7; red, CD45RA; green, CD127). (C) éLTNP patients had fewer regulatory T cells expressing HLA-DR, higher numbers of sjTREC+ cells, and less intracellular HIV-1 DNA within CD4+ T lymphocytes than naive patients. Boxes limit the first and third quartile, the line in the box represents the median value; whiskers indicate minimum and maximum.
miRNAs discriminate infected from uninfected subjects but do not distinguish éLTNP from naive subjects
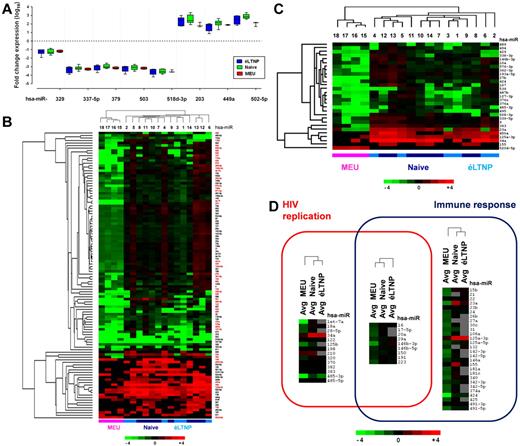
We investigated the expression levels of 377 miRNAs using real-time quantitative PCR-based arrays and 2−ΔΔCt method that relates the PCR signal of patient cell miRNA transcripts to that of normal cells collected from a pool of 6 healthy donors.10 To provide a criterion for comparison, all miRNAs were reviewed to ensure that there was systematic replication in more than 70% of the patients in at least 1 of the 3 patient classes. In the selection of 194 miRNAs, 114 varied by at least 1 log 10 in either direction compared with the healthy donors. Among 114 miRNAs, 3 (miR-203, miR-449a, and miR-502-5p) were up-regulated and 5 (miR-329, miR-337-5p, miR-379, miR-503, and miR-518d-3p) were down-regulated in all 3 patient classes, suggesting a retroviral exposure signature (Figure 2A). An additional 23 up-regulated miRNAs (2 and 10 selectively expressed in éLTNP and naive patients, respectively; 8 in both éLTNP and naive patients; and 3 in MEU patients) and 83 down-regulated miRNAs (5 in éLTNP patients, 3 in naive patients, 82 in the MEU patients with 4 miRNAs in common with éLTNP patients and 3 with naive patients) were found (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). None of the up-regulated miRNAs were detected in normal cells.
miRNA relative expression estimates and their involvement in immunity and/or viral replication. (A) Expression of miRNAs were determined by TaqMan MicroRNA qRT-PCR. Fold-change expression of miRNAs that replicated in the 70% of patients in all 3 classes were visualized as box-and-whiskers plots. Three miRNAs were up-regulated, and 5 miRNAs were down-regulated. (B) Only miRNAs (N = 114), which replicated in more than 70% of patients in at least one class and varied by at least 1 log 10 from healthy controls, were displayed in a heat map generated by Java TreeView 1.60 software. The heat map shows the fold-changes in miRNA expression in CD4+ T cells of HIV-1–infected (6 éLTNP and 8 naive) and exposed (4 MEU) patients compared with a pool of 6 healthy donors. Each colored block represents the expression of 1 miRNA (labeled on the right) in the indicated sample. PCR expression signals are converted into color (red, high signal; green, low signal). Color intensities are proportional to the variation of expression as indicated in the scale bar: values ranged from log10(−4) to log10(+4). Data represent 3 independent experiments. miRNAs and samples of patients were analyzed by average-linkage hierarchical clustering.25 The clustering, performed using average linkage and Pearson correlation, shows that éLTNP and naive subjects have similar expression profiles of miRNAs. The miRNAs, differently expressed in at least 2 groups, are marked in red. The miR-17/92 cluster was composed of miR-17-5p, miR-17-3p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a-1. (C) Heat map shows miRNAs for which statistical analyses (ANOVA followed by Bonferroni posthoc test) indicated significant differences in expression changes among the 3 classes. Only miRNAs with transcript levels replicated in more than 70% of the patients of each class were considered for statistical analysis. The hierarchical clustering analysis was performed on tested miRNAs and samples of patients. (D) Expression profile analysis of 377 miRNAs implicated in either viral replication or the immune response by literature analysis cluster together naive and éLTNP patients. The analysis of miRNAs, which share both functions, results in naive and MEU patient clusters. The identified miRNAs are divided according to their reported function through Venn analysis. The overlapping area shows miRNAs involved in both fields. Into the Venn diagram the average (avg) of miRNA relative expression of each subject class is displayed using a heat map representation. The hierarchical clustering dendrogram shows the similarity of miRNA patterns between groups of patients. This analysis performed was average linkage and Pearson correlation. miRNAs expression with more than 30% missing values are in gray.
miRNA relative expression estimates and their involvement in immunity and/or viral replication. (A) Expression of miRNAs were determined by TaqMan MicroRNA qRT-PCR. Fold-change expression of miRNAs that replicated in the 70% of patients in all 3 classes were visualized as box-and-whiskers plots. Three miRNAs were up-regulated, and 5 miRNAs were down-regulated. (B) Only miRNAs (N = 114), which replicated in more than 70% of patients in at least one class and varied by at least 1 log 10 from healthy controls, were displayed in a heat map generated by Java TreeView 1.60 software. The heat map shows the fold-changes in miRNA expression in CD4+ T cells of HIV-1–infected (6 éLTNP and 8 naive) and exposed (4 MEU) patients compared with a pool of 6 healthy donors. Each colored block represents the expression of 1 miRNA (labeled on the right) in the indicated sample. PCR expression signals are converted into color (red, high signal; green, low signal). Color intensities are proportional to the variation of expression as indicated in the scale bar: values ranged from log10(−4) to log10(+4). Data represent 3 independent experiments. miRNAs and samples of patients were analyzed by average-linkage hierarchical clustering.25 The clustering, performed using average linkage and Pearson correlation, shows that éLTNP and naive subjects have similar expression profiles of miRNAs. The miRNAs, differently expressed in at least 2 groups, are marked in red. The miR-17/92 cluster was composed of miR-17-5p, miR-17-3p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a-1. (C) Heat map shows miRNAs for which statistical analyses (ANOVA followed by Bonferroni posthoc test) indicated significant differences in expression changes among the 3 classes. Only miRNAs with transcript levels replicated in more than 70% of the patients of each class were considered for statistical analysis. The hierarchical clustering analysis was performed on tested miRNAs and samples of patients. (D) Expression profile analysis of 377 miRNAs implicated in either viral replication or the immune response by literature analysis cluster together naive and éLTNP patients. The analysis of miRNAs, which share both functions, results in naive and MEU patient clusters. The identified miRNAs are divided according to their reported function through Venn analysis. The overlapping area shows miRNAs involved in both fields. Into the Venn diagram the average (avg) of miRNA relative expression of each subject class is displayed using a heat map representation. The hierarchical clustering dendrogram shows the similarity of miRNA patterns between groups of patients. This analysis performed was average linkage and Pearson correlation. miRNAs expression with more than 30% missing values are in gray.
We evaluated significant differences in expression of miRNAs in either 2 or 3 patient classes using a linear mixed effects model of 2-way ANOVA followed by Bonferroni posthoc tests (Table 1). By this analysis, 51 differentially expressed miRNAs were revealed in the 2 patient class group and 29 differences occurred in the 3 patient classification. All changes were of absolute value greater than 1 log10. Analyzing the 29 miRNAs identified by the comparison of the 3 patient groups, éLTNP patients were differentiated from MEU patients by 21 miRNAs and naive patients were differentiated from MEU patients by 23 miRNAs. Sixteen miRNAs discriminated éLTNP and naive patients from MEU patients. The miR-155 only distinguished éLTNP from naive patients. By average linkage the hierarchical clustering of patients segregated infected and uninfected subjects perfectly based on global similarities in miRNA expression patterns (Figure 2B) as well as using significant miRNAs that were differentially expressed in the 3 patients' classes (Figure 2C). The finding that éLTNP patients clustered together with naive patients supports the hypothesis that miRNAs may function as HIV-1–related genetic factors. The data point to a complex change in regulation of CD4+ T cell miRNA, even in patients who are exposed to HIV-1 but fail to seroconvert.
Differentially expressed miRNAs in either 2 or 3 patient classes
| hsa-miRNA . | éLTNP vs MEU . | Naive vs MEU . | éLTNP vs naive . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference . | t . | Significance . | Difference . | t . | Significance . | Difference . | t . | Significance . | |
| 9 | −1.935 | 4.021 | ** | 1.863 | 4.081 | ** | −0.072 | 0.179 | ns |
| 10a | −1.714 | 3.562 | * | −1.208 | 2.646 | ns | −0.506 | 1.257 | ns |
| 23a | −1.853 | 3.850 | * | −3.070 | 6.726 | *** | 1.218 | 3.025 | ns |
| 27b | −3.730 | 7.753 | *** | −3.606 | 7.899 | *** | −0.125 | 0.309 | ns |
| 34a | −2.048 | 4.256 | ** | −2.295 | 5.029 | *** | 0.247 | 0.615 | ns |
| 107 | −2.722 | 5.658 | *** | −2.574 | 5.638 | *** | −0.149 | 0.370 | ns |
| 125a-3p | −1.754 | 3.646 | * | −1.744 | 3.820 | * | −0.010 | 0.025 | ns |
| 127-3p | −1.675 | 3.481 | ns | −1.692 | 3.708 | * | 0.018 | 0.044 | ns |
| 146b-3p | −2.443 | 5.078 | *** | −1.987 | 4.353 | ** | −0.456 | 1.133 | ns |
| 155 | 0.000 | 0.000 | ns | −1.502 | 3.291 | ns | 1.502 | 3.731 | * |
| 193a-5p | −1.528 | 3.177 | ns | −1.736 | 3.802 | * | 0.207 | 0.514 | ns |
| 338-3p | −1.640 | 3.409 | ns | −2.023 | 4.432 | ** | 0.383 | 0.952 | ns |
| 339-5p | −2.004 | 4.164 | ** | −1.824 | 3.995 | ** | −0.180 | 0.447 | ns |
| 362-3p | −2.311 | 4.804 | *** | −2.161 | 4.733 | *** | −0.151 | 0.375 | ns |
| 363 | −1.850 | 3.845 | * | −1.826 | 4.000 | ** | −0.025 | 0.061 | ns |
| 376a | −1.816 | 3.774 | * | −1.679 | 3.679 | * | −0.136 | 0.339 | ns |
| 376c | −1.546 | 3.214 | ns | −1.718 | 3.764 | * | 0.172 | 0.427 | ns |
| 424 | −2.253 | 4.683 | *** | −2.373 | 5.199 | *** | 0.120 | 0.299 | ns |
| 429 | −1.622 | 3.370 | ns | −2.335 | 5.115 | *** | 0.713 | 1.772 | ns |
| 450a | −2.132 | 4.430 | ** | −1.480 | 3.242 | ns | −0.652 | 1.619 | ns |
| 485−3p | −1.790 | 3.719 | * | −2.798 | 6.130 | *** | 1.008 | 2.505 | ns |
| 487b | −1.766 | 3.670 | * | −1.345 | 2.946 | ns | −0.421 | 1.046 | ns |
| 489 | 1.829 | 3.800 | * | 0.693 | 1.519 | ns | 1.135 | 2.820 | ns |
| 494 | −0.707 | 1.470 | ns | −1.688 | 3.698 | * | 0.981 | 2.436 | ns |
| 495 | −0.933 | 1.939 | ns | −1.686 | 3.694 | * | 0.753 | 1.871 | ns |
| 508-3p | −1.812 | 3.766 | * | −1.847 | 4.046 | ** | 0.035 | 0.087 | ns |
| 520d-5p | 2.333 | 4.848 | *** | 2.333 | 5.111 | *** | 0.000 | 0.000 | ns |
| 539 | −1.839 | 3.823 | * | −1.005 | 2.201 | ns | −0.835 | 2.073 | ns |
| 576-3p | −2.148 | 4.465 | ** | −2.263 | 4.958 | *** | 0.115 | 0.285 | ns |
| let-7a | nd | nd | nd | −1.460 | 3.738 | * | nd | nd | nd |
| let-7f | nd | nd | nd | −1.417 | 3.627 | * | nd | nd | nd |
| 21 | 1.307 | 3.197 | * | nd | nd | nd | nd | nd | nd |
| 99b | nd | nd | nd | −3.569 | 9.136 | *** | nd | nd | nd |
| 130a | nd | nd | nd | −1.380 | 3.532 | * | nd | nd | nd |
| 135b | −1.726 | 4.223 | *** | nd | nd | nd | nd | nd | nd |
| 143 | nd | nd | nd | −1.409 | 3.606 | * | nd | nd | nd |
| 148a | nd | nd | nd | −1.491 | 3.818 | ** | nd | nd | nd |
| 149 | −2.260 | 5.529 | *** | nd | nd | nd | nd | nd | nd |
| 152 | −2.464 | 6.027 | *** | nd | nd | nd | nd | nd | nd |
| 296-5p | nd | nd | nd | −1.525 | 3.904 | ** | nd | nd | nd |
| 301a | nd | nd | nd | −1.619 | 4.145 | ** | nd | nd | nd |
| 335 | nd | nd | nd | −4.265 | 10.920 | *** | nd | nd | nd |
| 361-5p | nd | nd | nd | −1.707 | 4.371 | *** | nd | nd | nd |
| 450b-5p | nd | nd | nd | −2.532 | 6.483 | *** | nd | nd | nd |
| 451 | nd | nd | nd | −2.670 | 6.835 | *** | nd | nd | nd |
| 502-3p | nd | nd | nd | −2.201 | 5.635 | *** | nd | nd | nd |
| 505 | nd | nd | nd | −1.859 | 4.759 | *** | nd | nd | nd |
| 579 | −1.288 | 3.152 | * | nd | nd | nd | nd | nd | nd |
| 618 | nd | nd | nd | −2.706 | 6.927 | *** | nd | nd | nd |
| 629 | nd | nd | nd | −4.082 | 10.450 | *** | nd | nd | nd |
| 671-3p | nd | nd | nd | −2.717 | 6.954 | *** | nd | nd | nd |
| hsa-miRNA . | éLTNP vs MEU . | Naive vs MEU . | éLTNP vs naive . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference . | t . | Significance . | Difference . | t . | Significance . | Difference . | t . | Significance . | |
| 9 | −1.935 | 4.021 | ** | 1.863 | 4.081 | ** | −0.072 | 0.179 | ns |
| 10a | −1.714 | 3.562 | * | −1.208 | 2.646 | ns | −0.506 | 1.257 | ns |
| 23a | −1.853 | 3.850 | * | −3.070 | 6.726 | *** | 1.218 | 3.025 | ns |
| 27b | −3.730 | 7.753 | *** | −3.606 | 7.899 | *** | −0.125 | 0.309 | ns |
| 34a | −2.048 | 4.256 | ** | −2.295 | 5.029 | *** | 0.247 | 0.615 | ns |
| 107 | −2.722 | 5.658 | *** | −2.574 | 5.638 | *** | −0.149 | 0.370 | ns |
| 125a-3p | −1.754 | 3.646 | * | −1.744 | 3.820 | * | −0.010 | 0.025 | ns |
| 127-3p | −1.675 | 3.481 | ns | −1.692 | 3.708 | * | 0.018 | 0.044 | ns |
| 146b-3p | −2.443 | 5.078 | *** | −1.987 | 4.353 | ** | −0.456 | 1.133 | ns |
| 155 | 0.000 | 0.000 | ns | −1.502 | 3.291 | ns | 1.502 | 3.731 | * |
| 193a-5p | −1.528 | 3.177 | ns | −1.736 | 3.802 | * | 0.207 | 0.514 | ns |
| 338-3p | −1.640 | 3.409 | ns | −2.023 | 4.432 | ** | 0.383 | 0.952 | ns |
| 339-5p | −2.004 | 4.164 | ** | −1.824 | 3.995 | ** | −0.180 | 0.447 | ns |
| 362-3p | −2.311 | 4.804 | *** | −2.161 | 4.733 | *** | −0.151 | 0.375 | ns |
| 363 | −1.850 | 3.845 | * | −1.826 | 4.000 | ** | −0.025 | 0.061 | ns |
| 376a | −1.816 | 3.774 | * | −1.679 | 3.679 | * | −0.136 | 0.339 | ns |
| 376c | −1.546 | 3.214 | ns | −1.718 | 3.764 | * | 0.172 | 0.427 | ns |
| 424 | −2.253 | 4.683 | *** | −2.373 | 5.199 | *** | 0.120 | 0.299 | ns |
| 429 | −1.622 | 3.370 | ns | −2.335 | 5.115 | *** | 0.713 | 1.772 | ns |
| 450a | −2.132 | 4.430 | ** | −1.480 | 3.242 | ns | −0.652 | 1.619 | ns |
| 485−3p | −1.790 | 3.719 | * | −2.798 | 6.130 | *** | 1.008 | 2.505 | ns |
| 487b | −1.766 | 3.670 | * | −1.345 | 2.946 | ns | −0.421 | 1.046 | ns |
| 489 | 1.829 | 3.800 | * | 0.693 | 1.519 | ns | 1.135 | 2.820 | ns |
| 494 | −0.707 | 1.470 | ns | −1.688 | 3.698 | * | 0.981 | 2.436 | ns |
| 495 | −0.933 | 1.939 | ns | −1.686 | 3.694 | * | 0.753 | 1.871 | ns |
| 508-3p | −1.812 | 3.766 | * | −1.847 | 4.046 | ** | 0.035 | 0.087 | ns |
| 520d-5p | 2.333 | 4.848 | *** | 2.333 | 5.111 | *** | 0.000 | 0.000 | ns |
| 539 | −1.839 | 3.823 | * | −1.005 | 2.201 | ns | −0.835 | 2.073 | ns |
| 576-3p | −2.148 | 4.465 | ** | −2.263 | 4.958 | *** | 0.115 | 0.285 | ns |
| let-7a | nd | nd | nd | −1.460 | 3.738 | * | nd | nd | nd |
| let-7f | nd | nd | nd | −1.417 | 3.627 | * | nd | nd | nd |
| 21 | 1.307 | 3.197 | * | nd | nd | nd | nd | nd | nd |
| 99b | nd | nd | nd | −3.569 | 9.136 | *** | nd | nd | nd |
| 130a | nd | nd | nd | −1.380 | 3.532 | * | nd | nd | nd |
| 135b | −1.726 | 4.223 | *** | nd | nd | nd | nd | nd | nd |
| 143 | nd | nd | nd | −1.409 | 3.606 | * | nd | nd | nd |
| 148a | nd | nd | nd | −1.491 | 3.818 | ** | nd | nd | nd |
| 149 | −2.260 | 5.529 | *** | nd | nd | nd | nd | nd | nd |
| 152 | −2.464 | 6.027 | *** | nd | nd | nd | nd | nd | nd |
| 296-5p | nd | nd | nd | −1.525 | 3.904 | ** | nd | nd | nd |
| 301a | nd | nd | nd | −1.619 | 4.145 | ** | nd | nd | nd |
| 335 | nd | nd | nd | −4.265 | 10.920 | *** | nd | nd | nd |
| 361-5p | nd | nd | nd | −1.707 | 4.371 | *** | nd | nd | nd |
| 450b-5p | nd | nd | nd | −2.532 | 6.483 | *** | nd | nd | nd |
| 451 | nd | nd | nd | −2.670 | 6.835 | *** | nd | nd | nd |
| 502-3p | nd | nd | nd | −2.201 | 5.635 | *** | nd | nd | nd |
| 505 | nd | nd | nd | −1.859 | 4.759 | *** | nd | nd | nd |
| 579 | −1.288 | 3.152 | * | nd | nd | nd | nd | nd | nd |
| 618 | nd | nd | nd | −2.706 | 6.927 | *** | nd | nd | nd |
| 629 | nd | nd | nd | −4.082 | 10.450 | *** | nd | nd | nd |
| 671-3p | nd | nd | nd | −2.717 | 6.954 | *** | nd | nd | nd |
ns indicates not significant; and nd, not determined.
The test was not applied when the miRNA expression change was not replicated in more than 70% of the patients in 1 of the 2 classes; gray box visualized differentially expressed miRNAs in the 2 patient class group (*P < .05, **P < .01, ***P < .001).
Among miRNAs known to be involved in HIV-1 replication, immune response, or both, only a few are differentially expressed in patient groups
The role of several miRNAs in the regulation of CD4+ T activity (supplemental Table 2), control of HIV-1 replication (supplemental Table 3), or both has been documented. All 377 tested miRNAs were screened for their involvement in the above-mentioned functions. Subsequent classification was in a 2-set Venn diagram, in which the relative expression of miRNAs for each class of subjects also was represented using hierarchical clustering (Figure 2D). This functional analysis revealed that among 51 miRNAs, whose expression showed significant differences between at least 2 groups, 3 were involved in viral replication (let-7a, miR-34a, miR-485-3p), 5 in immune response (miR-21, miR-23a, miR-125a-3p, miR-155, and miR-424), and 1 exhibited both functions (miR-146b-3p). Analysis of similarities in miRNA profile showed that éLTNP and naive patients clustered according to the expression of miRNAs linked to either viral replication or the immune response. In contrast, naive and MEU patients clustered according to miRNAs involved both in viral replication and immune response.
Furthermore, we investigated functional aspects of 114 miRNAs, variously expressed in all subjects, by analyzing their predicted and validated candidate target genes.10 Among the 114 miRNAs, 36 miRNAs characterized éLTNP/naive groups and 94 characterized the MEU group. Validated target genes of these miRNAs were identified by the Validated Targets component of miRECORDS,13 whereas the predicted target genes were identified by the intersection of the Predicted Targets component of miRECORDS and miRBase Targets 5 programs.14 Thus, among the 36 miRNAs distinctive of éLTNP/naive, 6 interact with 21 validated targets, and 30 with 534 predicted targets, whereas among the 94 miRNAs peculiar to MEU, 40 interact with 163 validated targets and 54 with 759 predicted targets. According to TAGGO results for predicted and validated genes, the most abundant GO terms for molecular function GO are the catalytic and binding activities, respectively, whereas for cellular component GO is the intracellular region. Comparing MEU with the éLTNP/naive groups, the TAGGO results showed that the percentage of the most abundant GO terms resulted similarly when the predicted genes were analyzed, and it increased in the éLTNP/naive groups when the validated genes were clustered (supplemental Figures 1 and 2, respectively; supplemental Table 4).
Exposure to HIV-1 antigen is sufficient to change miRNAs profile in CD4+ T cells
To evaluate whether a similar effect could be reproduced in healthy CD4+ T lymphocytes, we examined miRNA expressions after exposure of the cells to gp120 in vitro (Figure 3). Healthy CD4+ and HeLa-LAI–transformed cells were cocultured in a transwell for 6 hours. Notably, in HeLa-LAI supernatants natural gp120 molecules were secreted at the same concentration level detected in sera of AIDS patients after 36 hours of culture (12-85 ng/mL; data not shown).
Comparison of miRNA expression profile in CD4+ T cells exposed to natural or rgp120 in vitro in absence or presence of anti-gp120 mAbs. (A) Heat map shows the miRNAs whose expression was up-regulated (red) and down-regulated (green) by at least 1 log10 in treated CD4+ T cells in comparison with untreated cells. (B) Venn diagram of miRNAs (black) that reverted to the control level, healthy CD4+ T lymphocytes after gp120 neutralization by anti-gp120 mAbs and that are down-regulated (green) or up-regulated (red) in treated versus untreated cells.
Comparison of miRNA expression profile in CD4+ T cells exposed to natural or rgp120 in vitro in absence or presence of anti-gp120 mAbs. (A) Heat map shows the miRNAs whose expression was up-regulated (red) and down-regulated (green) by at least 1 log10 in treated CD4+ T cells in comparison with untreated cells. (B) Venn diagram of miRNAs (black) that reverted to the control level, healthy CD4+ T lymphocytes after gp120 neutralization by anti-gp120 mAbs and that are down-regulated (green) or up-regulated (red) in treated versus untreated cells.
Engagement of recombinant or natural gp120 molecules with its CD4 receptor resulted in the modulation of 49 and 51 miRNAs, respectively, of which 28 exhibited the same expression change and 11 were differentially expressed in the 3 patient classes. Significantly, when the gp120 action was neutralized with specific HIV-1 gp120 mAbs (such as IgG1 b12 that maps to the CD4 binding site and 2G12 that neutralizes laboratory HIV-1 strains IIIB and primary isolates), 1/3 of altered miRNAs were back to the control levels. These findings suggest that this glycoprotein activates CD4+ T lymphocytes not only by the CD4 binding site but also by other pathway signaling according to the protein folding. Considering that an approach for therapeutic vaccination is to use HIV-1 proteins as antigen, our findings may contribute to better understanding the lack of efficacy demonstrated by HIV vaccine trials.15
Expression level of the miRNA-processing enzymes seems to be influenced by the extent of antigen exposure
Dicer and Drosha, 2 RNApol III enzymes that are required for the maturation of miRNAs, have been demonstrated to inhibit HIV-1 replication both in PBMCs from HIV-1–infected donors and in latently infected cells.4
To estimate DICER and DROSHA mRNA expression in purified CD4+ T lymphocytes, we performed a real-time PCR assay. Comparing MEU group with éLTNP/naive groups, the results seem to explain the miRNAs changes observed, indicating that a low miRNAs expression is linked to a low enzyme expression (Figure 4A). However, no significant differences in DICER and DROSHA mRNA levels were observed in donor CD4+ T lymphocytes exposed to gp120 molecules compared with the control. In vitro data suggest that a single exposure to a HIV-1 antigen seems to change miRNAs profile by interfering with other protein than Dicer and Drosha (Figure 4B).
Analysis of expression of DICER and DROSHA transcripts by real-time PCR and ΔΔCt methods. (A) mRNA expression levels were determined in CD4+ T cells from HIV-1–infected or MEU patient groups and visualized as box-and-whiskers plots. Using Mann-Whitney statistical analysis, pairwise comparisons were performed. A 2-tailed P value of < .05 is statistically significant. (B) Histograms represent the transcript expression levels in CD4+ T cells exposed to HeLa-LAI cells or recombinant gp120 (rgp120) in presence or absence of anti-gp120 mAbs.
Analysis of expression of DICER and DROSHA transcripts by real-time PCR and ΔΔCt methods. (A) mRNA expression levels were determined in CD4+ T cells from HIV-1–infected or MEU patient groups and visualized as box-and-whiskers plots. Using Mann-Whitney statistical analysis, pairwise comparisons were performed. A 2-tailed P value of < .05 is statistically significant. (B) Histograms represent the transcript expression levels in CD4+ T cells exposed to HeLa-LAI cells or recombinant gp120 (rgp120) in presence or absence of anti-gp120 mAbs.
Discussion
In this study, we have shown that exists a miRNA signature that discriminate infected from exposed uninfected subjects. These data confirm the role of miRNAs as critical regulators of the complex interaction between HIV-1 and immune system. We further evaluated the functionality of altered miRNAs in each patient class by computational analysis of their predicted and validated target genes, and we found that these target genes are involved in many biologic functions. The alteration of miRNAs expression occurred in patient class also was explained by considering the role of miRNAs as inhibitors or promoters of HIV-1 replication as reported in the scientific literature.
Members of the cellular apolipoprotein B enzyme catalytic (APOBEC) family, including the APOBEC polypeptide–like 3G protein (APOBEC3G) are potent antiretroviral factors that counteract the translational inhibition of let-7a miRNA in human cells.16,17 The normal expression of let-7a miRNA in éLTNP and in naive patients but significantly decreased levels in MEU patients points to a possible high level of APOBEC activity in MEU subjects, in according to Vazquez-Perez et al.18
HIV-1 suppresses the expression of the miR-17/92 cluster in the Jurkat human tumor cell line.4 We observed a down-regulation of the miR-17/92 cluster in MEU patients. This suggests that changes in expression of the cluster can also occur after the contact with the virion or with its products and not necessarily in the presence of productive infection of normal CD4+ T cells. With ongoing infection, we found that 17/92 cluster expression levels return to normal values. Other miRNAs, such as miR-28-5p, miR-125b, miR-150, miR-223, and miR-382, seem to be enriched in resting rather than activated CD4+ T cells.5 The miRNA levels were normal in resting or activated CD4+ T cells of infected patients and down-regulated in resting CD4+T cells of MEU patients (Figure 1A). Specifically, MEU patients, known to have low number of activated T cells,19 showed a decreased expression of miR-28-5p, miR-125-b, and miR-223. Levels of miR-150 and miR-382 were unaltered. Our findings are at odds with recently published data,5 because in our study the expression profiles of such miRNAs were not associated with HIV-1 infection or replication.
An augmented expression of miR-34a and miR-210 has been found in Jurkat cells after HIV-1 infection.4 Similarly, the increased expression of these miRNAs in HIV-1–positive patients (either éLTNP or naive) but not in MEU patients suggests their role in the in vivo HIV-1 infection.
miR-23a, miR-125a-3p, and miR-155 expression was up-regulated in naive subjects, normal in éLTNP subjects, and down-regulated in MEU subjects. miR-23a expression is regulated by the transcription factor NFATC3,20,21 whereas miR-155 regulates central elements of the adaptive immune response and is highly expressed in regulatory T cells, as a direct target of Foxp3,22 as is miR-125a.23 The CD4 phenotype in the enrolled groups suggests that the up-regulation of miR-155 may contribute to the pathogenesis of HIV-1 infection. The decrease of miR-125a expression may reflect reduced immune function in MEU cells, with the ultimate result of limiting immune-mediate damage.
These findings highlight some intriguing issues relative to the cellular programming process in CD4+ T cells. First, the CD4+ T cells from infected patients expressed similar miRNA profiles, and these profiles were independent of parameters such as plasma viral load and intracellular HIV-1 DNA content, infection time, and CD4+ T cell number. Moreover, a few naive patients who were successfully treated with antiretroviral therapy for 3 months showed similar miRNA expression to that of naive and éLTNP patients (data not shown). Second, the CD4+ T cells from MEU subjects showed a complex down-regulation of miRNA after HIV-1 antigen exposure, pointing to the possibility that resetting of the cellular programming process may occur without a productive infectious event.
We also determined the expression of Dicer and Drosha, the main miRNAs processing enzymes, both in patient classes and in vitro experiments, and we found a correspondence between the altered expression of enzymes and miRNAs in ex vivo but not in vitro condition. This discrepancy on the role of enzymes points to a possible alternative mechanism on the miRNA biogenesis, and future studies will clarify this issue.
In conclusion, data reported in this study reveal how the constant exposure of CD4+ T cells to the virus can affect cellular processes at the level of miRNA. It is possible that the miRNA profiles observed in HIV-1–positive and –exposed uninfected subjects might be the result of a bystander phenomenon24 rather than a direct effect of HIV-1 infection on CD4+ T cells. Specifically, the miRNA profile observed in all HIV-1–infected subjects and in MEU subjects might be caused not only by a productive infection but also by the only exposure to viral products (eg, gp120 that binds CD4+ T cells) that leave stable marks. Considering that in MEU subjects the exposure to the virus was before their enrollment in this study, it can be argued that changes in miRNAs are a stable and nonreversible phenomenon. Furthermore, the evidence that HIV-1 antigen exposure (as observed both in ex vivo and in vitro condition) causes a significant change in miRNA expression profile, is particularly intriguing because of its possible implication for understanding the inefficacy of some HIV-1 vaccine based on viral proteins as antigens. In addition, the observation that there seemed to be a general down-regulation of miRNAs in MEU subjects, led us to support the hypothesis that inhibition of some miRNAs could be a new strategic approach in the fight against HIV-1 infection.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lucy Rasmussen (Stanford University) for critical reading of the manuscript.
This work has been founded by Istituto Superiore di Sanità, National Research Project on AIDS (grants 40H.15, 40D.14 and 40D.62, ELVIS Italian Network on LTNP), EC project LSHP-CT-2007-037616 (Genetic and Immunologic Studies of European and African HIV-1+ Long Term Non-Progressors, GISHEAL), and the Italian Cariparma Bank Foundaction (grant 2010.0382).
Authorship
Contribution: F.B. and E.P. were involved in experimental work and data analysis; L.B., P.R. M.G., and M.M. were involved in experimental work; R.R. was involved in the statistical data analysis; N.M. and A.C. were involved in project planning; G.M., M.P., L.L., C.M., and M.G. were involved in the enrollment and follow-up of patients; and C.C. designed and planned the project, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Casoli, GEMIB Biomedical Research Laboratory, Vicolo delle Asse 1, I-43121 Parma, Italy; e-mail: claudio.casoli@gemiblab.com.
References
Author notes
F.B. and E.P. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal