Abstract
The Nef protein of HIV-1 facilitates viral replication and disease progression in vivo. Nef disturbs the organization of immunological synapses between infected CD4+ T lymphocytes and antigen-presenting B-lymphocytes to interfere with TCR proximal signaling. Paradoxically, Nef enhances distal TCR signaling in infected CD4+ T lymphocytes, an effect thought to be involved in its role in AIDS pathogenesis. Using quantitative confocal microscopy and cell fractionation of Nef-expressing cells and HIV-1–infected primary human T lymphocytes, we found that Nef induces intracellular compartmentalization of TCR signaling to adjust TCR responses to antigenic stimulation. Nef reroutes kinase-active pools of the TCR signaling master switch Lck away from the plasma membrane (PM) to the trans-Golgi network (TGN), thereby preventing the recruitment of active Lck to the immunological synapse after TCR engagement and limiting signal initiation at the PM. Instead, Nef triggers Lck-dependent activation of TGN-associated Ras-Erk signaling to promote the production of the T lymphocyte survival factor IL-2 and to enhance virus spread. Overexpression of the Lck PM transporter Unc119 restores Nef-induced subversions of Lck trafficking and TCR signaling. Nef therefore hijacks Lck sorting to selectively activate TGN-associated arms of compartmentalized TCR signaling. By tailoring T-lymphocyte responses to antigenic stimulation, Nef optimizes the environment for HIV-1 replication.
Introduction
Replication of HIV-1 in primary human T lymphocytes is tightly coupled to their activation state. Whereas HIV-1 undergoes early replication events in quiescent CD4+ T lymphocytes, subsequent steps in the viral life cycle require cell activation.1 T lymphocyte activation is primarily governed by signaling through the TCR complex after engagement in a tight contact with APCs; this is referred to as the immunological synapse (IS).
TCR engagement by specific MHC-presented peptides launches highly dynamic and coordinated transport events that recruit specific factors to the IS and exclude others from it. This signal initiation triggers a broad cascade of downstream signaling that include dynamic F-actin remodeling at the IS, tyrosine phosphorylation, release of calcium flux, and activation of transcription. These events increase production of the T-cell survival cytokine IL-2 and are coordinated by the TCR proximal tyrosine kinase Lck, a master switch of TCR signaling. Immediately after TCR engagement, active Lck is recruited to the IS.2-4 Whereas signal diversification and enhancement occur at the plasma membrane (PM), subsequent TCR signaling is compartmentalized and also occurs at intracellular membranes. An important intracellular arm of the TCR response is regulated by the N-Ras GTPase that is activated at Golgi membranes downstream of Lck.5-9
T-cell activation is thought to be beneficial to HIV-1 because it allows transcriptional activation of latent provirus and progression of the life cycle. However, activation-induced cell death after TCR engagement runs the risk of limiting the lifespan of productively infected cells and thus the amount of viral progeny produced. Consequently, HIV-1 encodes gene products such as Nef to fine-tune the activation states of infected T lymphocytes.10,11
Nef is a 25- to 34-kDa myristoylated accessory protein encoded by HIV-1, HIV-2, and SIV. Ex vivo, Nef enhances the single-round infectivity of virus particles and moderately accelerates virus spread over multiple rounds.12 In vivo, Nef strongly boosts virus replication, particularly during primary infection, when the presence of Nef can elevate virus titers by more than 2 logs, and is critical for rapid disease progression.13-15 This role of Nef as a pathogenicity factor is also revealed in transgenic mice, in which Nef expression induces AIDS-like depletion of CD4+ T lymphocytes.16
Delineating the mechanisms of Nef action has been hampered by the multitude of interactions with host T-cell proteins suggested to modulate various intracellular transport and signaling pathways.17,18 This includes modulating exposure of cell-surface receptors such as MHC-I and II, CD4, and chemokine receptors to evade immune recognition and to prevent superinfection of infected cells, respectively (reviewed in Laguette et al12 ). In addition, Nef affects the basal states of T-cell activation and the responsiveness of T lymphocytes to TCR signaling.19-22
Initial studies with overexpression strategies in cell lines reached contradicting conclusions about the effects of Nef on TCR signaling. More recent work with viral infection and/or primary target T cells revealed that Nef moderately enhances distal responses to exogenous TCR stimulation by mitogens or anti-TCR Abs.11,23-29 In contrast to HIV-1 Nef, HIV-2 Nef and most SIV Nef proteins down-modulate TCR-CD3 cell-surface levels to induce a significantly more potent block to TCR signaling, and this is partially correlated with a lack of pathogenesis in the natural host. Therefore, facilitation of T-cell activation by Nef is thought to contribute to its role as a pathogenesis factor.11,30 In sharp contradiction to such elevated T-cell activation, Nef severely impairs the formation and organization of IS structures between Nef-expressing T lymphocytes and APCs by reducing the frequency of IS formation, blocking F-actin polarization at cell-cell contacts, and inducing mislocalization of the TCR itself and its effector kinase, Lck.23,30-34 These morphological alterations at the IS were accompanied by interference with early TCR signaling, such as induction of tyrosine phosphorylation.32-34
How HIV-1 Nef induces this paradox scenario of enhanced downstream TCR signaling on disruption of signal initiation is unclear. Effects of Nef on TCR-induced actin remodeling involve its association with the cellular kinase Pak2; however, this does not account for the Nef-mediated mislocalization of Lck.31,33,35 Nef also affects Lck localization independently of the changes induced to the intracellular transport of the Lck-binding partner CD4.34 Relocalization by Nef results in pronounced retargeting of Lck from the PM to a perinuclear membrane compartment positive for transferrin receptor (TfR).34 These effects are readily observed in virally infected primary T lymphocytes, and Nef is sufficient to induce rerouting of Lck.30,31,33,34 In the present study, we sought to determine whether, in addition to removing the kinase from the IS, rerouting of Lck by HIV-1 Nef is actively involved in shaping TCR signaling responses of infected T lymphocytes.
Methods
IS formation
IS formation between Staphylococcus aureus entertoxin E (SEE; Toxin Technology)–pulsed Raji B cells and Jurkat T cells or PBLs was induced as described previously.33 Briefly, Raji B cells were stained with CellTracker Blue CMAC (Molecular Probes) and incubated with SEE (10 ng/mL) in 0.5% FCS-RPMI GlutaMAX-I for 1 hour. For IS formation, SEE-pulsed Raji B cells were mixed at a 1:1 ratio with Jurkat T lymphocytes or PBLs in 0.5% FCS-RPMI GlutaMAX-I, centrifuged at low speed (≤ 50g) for 2 minutes (PBLs) or 5 minutes (Jurkat T lymphocytes) at room temperature and then incubated at 37°C for 10 minutes.
Virus production, infection, and replication
Generation of virus stocks by transfection of HEK293T cells with proviral plasmids, and isolation, activation, and HIV-1 infection of PBLs was carried out as described previously.32 On day 3 after infection, cells were used for IS formation.
For assaying virus replication, 2 × 106 freshly isolated PBMCs were infected directly with 300 ng of virus and cultured in the absence of IL-2. Forty-eight hours after infection, PBMCs were mixed with 7 × 105 Raji B cells pulsed or unpulsed with SEE. Supernatants were collected every 2 days and virus production was quantified by p24 ELISA.
For additional details on methods, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Image acquisition
For details regarding image acquistion, please see supplemental Methods.
Results
Nef causes accumulation of Lck at recycling endosomes and the TGN
We first characterized the effects Nef exerts on Lck localization. In the absence of Nef, the majority of endogenous Lck localized to the inner leaflet of the PM of Jurkat T lymphocytes, but subpopulations also resided in an intracellular membrane compartment distinct from the PM (henceforth referred to as intracellular) and diffusely distributed throughout the cytoplasm. As reported previously,31,33,34 the expression of Nef of the HIV-1 strain SF2 fused to green fluorescent protein (Nef.GFP, a functional analog of nonfusion Nef) caused a pronounced retargeting of Lck away from the PM into large intracellular structures (Figure 1A-B). Examining the magnitude (ie, the relative amount of total Lck per cell in intracellular accumulations, see supplemental Figure 1A) we found that Nef caused an almost 10-fold enrichment of Lck at this intracellular compartment at the single-cell level (8% ± 6.1% vs 76.6% ± 2.7% of total Lck per cell in intracellular accumulation in the absence or presence of Nef, respectively; Figure 1C). Frequency analysis (ie, the percentage of cells that displayed obvious intracellular accumulation of Lck) revealed that this retargeting occurred in more than 65% of Nef-expressing cells (Figure 1G). Similar results were obtained using ectopically expressed Lck (data not shown).
HIV-1 Nef targets Lck to the TGN and RE and prevents IS recruitment of the kinase. Scale bars indicate 10 μm. (A) Shown are representative confocal micrographs of Jurkat T lymphocytes transiently expressing GFP or Nef.GFP after staining for endogenous Lck. (B) 3D deconvolution of confocal micrographs scanning through Jurkat T lymphocytes expressing Lck.GFP in the presence of RFP (control) or Nef.RFP (+ Nef.RFP). (C) Quantification of Lck distribution in single cells. Depicted are the percentages of the total per-cell Lck signal detected in intracellular accumulation (see also supplemental Figure 1A). Each symbol designates a value for an individual cell. Bars indicate the mean values of all cells analyzed. (D) Representative confocal micrographs of Jurkat T lymphocytes expressing Lck.GFP in the absence (control) or presence of Nef.myc (+ Nef.myc). Lck is shown in green and subcellular markers in red. Subcellular markers were detected with staining by the respective Ab for endogenous (end.) proteins or by the fluorescent tag of coexpressed marker proteins. GalT.CFP is a Golgi marker; PDI, ER marker; EEA1,EE marker; Rab11.GFP and TfR, RE marker; TGN38.GFP, TGN marker; and LAMP1.GFP, lysosome marker. (E) Quantification of Lck colocalization with subcellular markers. Depicted are Manders coefficients of Lck overlapping with the indicated subcellular markers. Each symbol designates a value for an individual cell. Bars indicate the mean values of all cells analyzed. (F) Shown are representative merged micrographs of Jurkat T lymphocytes expressing GFP or the indicated Nef.GFP fusion proteins (green) in conjugates with SEE-pulsed Raji B cells (blue). Endogenous Lck is depicted in red. Note that expression of Nef prevents polarization of Lck to the IS and instead induces targeting of Lck to RE/TGN compartments. (G) Frequencies of RE/TGN accumulation versus IS recruitment of Lck on the expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD, in which more than 100 conjugates were analyzed for the predominant localization of Lck per condition. Micrographs for those Nef proteins not shown in panel F are depicted in supplemental Figure 3A. (H) Micrographs of primary human T lymphocytes infected with WT ΔNef HIV-1 IRES.GFP reporter viruses (infected cells are shown in green). (I) Frequencies of RE/TGN accumulation and IS recruitment of Lck in HIV-1–infected primary human T lymphocytes from 2 donors.
HIV-1 Nef targets Lck to the TGN and RE and prevents IS recruitment of the kinase. Scale bars indicate 10 μm. (A) Shown are representative confocal micrographs of Jurkat T lymphocytes transiently expressing GFP or Nef.GFP after staining for endogenous Lck. (B) 3D deconvolution of confocal micrographs scanning through Jurkat T lymphocytes expressing Lck.GFP in the presence of RFP (control) or Nef.RFP (+ Nef.RFP). (C) Quantification of Lck distribution in single cells. Depicted are the percentages of the total per-cell Lck signal detected in intracellular accumulation (see also supplemental Figure 1A). Each symbol designates a value for an individual cell. Bars indicate the mean values of all cells analyzed. (D) Representative confocal micrographs of Jurkat T lymphocytes expressing Lck.GFP in the absence (control) or presence of Nef.myc (+ Nef.myc). Lck is shown in green and subcellular markers in red. Subcellular markers were detected with staining by the respective Ab for endogenous (end.) proteins or by the fluorescent tag of coexpressed marker proteins. GalT.CFP is a Golgi marker; PDI, ER marker; EEA1,EE marker; Rab11.GFP and TfR, RE marker; TGN38.GFP, TGN marker; and LAMP1.GFP, lysosome marker. (E) Quantification of Lck colocalization with subcellular markers. Depicted are Manders coefficients of Lck overlapping with the indicated subcellular markers. Each symbol designates a value for an individual cell. Bars indicate the mean values of all cells analyzed. (F) Shown are representative merged micrographs of Jurkat T lymphocytes expressing GFP or the indicated Nef.GFP fusion proteins (green) in conjugates with SEE-pulsed Raji B cells (blue). Endogenous Lck is depicted in red. Note that expression of Nef prevents polarization of Lck to the IS and instead induces targeting of Lck to RE/TGN compartments. (G) Frequencies of RE/TGN accumulation versus IS recruitment of Lck on the expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD, in which more than 100 conjugates were analyzed for the predominant localization of Lck per condition. Micrographs for those Nef proteins not shown in panel F are depicted in supplemental Figure 3A. (H) Micrographs of primary human T lymphocytes infected with WT ΔNef HIV-1 IRES.GFP reporter viruses (infected cells are shown in green). (I) Frequencies of RE/TGN accumulation and IS recruitment of Lck in HIV-1–infected primary human T lymphocytes from 2 donors.
To identify the compartment that Lck associates with in the presence of Nef, we examined the colocalization of Lck with endogenous or ectopically expressed subcellular markers by confocal microscopy in Jurkat T lymphocytes (Figure 1D). Lck showed no association with markers of the endoplasmic reticulum (PDI), the Golgi apparatus (GalT.CFP), or lysosomes (LAMP1.GFP), whereas at intracellular membranes, Lck partially colocalized with the early endosome (EE) and recycling endosome (RE) markers EEA1, Rab11.GFP, and TfR, both with and without Nef.GFP. Nef expression did not affect the extent of colocalization with TfR (Figure 1E), but appeared to cause expansion of the TfR-positive compartment. Nef expression induced a strong colocalization of Lck with TGN38.GFP at the trans-Golgi network (TGN) that was not detected in the absence of Nef (Figure 1D-E). Immune-electron microscopy of CHO cells expressing Nef.GFP and Lck.RFP confirmed that Nef caused a pronounced depletion of Lck from the PM and an enrichment of the kinase in intracellular vesicles that resembled EE-, RE-, or TGN-associated vesicles (supplemental Figure 2). Therefore, Nef induces retargeting of Lck away from the PM to REs and, most prominently, to the TGN.
RE/TGN retargeting by Nef prevents recruitment of Lck to the IS
Consistent with its rapid recruitment from intracellular pools of T lymphocytes to newly initiated IS contacts with APCs,36 IS formation resulted in a marked recruitment of Lck to cell-cell contacts after incubation of GFP-expressing Jurkat T lymphocytes with SEE-pulsed Raji B cells (Figure 1F-G). The presence of Nef markedly reduced IS recruitment of Lck, because RE/TGN targeting of Lck was maintained even in cells with close cell-cell contacts. Similar Nef-specific effects were observed after infection of primary human T lymphocytes with HIV-1 IRES.GFP reporter viruses (Figure 1H-I). The molecular determinants of Nef that govern the prevention of Lck recruitment to the IS matched those for RE/TGN targeting of the kinase and, expectedly,31 most prominently involved the SH3-domain binding motif of Nef (mutant AxxA in Figure 1G and supplemental Figure 3A). Two Nef alleles (from isolates HIV-1 8161K9 and HIV-2 Ben) that display reduced ability for RE/TGN retargeting of Lck33 were also partially inactive in inhibiting IS recruitment of Lck. Overall, the ability of Nef to target Lck to RE/TGN was strongly correlated with the prevention of IS recruitment (P < .0001, R2 = 0.9138, supplemental Figure 3B). These findings suggest that Nef-induced retargeting of Lck determines the reduction of its IS recruitment after TCR engagement.
Active Lck is potently targeted by Nef
Lck activity is subject to complex posttranslational regulation, primarily by phosphorylation. Whereas Lck is activated by autophosphorylation of Y394, phosphorylation at Y505 by Csk causes kinase inactivation.37 However, Lck exists in several conformations with distinct activity states and accessibility to immunohistological detection by phosphospecific Abs.4 Because p-Y505 is not detectable by immunohistology on Lck molecules in the inactive conformation, staining with the p-Y505–specific Ab detects only the dually phosphorylated active Lck species (designated p-Lck505&394).4 Staining with Abs specific for phospho-Y394 detects both monophosphorylated p-Lck394 and dually phosphorylated p-Lck505&394 active Lck species. Quantitative Western blotting (in which protein denaturation overrides conformation-dependent differences in protein detection) revealed that the overall ratio of each phosphorylated species versus total Lck (endogenous or Lck.GFP) was not significantly altered by coexpression of Nef.RFP in unstimulated T lymphocytes (Figure 2A-B). Microscopy-based quantification of per-cell levels of total Lck, p-Lck505&394, and p-Lck394 confirmed that Nef did not markedly affect the overall activity of Lck in our experimental system (Figure 2C). Instead, Nef.GFP caused a pronounced subcellular redistribution of active Lck (p-Lck394 and p-Lck505&394) that resembled its effect on total Lck (Figure 2D): whereas present almost exclusively at the PM in the absence of Nef, both active Lck species were enriched at RE/TGN compartments in a manner that depended on the SH3-domain binding motif of Nef. The retargeting frequency of active Lck was in a similar range as that observed for total Lck (Figure 2E), and single-cell quantification of Lck distribution revealed that more than 50% of the total active Lck population per cell resided in the RE/TGN compartments in Nef-expressing cells (Figure 2F).
Nef affects intracellular distribution of catalytically active Lck. (A) Western blot analysis of Jurkat T lymphocytes expressing Lck.GFP with RFP or Nef.RFP. All samples were generated within the same experiment and run on the identical gel but are shown as individual boxes to illustrate that lanes in between those shown were removed. Total and active Lck was detected by Abs that recognize all Lck species or are specific for the indicated Lck phospho-species, respectively. (B) LI-COR quantification of the Western blots shown in panel A. Depicted are the ratios of phosphorylated versus total Lck signal intensity. Note that the presence of Nef does not affect abundance of both phosphorylated Lck forms investigated. Values are the arithmetic means of at least 3 independent experiments ± SD (C) Pixel quantification of total or active Lck populations in the presence of the indicated GFP fusion proteins. Jurkat T lymphocytes expressing the indicated proteins were subjected to z-stacks of confocal microscopy, and the total amounts of Lck per cell were determined with ImageJ Version 1.42q software. Values for individual cells are presented with the arithmetic mean indicated by the black line, and are relative to ratios obtained for neighboring untransfected cells that were arbitrarily set to 1. (D) Representative confocal micrographs of Jurkat T lymphocytes expressing the indicated GFP proteins. Shown is the staining for the indicated phosphorylated Lck species. Asterisks indicate GFP-positive cells. Scale bar indicates 10 μm. (E) Numbers of cells that display RE/TGN accumulation of total and active Lck populations. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (F) Magnitude of Lck RE/TGN targeting per cell. Depicted are the percentages of Lck signal per cell in RE/TGN compartments. Values for individual cells are presented, with the arithmetic mean indicated by the black line.
Nef affects intracellular distribution of catalytically active Lck. (A) Western blot analysis of Jurkat T lymphocytes expressing Lck.GFP with RFP or Nef.RFP. All samples were generated within the same experiment and run on the identical gel but are shown as individual boxes to illustrate that lanes in between those shown were removed. Total and active Lck was detected by Abs that recognize all Lck species or are specific for the indicated Lck phospho-species, respectively. (B) LI-COR quantification of the Western blots shown in panel A. Depicted are the ratios of phosphorylated versus total Lck signal intensity. Note that the presence of Nef does not affect abundance of both phosphorylated Lck forms investigated. Values are the arithmetic means of at least 3 independent experiments ± SD (C) Pixel quantification of total or active Lck populations in the presence of the indicated GFP fusion proteins. Jurkat T lymphocytes expressing the indicated proteins were subjected to z-stacks of confocal microscopy, and the total amounts of Lck per cell were determined with ImageJ Version 1.42q software. Values for individual cells are presented with the arithmetic mean indicated by the black line, and are relative to ratios obtained for neighboring untransfected cells that were arbitrarily set to 1. (D) Representative confocal micrographs of Jurkat T lymphocytes expressing the indicated GFP proteins. Shown is the staining for the indicated phosphorylated Lck species. Asterisks indicate GFP-positive cells. Scale bar indicates 10 μm. (E) Numbers of cells that display RE/TGN accumulation of total and active Lck populations. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (F) Magnitude of Lck RE/TGN targeting per cell. Depicted are the percentages of Lck signal per cell in RE/TGN compartments. Values for individual cells are presented, with the arithmetic mean indicated by the black line.
In agreement with the results obtained for total Lck, active Lck efficiently localized at T-cell/B-cell contacts in the presence of GFP or the Nef mutant AxxA.GFP, whereas coexpression of Nef.GFP resulted in RE/TGN targeting of the active kinase without recruitment to IS contacts (Figure 3A; frequency is shown in Figure 3B). Pixel quantification revealed that almost 50% of active Lck per cell was recruited to IS contacts in the absence of Nef, whereas Nef expression efficiently prevented IS recruitment of the active kinase (Figure 3C and supplemental Figures 1B and 4A-C). These results were confirmed in primary human T lymphocytes infected with HIV-1 IRES.GFP reporter viruses (supplemental Figure 4D-E for p-Lck394 and F-G for p-Lck505&394). T lymphocytes express Fyn as second major Src family kinase, with important roles in early TCR signaling,38 and active p-Fyn528 was efficiently recruited to the T-cell/B-cell IS in GFP- or AxxA.GFP-expressing control cells (Figure 3D-F). In contrast to its effects on Lck, Nef only slightly affected IS recruitment frequency and magnitude of Fyn and did not cause any appreciable retargeting to RE/TGN compartments. Effects of Nef on subcellular localization and IS recruitment of Src kinases are therefore selective for individual family members, and Nef-mediated rerouting of catalytically active Lck away from the PM generates a RE/TGN-associated pool of active, signaling competent Lck.
Nef specifically prevents IS recruitment of active Lck but not of Fyn. (A) Representative micrographs of active Lck (p-Lck505&394) in the presence of the indicated GFP fusion proteins in T lymphocytes in conjugates with SEE-pulsed Raji B cells (in blue). Scale bar indicates 10 μm. (B) Frequencies of cells with pronounced RE/TGN accumulation (gray bars) or IS recruitment (black bars) of p-Lck505&394 on expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 conjugates were analyzed per condition. (C) Magnitude of RE/TGN accumulation (gray symbols) or IS recruitment (black symbols) of p-Lck505&394 per cell. Values for individual cells are presented, with the arithmetic mean indicated by the black line, and depict the percentage of total Lck signal per cell detected in RE/TGN compartments or at the IS, respectively (see also supplemental Figure 1B). (D) Representative micrographs of p-Fyn528 in the presence of the indicated GFP fusion proteins in T lymphocytes in conjugates with SEE-pulsed Raji B cells (in blue). Scale bar indicates 10 μm. (E) Frequencies of cells with pronounced RE/TGN targeting (gray bars) or IS recruitment (black bars) of p-Fyn528 on expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 conjugates were analyzed per condition. (F) Magnitude of RE/TGN targeting (gray symbols) or IS recruitment (black symbols) of p-Fyn528 per cell. Values for individual cells are presented, with the arithmetic mean indicated by the black line, and depict the percentage of total p-Fyn528 signal per cell detected in RE/TGN compartments or at the IS, respectively.
Nef specifically prevents IS recruitment of active Lck but not of Fyn. (A) Representative micrographs of active Lck (p-Lck505&394) in the presence of the indicated GFP fusion proteins in T lymphocytes in conjugates with SEE-pulsed Raji B cells (in blue). Scale bar indicates 10 μm. (B) Frequencies of cells with pronounced RE/TGN accumulation (gray bars) or IS recruitment (black bars) of p-Lck505&394 on expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 conjugates were analyzed per condition. (C) Magnitude of RE/TGN accumulation (gray symbols) or IS recruitment (black symbols) of p-Lck505&394 per cell. Values for individual cells are presented, with the arithmetic mean indicated by the black line, and depict the percentage of total Lck signal per cell detected in RE/TGN compartments or at the IS, respectively (see also supplemental Figure 1B). (D) Representative micrographs of p-Fyn528 in the presence of the indicated GFP fusion proteins in T lymphocytes in conjugates with SEE-pulsed Raji B cells (in blue). Scale bar indicates 10 μm. (E) Frequencies of cells with pronounced RE/TGN targeting (gray bars) or IS recruitment (black bars) of p-Fyn528 on expression of the indicated GFP fusion proteins. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 conjugates were analyzed per condition. (F) Magnitude of RE/TGN targeting (gray symbols) or IS recruitment (black symbols) of p-Fyn528 per cell. Values for individual cells are presented, with the arithmetic mean indicated by the black line, and depict the percentage of total p-Fyn528 signal per cell detected in RE/TGN compartments or at the IS, respectively.
Nef induces formation of TGN-associated Ras signaling downstream of Lck
We next investigated whether Nef induces active intracellular signaling in the absence of exogenous stimulation. Whereas TCR proximal signaling typically occurs predominately at the PM, activation of N-Ras and mediation of downstream signaling in response to TCR stimulation can occur at intracellular membranes, including the Golgi.6-9 Because activation of N-Ras requires upstream activity of Lck,39 we investigated whether retargeting of active Lck to RE/TGN compartments in the presence of Nef is sufficient to cause enrichment of active N-Ras at this subcellular site. In unstimulated Jurkat T lymphocytes, total CFP.N-Ras predominately localized to a small and well-defined intracellular compartment reminiscent of the distribution of the Golgi apparatus (Figure 4A RFP). In contrast, the presence of Nef.RFP, but not its AxxA mutant, caused a subtle but significant dispersion of N-Ras into a more diffuse cytoplasmic distribution (Figure 4A Nef.RFP). This retargeted N-Ras pool partially colocalized with TGN markers (data not shown). Nef-mediated retargeting of N-Ras occurred in more than 70% of cells analyzed and was not impaired after pharmacologic inhibition of Lck activity with the Lck inhibitor 4-amino-5-(4-phenoxyphenyl)-7H-pyrrolo[3,2-d]pyrimidin-7-yl-cyclopentane (Figure 4B). Effects of Nef on the localization of N-Ras became more apparent when the GTPase-binding domain of cRaf-1 fused to GFP (RBD.GFP) was used as a biosensor for active, GTP-loaded Ras7 (Figure 4C). Whereas RBD.GFP was distributed homogenously in the cytoplasm in the absence of Nef, expression of wild-type (WT), but not AxxA, Nef caused a marked accumulation of RBD.GFP to RE/TGN compartments, which is indicative of N-Ras activation in more than 70% of the cells analyzed (Figure 4C-D). The effect of Nef on N-Ras activation was abrogated in the presence of Lck inhibitor and thus depended on Lck activity (Figure 4D).
Nef TGN-association of active N-Ras downstream of Lck. (A) Representative confocal micrographs of Jurkat T lymphocytes expressing CFP.N-Ras (blue) together with the indicated RFP fusion proteins (red). Arrows indicate discrete or less compact localization of CFP.N-Ras in the presence of RFP/AxxA.RFP (1 arrow) or Nef.RFP (2 arrows), respectively. Scale bar indicates 10 μm. (B) Frequencies of cells that display dispersion of N-Ras into the expanded cytoplasmic pattern shown in the presence of Nef in panel A. Where indicated, Lck inhibitor (1μM) was added 3 hours before the analysis. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Representative confocal micrographs of Jurkat T lymphocytes expressing HA.N-Ras (not shown) with the indicated RFP fusion proteins (red) and the Ras-binding domain of cRaf-1 fused to GFP as biosensor of Ras activity (RBD.GFP, green). Note that expression of Nef leads to a marked enrichment of active Ras at the TGN (indicated by the arrow). Scale bar indicates 10 μm. (D) Frequencies of cells that display accumulation of RBD.GFP at the TGN as shown in the presence of Nef in panel C. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. Where indicated, Lck inhibitor (1μM) was added 3 hours before the analysis. (E) Membrane flotation analysis of Jurkat T lymphocytes expressing Lck.GFP with RFP or Nef.RFP. Lck.GFP and endogenous Lck were detected by an anti–Lck Ab. M indicates the membrane fraction; S, soluble fraction. TfR and MLC were used as markers for the membrane and soluble fractions, respectively. All samples were generated within the same experiment and run on the identical gel, but are separated by a vertical line to illustrate that lanes in between those shown were removed. (F) Subcellular fractionation. Postnuclear supernatants of homogenates of Jurkat T lymphocytes that were lentivirally transduced for expression of GFP or Nef.GFP (transduction efficiency above 98%) were subjected to sucrose density gradient centrifugation. Ten fractions were collected from the top of the gradient and analyzed by Western blotting for distribution of endogenous Lck, p-Lck394, p-Lck505, and p-Fyn409. For detection of N-Ras, Jurkat T lymphocytes were transfected with expression plasmids for HA.N-Ras and GFP or Nef.GFP plasmids. TGN46, TfR, and PLSCR1 serve as markers for gradient fractions containing TGN, RE, and PM, respectively.
Nef TGN-association of active N-Ras downstream of Lck. (A) Representative confocal micrographs of Jurkat T lymphocytes expressing CFP.N-Ras (blue) together with the indicated RFP fusion proteins (red). Arrows indicate discrete or less compact localization of CFP.N-Ras in the presence of RFP/AxxA.RFP (1 arrow) or Nef.RFP (2 arrows), respectively. Scale bar indicates 10 μm. (B) Frequencies of cells that display dispersion of N-Ras into the expanded cytoplasmic pattern shown in the presence of Nef in panel A. Where indicated, Lck inhibitor (1μM) was added 3 hours before the analysis. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Representative confocal micrographs of Jurkat T lymphocytes expressing HA.N-Ras (not shown) with the indicated RFP fusion proteins (red) and the Ras-binding domain of cRaf-1 fused to GFP as biosensor of Ras activity (RBD.GFP, green). Note that expression of Nef leads to a marked enrichment of active Ras at the TGN (indicated by the arrow). Scale bar indicates 10 μm. (D) Frequencies of cells that display accumulation of RBD.GFP at the TGN as shown in the presence of Nef in panel C. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. Where indicated, Lck inhibitor (1μM) was added 3 hours before the analysis. (E) Membrane flotation analysis of Jurkat T lymphocytes expressing Lck.GFP with RFP or Nef.RFP. Lck.GFP and endogenous Lck were detected by an anti–Lck Ab. M indicates the membrane fraction; S, soluble fraction. TfR and MLC were used as markers for the membrane and soluble fractions, respectively. All samples were generated within the same experiment and run on the identical gel, but are separated by a vertical line to illustrate that lanes in between those shown were removed. (F) Subcellular fractionation. Postnuclear supernatants of homogenates of Jurkat T lymphocytes that were lentivirally transduced for expression of GFP or Nef.GFP (transduction efficiency above 98%) were subjected to sucrose density gradient centrifugation. Ten fractions were collected from the top of the gradient and analyzed by Western blotting for distribution of endogenous Lck, p-Lck394, p-Lck505, and p-Fyn409. For detection of N-Ras, Jurkat T lymphocytes were transfected with expression plasmids for HA.N-Ras and GFP or Nef.GFP plasmids. TGN46, TfR, and PLSCR1 serve as markers for gradient fractions containing TGN, RE, and PM, respectively.
We next sought to validate this Nef-mediated retargeting of TCR signaling machinery by biochemical means. Flotation of total cellular membranes revealed that Nef did not affect the overall association of Lck with cellular membranes (Figure 4E compare distribution of Lck.GFP between membrane and soluble fractions). To differentiate between individual cellular membrane systems, we carried out fractionation experiments by discontinuous sucrose density gradient centrifugation.40 In these fractionations, the PM marker phospholipid scramblase-1 (PLSCR1) was detected in fractions 1 and 2 and clearly segregated from TGN46 and TfR, which spread over fractions 3-9 (Figure 4F). In GFP-expressing control cells, the majority of endogenous total Lck and p-Lck505 were associated with PM fractions, whereas active p-Lck394 was found to be evenly distributed between the PM and RE/TGN fractions. Consistent with our previous morphometric analysis, expression of Nef.GFP caused a partial shift of Lck and p-Lck505 to RE/TGN fractions and p-Lck394 was exclusively detected in these PM-free fractions. p-Fyn associated exclusively with PM fractions both in absence and presence of Nef. Consistent with our microscopy analysis, HA.N-Ras was efficiently shifted to RE/TGN fractions in Nef-expressing cells. These results demonstrate that Nef-mediated retargeting of active Lck is correlated with N-Ras activation at RE/TGN compartments.
Nef-induced retargeting of Lck sensitizes T lymphocytes for activation of Erk1/2
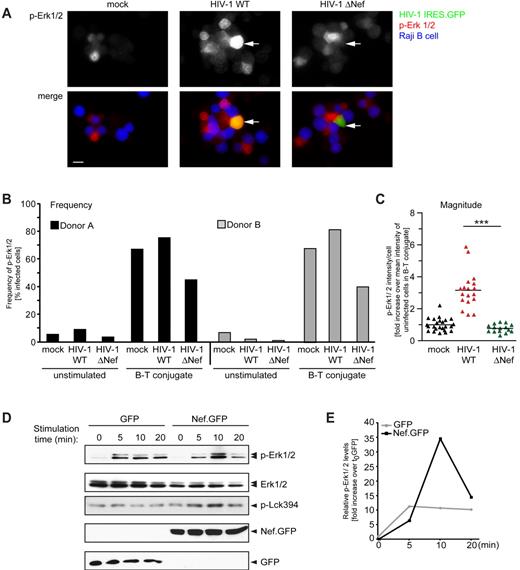
We next investigated whether the activity of Erk1/2 kinase, the best-characterized downstream effector of N-Ras in TCR signaling,5-9 was activated by this Nef-induced intracellular signaling. Levels of active Erk1/2 (p-Erk1/2) were analyzed by microscopy in primary human T lymphocytes after infection with HIV-1 IRESGFP reporter viruses in conjugation with SEE-pulsed Raji B cells (Figure 5A-C). Regardless of their infection status, unstimulated cells not engaged in an IS displayed low levels of p-Erk1/2, indicating that Nef-mediated intracellular accumulation of active Lck and N-Ras is not sufficient to trigger activation of Erk1/2. In contrast, IS engagement of T cells resulted in efficient activation of Erk1/2 in mock or HIV-1 WT–infected cells. Elevated p-Erk1/2 levels were much less apparent in T cells infected with HIV-1 ΔNef (Figure 5A right panel). Quantification of cells from 2 independent donors revealed that elevated p-Erk1/2 levels were up to 2-fold more frequent in HIV-1 WT- than in ΔNef-infected cells (Figure 5B). Quantification of p-Erk1/2 signal intensities in individual cells revealed that Erk activation was approximately 3-fold higher in cells infected with WT relative to ΔNef HIV-1 (Figure 5C). We also assessed the impact of Nef on activation of Erk1/2 by Western blotting. Because the use of T-cell/B-cell conjugates for these bulk measurements does not distinguish between p-Erk1/2 levels in the T and B cells, soluble anti–CD3 Ab stimulation of primary human T lymphocytes after retroviral transduction for expression of GFP or Nef.GFP was used. Nef-mediated RE/TGN targeting of Lck was preserved under these conditions (data not shown). In GFP-expressing control cells, p-Erk1/2 was rapidly induced from low basal levels and remained elevated over the 20-minute observation period (Figure 5D and E for quantification of p-Erk1/2 vs total Erk levels). Consistent with earlier results,11,23-29 expression of Nef did not significantly alter basal p-Erk1/2 levels, but resulted in a more potent but transient increase of p-Erk1/2 at 10 minutes after stimulation. This Nef-mediated increase in p-Erk1/2 induction varied between cells from different donors and was not observed when cells displayed elevated basal p-Erk1/2 levels before stimulation (data not shown). Consistent with a recent study,4 Lck activity was constantly present during these activation kinetics (p-Lck394). This activity was maintained and even slightly increased in the presence of Nef. Therefore, Nef induces RE/TGN recruitment of active N-Ras to increase the frequency and magnitude of p-Erk1/2 induction after antigenic or Ab-mediated stimulation in HIV-1–infected primary T lymphocytes.
Nef expression increases p-Erk1/2 induction in infected and transduced primary T cells. (A) Representative micrographs of uninfected (mock) primary human T lymphocytes or after infection with the indicated HIV-1 IRES.GFP reporter viruses (infected cells in green) in conjugates with SEE-pulsed Raji B cells (in blue) after staining for p-Erk1/2 (in red). Arrows indicate infected cells in B-cell/T-cell conjugates. Note the higher level of p-Erk1/2 in the HIV-1 WT–infected cell in conjugate with a B cell compared with the ΔNef–infected cell and uninfected cells. Scale bar indicates 10 μm. (B) As in panel A, the frequencies of primary human T lymphocytes from 2 different donors with elevated p-Erk1/2 levels were analyzed. (C) Total per-cell levels of p-Erk1/2 in primary human T lymphocytes infected with WT or ΔNef HIV-1. Values for individual cells are presented, with the arithmetic mean indicated by the black line relative to uninfected control cells in conjugates. Asterisks indicate statistical significance by Student t test analysis. ***P ≤ .001; **P < .005; *P < .05. (D) Primary CD4+ T lymphocytes were lentivirally transduced for expression of GFP or Nef.GFP, stimulated with anti–CD3 Ab for the indicated times 48 hours after transduction, and cell lysates were analyzed by Western blotting for the detection of total (Erk1/2), active (p-Erk1/2) Erk, or active Lck (p-Lck394). Expression of GFP and Nef.GFP was analyzed with an anti-GFP Ab. (E) Quantification of p-Erk1/2 levels in the Western blot shown in panel D. The intensities of the bands were analyzed using Quantity One Version 4.6.5 software and are plotted as the ratio of p-Erk1/2 to total Erk1/2 levels. The value for the GFP control at t0 was arbitrarily set to 1, and all other values are plotted relative to this.
Nef expression increases p-Erk1/2 induction in infected and transduced primary T cells. (A) Representative micrographs of uninfected (mock) primary human T lymphocytes or after infection with the indicated HIV-1 IRES.GFP reporter viruses (infected cells in green) in conjugates with SEE-pulsed Raji B cells (in blue) after staining for p-Erk1/2 (in red). Arrows indicate infected cells in B-cell/T-cell conjugates. Note the higher level of p-Erk1/2 in the HIV-1 WT–infected cell in conjugate with a B cell compared with the ΔNef–infected cell and uninfected cells. Scale bar indicates 10 μm. (B) As in panel A, the frequencies of primary human T lymphocytes from 2 different donors with elevated p-Erk1/2 levels were analyzed. (C) Total per-cell levels of p-Erk1/2 in primary human T lymphocytes infected with WT or ΔNef HIV-1. Values for individual cells are presented, with the arithmetic mean indicated by the black line relative to uninfected control cells in conjugates. Asterisks indicate statistical significance by Student t test analysis. ***P ≤ .001; **P < .005; *P < .05. (D) Primary CD4+ T lymphocytes were lentivirally transduced for expression of GFP or Nef.GFP, stimulated with anti–CD3 Ab for the indicated times 48 hours after transduction, and cell lysates were analyzed by Western blotting for the detection of total (Erk1/2), active (p-Erk1/2) Erk, or active Lck (p-Lck394). Expression of GFP and Nef.GFP was analyzed with an anti-GFP Ab. (E) Quantification of p-Erk1/2 levels in the Western blot shown in panel D. The intensities of the bands were analyzed using Quantity One Version 4.6.5 software and are plotted as the ratio of p-Erk1/2 to total Erk1/2 levels. The value for the GFP control at t0 was arbitrarily set to 1, and all other values are plotted relative to this.
Unc119 is limiting for Lck PM transport in the presence of Nef
We next sought to define T-cell components involved in Nef-mediated interference with PM transport of Lck. Transport routes of Lck to the PM are incompletely characterized; however, the proteins SH3/SH2 ligand-uncoordinated 119 (Unc119)41,42 and MAL43,44 were implicated in this process and Unc119 is essential for Lck-dependent TCR signaling.42 Because depletion of each of these factors causes Lck retargeting from the PM to intracellular membrane compartments in a manner similar to Nef,41,42 we attempted to overcome Nef-mediated Lck retargeting by ectopic expression of MAL or Unc119. Expression of these proteins alone did not affect cell viability, localization, or IS recruitment of total and active Lck or IS formation in general (supplemental Figure 5A-C and data not shown). Coexpression of Mal.GFP did not affect RE/TGN targeting and IS exclusion induced by Nef.myc (Figure 6A-B left panels and supplemental Figure 5B-C). In contrast, coexpression of Unc119.HA with Nef.RFP efficiently prevented RE/TGN targeting of Lck.GFP and resulted in a diffuse cytoplasmic localization of the kinase (Figure 6A right panels; 26.0% ± 7.6% of total Lck in RE/TGN compartments based on single-cell quantification). Coexpression of Unc119 affected neither Lck expression levels nor unrelated Nef activities such as down-regulation of cell-surface CD4 (data not shown). Coexpression of Unc119 reduced the frequency of cells with pronounced RE/TGN targeting to levels of Nef-negative control cells, even though it did not fully restore the natural subcellular localization of Lck (Figure 6B). The expression of Unc119 almost completely reversed the Nef-mediated inhibition of IS recruitment of p-Lck505&394 (Figure 6C-D and supplemental Figure 5B-C for p-Lck394). Similar results were obtained in HIV-1–infected primary human T lymphocytes (data not shown). We conclude that overexpression of Unc119 prevents RE/TGN targeting by Nef, suggesting that Unc119 may be limiting for PM transport of Lck in the presence of Nef.
Expression of Unc119 partially restores Lck trafficking in the presence of Nef. Effects of coexpression of MAL and Unc119 on Nef-mediated RE/TGN targeting of Lck. (A) Representative confocal micrographs of Jurkat T lymphocytes cotransfected with expression plasmids for the indicated proteins. Myc and HA refers to empty control plasmids that encode only for the respective epitope tag. Shown is the distribution of Lck; red asterisks indicate cotransfected cells. Scale bar indicates 10 μm. Note that the expression of Unc119.HA releases Lck from the Nef-induced RE/TGN accumulation into a diffuse cytoplasmic distribution. (B) Frequencies of cells with RE/TGN accumulation of Lck. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Effect of Unc119 coexpression on IS recruitment of Lck in the presence of Nef. Shown are representative micrographs of Jurkat T lymphocytes expressing GFP or Nef.GFP (green) in conjugates with SEE-pulsed Raji B cells (blue). Active p-Lck505&394 is depicted in red. Note that RE/TGN targeting and inhibition of IS recruitment of p-Lck505&394 by Nef is reversed by coexpression of Unc119.HA (dark blue). Scale bar indicates 10 μm. (D) Frequencies of RE/TGN accumulation versus IS recruitment of p-Lck505&394. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition.
Expression of Unc119 partially restores Lck trafficking in the presence of Nef. Effects of coexpression of MAL and Unc119 on Nef-mediated RE/TGN targeting of Lck. (A) Representative confocal micrographs of Jurkat T lymphocytes cotransfected with expression plasmids for the indicated proteins. Myc and HA refers to empty control plasmids that encode only for the respective epitope tag. Shown is the distribution of Lck; red asterisks indicate cotransfected cells. Scale bar indicates 10 μm. Note that the expression of Unc119.HA releases Lck from the Nef-induced RE/TGN accumulation into a diffuse cytoplasmic distribution. (B) Frequencies of cells with RE/TGN accumulation of Lck. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Effect of Unc119 coexpression on IS recruitment of Lck in the presence of Nef. Shown are representative micrographs of Jurkat T lymphocytes expressing GFP or Nef.GFP (green) in conjugates with SEE-pulsed Raji B cells (blue). Active p-Lck505&394 is depicted in red. Note that RE/TGN targeting and inhibition of IS recruitment of p-Lck505&394 by Nef is reversed by coexpression of Unc119.HA (dark blue). Scale bar indicates 10 μm. (D) Frequencies of RE/TGN accumulation versus IS recruitment of p-Lck505&394. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition.
Nef-Induced retargeting of Lck facilitates distal TCR signaling despite IS disorganization and facilitates HIV-1 replication
We next used ectopic expression of Unc119 as a tool to define the functional relevance of Nef-induced RE/TGN targeting of Lck in the context of TCR signaling in Jurkat T lymphocytes. As expected,31,32 total phosphotyrosine (p-Tyr) as a marker of early TCR signaling was potently reduced at the IS in the presence of Nef. Instead, Nef induced intracellular accumulation of p-Tyr species in a manner dependent on its SH3-domain–binding motif (Figure 7A-B). This block in p-Tyr localization to the IS by Nef was rescued by coexpression of Unc119, indicating that restoration of Lck IS recruitment overcomes the block imposed on early TCR signaling by HIV-1 Nef.
Retargeting of Lck is critically involved in Nef-induced alterations of TCR signaling and HIV-1 replication. (A) Microscopic analysis of p-Tyr (red) distribution in Jurkat T lymphocytes expressing the indicated GFP or Nef.GFP proteins in conjugates with SEE-pulsed Raji B cells (blue) Scale bar indicates 10 μm. (B) Frequencies of cells that displayed intracellular accumulation or IS recruitment of p-Tyr. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Enhanced IL-2 production by HIV-1 Nef is reversed by Unc119. Jurkat T lymphocytes were transfected with an expression plasmid for RFP or Unc119.RFP, followed by infection with WT or ΔNef HIV-1 IRES.GFP or the mock control. Cells were stimulated with SEE-pulsed Raji B cells and intracellular IL-2 was measured in infected cells positive for RFP/Unc119.RFP. CytoD refers to uninfected cells treated with the actin-disrupting drug cytochalasin D before stimulation (0.625μM for 1 hour). Depicted is the mean fold increase of IL-2 levels relative to HIV-1 ΔNef–infected cells ± SD from 3 independent experiments. (D) Representative micrographs of uninfected (mock) primary human T lymphocytes or after infection with the indicated HIV-1 IRES.GFP reporter viruses in conjugates with SEE-pulsed Raji B cells after staining for p-Erk1/2. Arrows indicate infected cells in B-cell/T-cell conjugates. Note the low level of p Erk1/2 in the HIV-1 WT IRES.Unc119.GFP-infected cell in conjugate with a B cell compared with the WT-infected cell. Scale bar indicates 10 μm. (E) Frequencies of primary human T lymphocytes with elevated p-Erk1/2 levels. (F) Total per-cell levels of p-Erk1/2 in primary human T lymphocytes infected with the indicated viruses. Values for individual cells are presented, with the arithmetic mean indicated by the black line relative to uninfected control cells in conjugates. (G) The positive effect of Nef on HIV-1 replication is antagonized by Unc119 coexpression. Replication kinetics of the indicated HIV-1 viruses in PBMC/Raji B-cell cocultures are shown. Results shown are arithmetic means ± SD from triplicate infections of p24 concentrations in the cell culture supernatants harvested at the indicated time points. (H) Depicted are the mean p24 concentrations at 8 days after infection from the experiment shown in panel G, with the SDs indicated. Statistical significance was analyzed by the Student t test. ***P ≤ .001, **P < .005, and *P < .05.
Retargeting of Lck is critically involved in Nef-induced alterations of TCR signaling and HIV-1 replication. (A) Microscopic analysis of p-Tyr (red) distribution in Jurkat T lymphocytes expressing the indicated GFP or Nef.GFP proteins in conjugates with SEE-pulsed Raji B cells (blue) Scale bar indicates 10 μm. (B) Frequencies of cells that displayed intracellular accumulation or IS recruitment of p-Tyr. Values are the arithmetic means of at least 3 independent experiments ± SD in which more than 100 cells were counted per condition. (C) Enhanced IL-2 production by HIV-1 Nef is reversed by Unc119. Jurkat T lymphocytes were transfected with an expression plasmid for RFP or Unc119.RFP, followed by infection with WT or ΔNef HIV-1 IRES.GFP or the mock control. Cells were stimulated with SEE-pulsed Raji B cells and intracellular IL-2 was measured in infected cells positive for RFP/Unc119.RFP. CytoD refers to uninfected cells treated with the actin-disrupting drug cytochalasin D before stimulation (0.625μM for 1 hour). Depicted is the mean fold increase of IL-2 levels relative to HIV-1 ΔNef–infected cells ± SD from 3 independent experiments. (D) Representative micrographs of uninfected (mock) primary human T lymphocytes or after infection with the indicated HIV-1 IRES.GFP reporter viruses in conjugates with SEE-pulsed Raji B cells after staining for p-Erk1/2. Arrows indicate infected cells in B-cell/T-cell conjugates. Note the low level of p Erk1/2 in the HIV-1 WT IRES.Unc119.GFP-infected cell in conjugate with a B cell compared with the WT-infected cell. Scale bar indicates 10 μm. (E) Frequencies of primary human T lymphocytes with elevated p-Erk1/2 levels. (F) Total per-cell levels of p-Erk1/2 in primary human T lymphocytes infected with the indicated viruses. Values for individual cells are presented, with the arithmetic mean indicated by the black line relative to uninfected control cells in conjugates. (G) The positive effect of Nef on HIV-1 replication is antagonized by Unc119 coexpression. Replication kinetics of the indicated HIV-1 viruses in PBMC/Raji B-cell cocultures are shown. Results shown are arithmetic means ± SD from triplicate infections of p24 concentrations in the cell culture supernatants harvested at the indicated time points. (H) Depicted are the mean p24 concentrations at 8 days after infection from the experiment shown in panel G, with the SDs indicated. Statistical significance was analyzed by the Student t test. ***P ≤ .001, **P < .005, and *P < .05.
Most previous studies suggesting that HIV-1 Nef is a promoter of distal TCR signaling relied on mitogenic or Ab-mediated stimulation protocols. We therefore compared the response of Jurkat T lymphocytes with stimulation with phytohemagglutinin- or SEE-pulsed Raji B cells and determined the cell-surface levels of the T-cell activation markers CD25 and CD69 and intracellular levels of IL-2 (the latter of which is highly dependent on Ras/Erk activation) as markers for T-cell activation (supplemental Figure 6A). Whereas CD69 cell-surface levels were induced to similar extents by both stimuli, surface expression of CD25 and IL-2 production were more potent with antigenic stimulation. Productive HIV-1 infection of control T lymphocytes expressing RFP did not significantly affect antigenic induction of CD69 and caused a slight but Nef-independent increase in surface expression of CD25 (supplemental Figure 6B-C). We therefore focused on the production of IL-2 as assessed by intracellular flow cytometry (Figure 7C). Because dynamic actin remodeling and IS recruitment of Lck is essential for proximal and distal TCR signaling,2 inhibition of these processes by HIV-1 Nef should strongly impair such T lymphocyte responses. Illustrating the requirement for F-actin integrity, IL-2 induction after TCR stimulation was more than 3-fold reduced after treatment with the actin-disrupting drug cytochalasin D (Figure 7C compare ΔNef and cytochalasin D). Despite the ability of Nef to disrupt early TCR-induced signaling and actin-remodeling events, infection with WT HIV-1 caused a moderate 1.6-fold increase rather than the expected significant decrease in IL-2 production. Therefore, in agreement with earlier studies,28 Nef triggered a slight but significant increase in IL-2 production after antigenic stimulation. This Nef-dependent increase in IL-2 production was abrogated by expression of Unc119.RFP (Figure 7C and supplemental Figure 6D).
To assess whether Nef-mediated Lck-Ras-Erk signaling affects HIV-1 replication in primary human T lymphocytes, we generated a HIV-1 IRES-GFP provirus that coexpresses Nef and Unc119.GFP (supplemental Figure 7A). Expression of Nef and Unc119.GFP was comparable to that of Nef and GFP from HIV-1 IRES-GFP WT, and the corresponding virions displayed similar relative infectivity (supplemental Figure 7B-C). We first investigated whether coexpression of Unc119 affects Erk activation downstream of Lck and Ras, and determined the frequency and magnitude of p-Erk1/2 induction in infected primary human T lymphocytes after antigenic stimulation (Figure 7D-F and supplemental Figure 7D). Coexpression of Unc119 fully prevented the Nef-mediated enhancement of pErk1/2 induction, but stimulated Erk activation in the absence of Nef. These results support the idea that Nef-mediated retargeting of Lck facilitates TCR-stimulated activation of Erk; they also show that overexpression of the transport factor can activate this pathway in the absence of Nef and that Nef appears to subvert this activity of Unc119, possibly reflecting an enhancement of Lck activity by Unc119.42 Finally, we investigated the effect of Nef-induced signaling on HIV-1 replication. Because the effects of Nef on HIV-1 replication in T lymphocytes are most pronounced when stimulated after infection, unstimulated PBMCs were infected and maintained in the absence of IL-2. Two days after infection, cells were incubated with SEE-pulsed or SEE-unpulsed B cells, respectively, and virus replication was determined by the release of p24 into the cell culture supernatant (supplemental Figure 7G). Reflecting the requirement of T-cell activation for propagation of HIV-1 in primary human T lymphocytes, no virus production was observed when B cells that were not SEE loaded were added to infected cultures (“unpulsed B cells”). Stimulation with SEE-pulsed Raji B cells triggered potent replication of HIV-1 IRES-GFP WT, whereas replication of HIV-1 IRES-GFP ΔNef was delayed (Figure 7G for replication kinetics and Figure 7H for peak p24 production at day 8 after infection). Coexpression of Unc119 slowed down HIV-1 replication and, dependent on the donor, almost fully (Figure 7G) or partially (supplemental Figure 7F-G) abrogated the Nef-mediated enhancement of virus spread. Consistent with the results on Erk activation, coexpression of Unc119 increased virus replication in the context of HIV-1ΔNef. These results reveal a correlation between Nef-mediated facilitation of Erk activation and HIV-1 replication in primary human T lymphocytes. We conclude that RE/TGN targeting of active Lck allows HIV-1 Nef to induce compartmentalization of TCR signaling and to shape TCR signal outputs. These effects contribute to the Nef-mediated increase of HIV-1 replication in primary human T lymphocytes.
Discussion
The HIV-1 pathogenicity factor Nef disrupts IS recruitment of the TCR proximal kinase and signaling master switch Lck, thus interfering with TCR proximal events, yet does thereby not diminish but even moderately enhance TCR downstream signaling. In the present study, we found that Nef subverts host-cell vesicular transport to induce intracellular compartmentalization of TCR signaling to adjust TCR responses to antigenic stimulation. By potently retargeting PM-associated Lck pools, Nef induces a marked accumulation of kinase-active Lck in the RE and TGN compartments. This mechanism prevents IS recruitment of active Lck after TCR engagement, thus limiting the availability of Lck for TCR signaling at the PM. Instead, RE/TGN-targeted active Lck triggers intracellular signaling by recruiting active N-Ras to sensitize Erk signaling in response to TCR engagement. Coexpression of the Lck transport adaptor Unc119 prevents Nef-mediated RE/TGN targeting of Lck, restores IS recruitment of active Lck, and reverses the Nef-mediated enhancement of Erk activity and IL-2 production and virus replication in primary human T lymphocytes after antigenic stimulation. Induction of RE/TGN-associated Lck-Ras-Erk signaling allows Nef to ensure TCR distal signaling that is beneficial for HIV-1 even though IS organization, and thus TCR proximal signaling events, are impaired in the presence of the viral protein.
For modulation of cell-surface receptor levels, Nef is thought to physically interact with the cytoplasmic tail of the receptor, resulting in altered internalization and/or recycling rates.12 With Hck in macrophages and Lck in T lymphocytes, Nef also affects the intracellular localization of peripheral membrane proteins.34,45 Extending the previous observation that Lck localizes to TfR-positive compartments,34 our microscopy and biochemical analyses identified these intracellular compartments as the RE and the TGN, but not the Golgi apparatus. Lck is targeted to the PM before undergoing internalization from and recycling back to this predominant subcellular localization.46 At which step Nef acts to affect the steady-state localization of the kinase is currently unclear, but the rescue of Lck PM transport on coexpression of Unc119 suggests that Nef might interfere with Lck trafficking by targeting the activity of Unc119. Physiologically, Unc119 serves as transport adaptor that interacts directly with Lck and, in complex with the GTPase Rab11, translocates the kinase from endosomal compartments to the PM.41,42 Rab11 has already been implicated in Nef-mediated manipulations of host-cell trafficking,47 but how the activities of Unc119 and MAL are integrated to facilitate PM transport of Lck is unclear. Colocalization analysis revealed that in Nef-expressing cells, MAL is enriched at subcellular sites of intracellular Lck accumulation (data not shown). Therefore, by virtue of interference with the adaptor function of Unc119, Nef may disrupt the transport of Lck- and MAL-containing vesicles to the PM. Because the subcellular localization of the closely related Src kinase Fyn was not affected by Nef, this mechanism appears to have specificity for Lck, indicating active and specific cargo selection in this pathway. Future studies will focus on the underlying molecular mechanisms and will use Nef as an important tool to decipher the hierarchy and cooperation between components of this PM transport route.
Addressing the functional consequences of Lck retargeting in the context of TCR signaling, we found that RE/TGN-targeted Lck is not accessible for IS recruitment and thus causes the severe reduction of Lck at sites of TCR engagement. Because these effects expand to kinase-active Lck populations and Lck is central for proximal TCR signaling, this mechanism should translate into profound defects on TCR output signals. However, Nef failed to suppress induction of T-cell activation markers after antigenic stimulation and even enhanced the IL-2 response of infected T lymphocytes. Because it leads to enrichment of active components of TCR signaling at the TGN, the Lck-retargeting mechanism resolves the paradox of Nef-mediated disruption of proximal and maintenance of distal TCR signaling in infected cells. Our results show that Nef, via the enrichment of active Lck at RE/TGN compartments, induces high local concentrations of active Ras that sensitize T lymphocytes for Erk signaling. Therefore, Nef exploits the regulation of this pathway by the subcellular localization of Lck activity to induce compartmentalization of TCR signaling4,36 and to uncouple downstream TCR signaling from the TCR itself, creating a shortcut for signal output.
Whereas active forms of Lck and N-Ras were efficiently assembled at the TGN by Nef in the absence of TCR activation, induction of Erk and production of IL-2 were only seen after TCR engagement. Because the Src kinase Fyn provides N-Ras with a costimulus required for activation of N-Ras and its downstream signaling,38 and because its subcellular localization and IS recruitment were resistant to Nef, this responsiveness to TCR stimulation likely reflects the activity of Fyn. In contrast to the coordinated action of Lck and Fyn in the absence of Nef, the exclusive involvement of Fyn in PM-associated TCR signaling in Nef-positive cells likely accounts for the reduced breadth of downstream TCR signaling in the context of HIV-1 infection.
This highly coordinated and selective manipulation of TCR signal transduction provides a molecular explanation for the longstanding observation that Nef selectively sensitizes T lymphocytes for Erk signaling and IL-2 production after TCR stimulation.25,27,28,30 Erk signaling is also targeted by Nef via an independent mechanism involving the NAKC complex that elevates HIV-1 transcription and exosome release.48 Conceivably, the induction of TGN-associated Lck-Ras-Erk signaling represents a prerequisite for these subsequent functional Nef-Erk interactions. Erk signaling thus emerges as a major target of Nef-mediated host-cell manipulation. The results presented herein identify enhanced virus replication after antigenic stimulation as one functional consequence of these Nef-induced alterations in T lymphocyte signaling. This mechanism might be relevant during the primary phase of lentiviral infection, during which antigen stimulation drives virus replication in the gut-associated lymphoid tissue and thus contributes to the Nef-mediated elevation of virus titers at that stage. In this scenario, the expression of small amounts of biologically active Nef before integration of proviral DNA into the host genome may be sufficient to exert this activity.28 Similarly, reactivation of virus production from latently infected cells may be facilitated by Nef-mediated RE/TGN targeting of Lck. The results of the present study suggest that Lck retargeting is a cardinal function of Nef in pathogenic HIV-1 infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Oliver Keppler, Hans-Georg Kräusslich, Bettina Stolp, and Andrea Imle for discussion and critical comments on the manuscript; Philipp Klein for help with lentiviral vector production; the FACS sorting facility of SFB638 and the Nikon Imaging Center at the University of Heidelberg for access and service; Nadine Tibroni for expert technical help; and Barbara Müller, Mark R. Philips, Rafick-Pierre Sekaly, Frank Kirchhoff, Nicole Schaeren-Wiemers, Michael Schindler, and Marietta L. Harrison for the kind gift of reagents.
This study was supported by the Deutsche Forschungsgemeinschaft (grants TRR83 and SFB 638 to O.T.F.).
Authorship
Contribution: X.P. and J.M.R. designed and performed the research and analyzed and interpreted the data; L.A., A.H, C.H., and J.K.-L. performed the research and analyzed the data; and O.T.F. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oliver T. Fackler, Department of Infectious Diseases, Virology, University Hospital Heidelberg, Im Neuenheimer Feld 324, 69120 Heidelberg, Germany; e-mail: oliver.fackler@med.uni-heidelberg.de.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal