Abstract
Although genetic and environmental factors explain approximately half of the interindividual variability in warfarin dose requirement in adults, there is limited information available in children. In a cross-sectional study of anticoagulated children from 5 tertiary care centers, 120 children with a stable warfarin dose were genotyped for VKORC1 (−1639G > A; rs9923231), CYP2C9 (*2 and *3 alleles; rs1799853 and rs1057910), and CYP4F2 (V433M; rs2108622) polymorphisms. Clinical and demographic features were recorded. Multiple regression analysis of the data showed that, although CYP4F2 made no contribution to the dose model, 72.4% of the variability in warfarin dose requirement is attributed to by patient height, genetic polymorphisms in VKORC1 and CYP2C9, and indication for warfarin. The recently published International Warfarin Pharmacogenetics Consortium pharmacogenetic-based warfarin dosing algorithm (based on data derived from anticoagulated adults) consistently overestimated warfarin dose for our cohort of children. A similar proportion of the interindividual variability in warfarin dose is explained by genetic factors in children compared with adult patients, although height is a greater predictor in children. A pharmacogenomic approach to warfarin dosing has the potential to improve the efficacy and safety of warfarin therapy in children. However, algorithms should be derived from data in children if their potential benefit is to be realized.
Introduction
Anticoagulant therapy is being increasingly used in children for the treatment and prevention of thromboembolic events, and warfarin remains the most frequently used agent for long-term anticoagulation in this patient group. Anticoagulation response to a fixed dose of warfarin is notoriously difficult to predict because of interindividual variability in dose requirement. This, together with the drug's narrow therapeutic window, necessitates maintenance of anticoagulation status within a tight therapeutic range facilitated by frequent international normalized ratio (INR) monitoring to ensure the efficacy and safety of therapy.
Oral anticoagulant therapy in children is complicated by numerous factors, including variable age-related dose-response rates, complex underlying health problems, multiple intercurrent illnesses, polypharmacy, and diet, and anticoagulation control is poor in this patient population.1-3 Studies in adults have shown that warfarin dose requirement is influenced by demographic and genetic factors. The latter include single nucleotide polymorphisms (SNPs) in the vitamin K epoxide reductase (VKORC1)4,5 and cytochrome P450 CYP2C9 genes6,7 and, to a lesser extent, other genes involved in vitamin K metabolism and the vitamin K cycle, such as the cytochrome P450 CYP4F2 gene.8,9 A pharmacogenomic approach to warfarin dosing has the potential to improve the efficacy and safety of warfarin therapy. This has resulted in the construct of pharmacogenetic-guided dosing algorithms for adult patients.10,11 Despite substantial interest from physicians who anticoagulate children with warfarin,12 a pharmacogenomic approach to warfarin therapy has not yet been developed and evaluated in a large pediatric population. We evaluated the effects of genetic, clinical, and demographic factors on maintenance warfarin dose in a cross-sectional design, multicenter study in children with stable anticoagulation.
Methods
Patient recruitment
Children were recruited from 4 United Kingdom sites (Birmingham Children's Hospital, Royal Manchester Children's Hospital, The Newcastle upon Tyne Hospitals NHS Trust, and Royal Hospital for Sick Children, Glasgow) and The Hospital for Sick Children, Toronto, ON. The study was approved by the Regional Ethics Committee, the Medicines and Healthcare products Regulatory Agency, and the institutional review boards at each of the study sites. The study recruited children, 18 years of age or younger, who were anticoagulated for at least 3 months after warfarin initiation and whose target INR range was either 2.0 to 3.0 or 2.5 to 3.5. The stability criterion for inclusion was that no change in warfarin dose had been made for at least the previous 3 consecutive INR measurements over a minimum period of 4 weeks. An additional cohort of children and young adults who had received warfarin when 18 years of age or younger, and for whom historical data regarding warfarin dose requirement and INR were available, were recruited from one of the participating United Kingdom centers.
Data collection and blood sampling
Written informed consent to take part in the study was obtained from patients 16 years of age or older and from parents/caregivers of children younger than 16 years, in accordance with the Declaration of Helsinki. Details of diet, indication for anticoagulation with warfarin, target INR range, current warfarin dose, and other medication(s) were obtained by questionnaire and review of patients' medical records. Height, weight, and gender were recorded. Ethnicity was reported by the patient or their parent/caregiver, according to categories defined by the investigators. For the cohort of children and young adults who had previously been treated with warfarin during childhood, the aforementioned details were collected by review of patients' medical records and warfarin-dosing records at a time point when the child had been stable on warfarin according to the aforementioned criterion. A venous blood sample (4-8 mL) was collected from each patient and stored in EDTA tubes at −80°C for later genotyping.
Genotyping
Genomic DNA was extracted from whole blood samples according to an established method.13 Genotyping for VKORC1 (−1639G > A; rs9923231), CYP2C9 (*2 and *3 alleles; rs1799853 and rs1057910), and CYP4F2 (V433M; rs2108622) was performed using the StepOne Real-Time PCR System with TaqMan SNP Genotyping Assays (Applied Biosystems). The VKORC1 and CYP2C9 genotype results were validated using control samples that had been genotyped as part of a previous study.14 CYP4F2 genotypes were validated by regenotyping 26 samples in duplicate by PCR-restriction fragment length polymorphism analysis as described by Cen et al.15
Statistical analysis
Sample size.
Because of the absence of data on the impact of genetic polymorphisms on warfarin dose in the pediatric population, a power calculation was based on comparable adult data. To detect a difference of approximately 1 mg in mean warfarin daily dose between the CYP2C9 polymorphisms, with significance at the .05 level and a power of 80%, it was estimated that a sample size of 120 patients would be required. As the frequency of each of the VKORC1 genotypes is greater than that of the CYP2C9 mutant alleles and the effect of VKORC1 on warfarin dose requirement in the adult population is larger than that of CYP2C9, a sample size of 120 was deemed adequate for detecting the effect of VKORC1 genotype and other significant variables.
Data analysis.
Statistical analyses were performed using MiniTab Version 15.0 (Coventry). Mean warfarin daily dose was transformed by taking the square root of each value to obtain a normal distribution, allowing parametric tests to be performed. Associations between warfarin dose and height, weight, body surface area, body mass index, and age were evaluated using the Pearson correlation test. The effect of genotype, indication for warfarin, target INR range, and ethnicity were evaluated using unpaired t test or ANOVA. Stepwise regression analysis was used to identify factors contributing to the transformed warfarin dose followed by linear regression to model the relationships of dose with other variables measured. Results are presented as mean ± SD unless stated otherwise. A P value of < .05 was taken as statistically significant.
Results
Patient characteristics
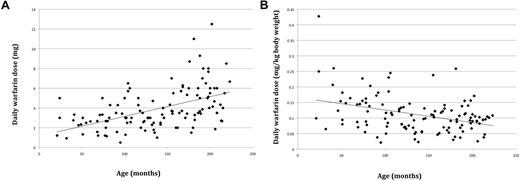
Recruitment occurred between April 2009 and December 2010. A total of 120 children with a median age of 11 years (range, 1-18 years) and a median duration of warfarin therapy of 49 months (range, 3-199 months) were recruited, including 8 subjects who were recruited retrospectively using historical data. The patients' demographics, indication for anticoagulation, and target INR range are shown in Table 1. Median warfarin daily dose was 3.4 mg (range, 0.5-12.5 mg). Figure 1 shows the relationship between warfarin daily dose and age.
Patient characteristics
| . | No. (%) of children . |
|---|---|
| Sex | |
| Male | 82 (68.3) |
| Female | 38 (31.7) |
| Age group | |
| 0-3 y | 7 (5.8) |
| 4-6 y | 20 (16.7) |
| 7-9 y | 20 (16.7) |
| 10-12 y | 21 (17.5) |
| 13-15 y | 29 (24.2) |
| 16-18 y | 23 (19.2) |
| Ethnic origin | |
| White | 91 (75.8) |
| Indian/Pakistani | 10 (8.3) |
| Chinese | 4 (3.3) |
| Black Caribbean | 3 (2.5) |
| Black African | 3 (2.5) |
| South-East Asian/Filipino | 2 (1.7) |
| Other* | 7 (5.8) |
| Indication for anticoagulation with warfarin | |
| Fontan procedure | 64 (53.3) |
| Prosthetic heart valve | 18 (15.0) |
| Coronary aneurysm | 11 (9.2) |
| Dilated cardiomyopathy | 6 (5.0) |
| Deep vein thrombosis/pulmonary embolism | 6 (5.0) |
| Pulmonary hypertension | 5 (4.2) |
| Stroke | 2 (1.7) |
| Other† | 8 (6.7) |
| Target INR range | |
| 2.0-3.0 | 101 (84.2) |
| 2.5-3.5 | 19 (15.8) |
| Total no. of children | 120 (100) |
| . | No. (%) of children . |
|---|---|
| Sex | |
| Male | 82 (68.3) |
| Female | 38 (31.7) |
| Age group | |
| 0-3 y | 7 (5.8) |
| 4-6 y | 20 (16.7) |
| 7-9 y | 20 (16.7) |
| 10-12 y | 21 (17.5) |
| 13-15 y | 29 (24.2) |
| 16-18 y | 23 (19.2) |
| Ethnic origin | |
| White | 91 (75.8) |
| Indian/Pakistani | 10 (8.3) |
| Chinese | 4 (3.3) |
| Black Caribbean | 3 (2.5) |
| Black African | 3 (2.5) |
| South-East Asian/Filipino | 2 (1.7) |
| Other* | 7 (5.8) |
| Indication for anticoagulation with warfarin | |
| Fontan procedure | 64 (53.3) |
| Prosthetic heart valve | 18 (15.0) |
| Coronary aneurysm | 11 (9.2) |
| Dilated cardiomyopathy | 6 (5.0) |
| Deep vein thrombosis/pulmonary embolism | 6 (5.0) |
| Pulmonary hypertension | 5 (4.2) |
| Stroke | 2 (1.7) |
| Other† | 8 (6.7) |
| Target INR range | |
| 2.0-3.0 | 101 (84.2) |
| 2.5-3.5 | 19 (15.8) |
| Total no. of children | 120 (100) |
Mixed race, n = 5; Canadian Aboriginal, n = 1; and Middle Eastern, n = 1.
One patient each with arrhythmia, bidirectional Glenn procedure, cerebral sinovenous thrombosis, left coronary artery to right ventricular fistula, recurrent transient ischemic attack, transposition of the great arteries, truncal valve replacement, and ventricular assist device.
Relationship between age and daily warfarin dose. (A) In milligrams. (B) In milligrams per kilogram body weight.
Relationship between age and daily warfarin dose. (A) In milligrams. (B) In milligrams per kilogram body weight.
Genotyping results
Genotype frequencies for VKORC1 (−1639G > A; rs9923231), CYP2C9 (*2 and *3; rs1799853 and rs1057910), and CYP4F2 (V433M; rs2108622) for the study population are shown in Table 2. All genotypes were in Hardy-Weinberg equilibrium.
Genetic characteristics of study population
| . | No. (%) of children . |
|---|---|
| VKORC1 genotype | |
| GG | 43 (35.8) |
| GA | 55 (45.8) |
| AA | 22 (18.3) |
| CYP2C9 genotype | |
| *1/*1 | 84 (70.0) |
| *1/*2 | 17 (14.2) |
| *1/*3 | 17 (14.2) |
| *2/*2 | 1 (0.8) |
| *2/*3 | 1 (0.8) |
| *3/*3 | 0 (0.0) |
| CYP4F2 genotype | |
| CC | 61 (50.8) |
| CT | 49 (40.8) |
| TT | 10 (8.3) |
| . | No. (%) of children . |
|---|---|
| VKORC1 genotype | |
| GG | 43 (35.8) |
| GA | 55 (45.8) |
| AA | 22 (18.3) |
| CYP2C9 genotype | |
| *1/*1 | 84 (70.0) |
| *1/*2 | 17 (14.2) |
| *1/*3 | 17 (14.2) |
| *2/*2 | 1 (0.8) |
| *2/*3 | 1 (0.8) |
| *3/*3 | 0 (0.0) |
| CYP4F2 genotype | |
| CC | 61 (50.8) |
| CT | 49 (40.8) |
| TT | 10 (8.3) |
Association of demographic and genetic variables with warfarin dose
The square root of warfarin daily dose was highly significantly correlated with body surface area (r = 0.56, P < .001, Pearson correlation coefficient), height (r = 0.55, P < .001), weight (r = 0.53, P < .001), and age (r = 0.53, P < .001), with body surface area and height being the most accurate predictors of warfarin dose requirement. Body mass index correlated less closely with warfarin daily dose (r = 0.32, P < .001).
The mean warfarin daily dose requirement in children with the VKORC1 (−1639) GG genotype (5.0 ± 2.2 mg) was significantly higher than in those with GA (3.7 ± 1.9 mg) or AA (2.2 ± 1.1 mg) genotype (P < .001, ANOVA; Figure 2). The mean warfarin daily dose requirement in children with homozygous wild-type CYP2C9 genotype (4.3 ± 2.1 mg) was significantly higher than in those with *1/*3 (2.2 ± 1.1 mg, P < .001), *1/*2 (3.7 ± 2.1 mg, P = 0.28), *2/*2 (1.3 mg, n = 1), or *2/*3 (1.6 mg, n = 1) genotype (Figure 2). Children who were anticoagulated after a Fontan procedure had a significantly lower mean warfarin daily dose (3.4 ± 1.6 mg) than those who were anticoagulated for other indications (4.4 ± 2.5 mg; P = .02, unpaired t test; Figure 2). Although children with a higher target INR range required a higher mean warfarin daily dose than those with a lower target INR range, the effect of target INR range on warfarin dose requirement was not significant (2.5-3.5, 4.5 ± 2.6 mg vs 2.0-3.0, 3.8 ± 2.0 mg, P = .23). The mean warfarin daily dose requirement was 5.1 ± 2.8 mg in children with CYP4F2 (rs2108633) TT genotype and was higher than in those with CT (4.0 ± 2.3 mg) or CC (3.6 ± 1.8 mg) genotype, but the differences were not statistically significant (P = .12). Children of Indian/Pakistani origin (n = 10) had a higher mean warfarin daily dose requirement (5.1 ± 2.6 mg) than children of different ethnic origin (3.8 ± 2.1 mg); this was accounted for by the higher frequency of VKORC1 (−1639) GG genotype in this ethnic group, 8 of 10 (80%) versus 35 of 110 (31.8%) for the rest of the cohort. However, the difference was not statistically significant (P = .153, t test). There were too few children for other ethnic groups to permit further subgroup analysis.
Box plots showing the influence of VKORC1 and CYP2C9 genotypes, and indication for warfarin, on warfarin dose. Boxes represent the median and interquartile ranges. Vertical lines above and below boxes indicate the minimum and maximum values. Indicated values are mean warfarin doses. FP indicates Fontan procedure; and O, other indication.
Box plots showing the influence of VKORC1 and CYP2C9 genotypes, and indication for warfarin, on warfarin dose. Boxes represent the median and interquartile ranges. Vertical lines above and below boxes indicate the minimum and maximum values. Indicated values are mean warfarin doses. FP indicates Fontan procedure; and O, other indication.
According to the regression model, height, indication, and VKORC1 and CYP2C9 genotypes made a significant contribution to the variability in warfarin dose requirement (R2 = 72.4%; Table 3). Both height and body surface area were found to be good predictors of the square root of dose, but in a model where the genetic parameters were included height was found to be the superior predictor and, once height was included, body surface area did not significantly improve the fit of the model. Regression coefficient, SE, and P value for target range was 0.065, 0.077 and P = .395, and for CYP4F2 genotype was 0.064, 0.040, and P = .114, respectively.
Contribution of height, VKORC1, CYP2C9*2 and *3 genotypes, and indication for warfarin to regression equation for modeling warfarin daily dose requirements in children
| x variable . | P . | Contribution to model, % . |
|---|---|---|
| Height | < .001 | 29.8 |
| VKORC1 | < .001 | 26.6 |
| CYP2C9*3 | < .001 | 12.8 |
| CYP2C9*2 | < .001 | |
| Indication | < .001 | 3.2 |
| Height, VKORC1, CYP2C9*2/*3, indication | < .001 | 72.4 |
| x variable . | P . | Contribution to model, % . |
|---|---|---|
| Height | < .001 | 29.8 |
| VKORC1 | < .001 | 26.6 |
| CYP2C9*3 | < .001 | 12.8 |
| CYP2C9*2 | < .001 | |
| Indication | < .001 | 3.2 |
| Height, VKORC1, CYP2C9*2/*3, indication | < .001 | 72.4 |
Regression equation: √dose = −0.009 + 0.011 (height) + 0.357 (VKORC1) − 0.478 (CYP2C9*3) − 0.277 (CYP2C9*2) + 0.186 (indication). Height: input height in centimeters; VKORC1 genotype: input 0 for AA, 1 for AG, and 2 for GG; CYP2C9*3 genotype: input 0, 1, or 2 for the number of *3 alleles; CYP2C9*2 genotype: input 0, 1, or 2 for the number of *2 alleles; indication: input 0 for Fontan procedure, 1 for other indication. The 95% confidence intervals for the coefficients are: intercept (−0.313, 0.294), height (0.009, 0.013), VKORC1 (0.284, 0.425), CYP2C9*3 (−0.335, −0.621), CYP2C9*2 (−0.148, −0.407), and indication (0.085, 0.29).
Subgroup analysis using 2 age groups (children < 10 years of age and children ≥ 10 years of age) showed that neither the contribution of each individual factor nor the dosing algorithm differed significantly between children of differing age.
Other variables
Concomitant drug therapy.
A total of 83 of 120 (69.2%) children were taking additional prescribed drug(s). Of these children, 16 (13.3%) were taking 1 or more drugs known or suspected to have an effect on warfarin metabolism. These included: trimethoprim/co-trimoxazole, 6 patients; omeprazole, 4 patients; erythromycin/azithromycin, 2 patients; amiodarone, 1 patient; imatinib, 1 patient; prednisolone, 1 patient; iloprost, 1 patient; amitryptiline, 1 patient; sodium valproate, 1 patient; and carbamazepine, 1 patient. Children receiving trimethoprim or co-trimoxazole required a slightly lower warfarin dose than those who were not, but this difference was not statistically significant.
Diet.
A total of 117 of 120 (97.5%) of the children reported a normal diet, meaning that they were not vegetarian or vegan or receiving enteral, parenteral, or infant formula feeds. Of the remainder, 1 child (4 years old) was receiving enteral feeding, 1 (14 years old) was vegetarian, and 1 (2 years old) was receiving infant formula feed. When the factors in the regression equation were accounted for, none of these children had a warfarin daily dose that was outside of the expected range.
Predictive value of IWPC algorithm
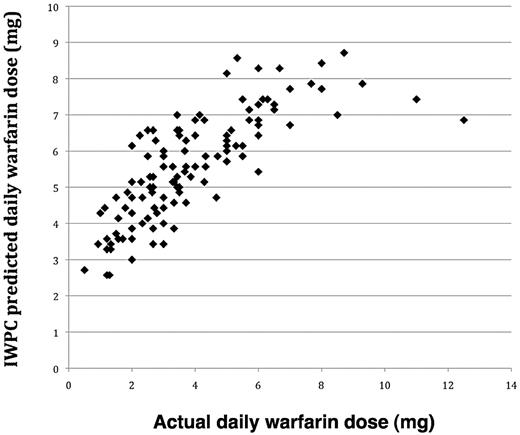
There was a close and highly significant correlation between the actual warfarin maintenance dose and the predicted maintenance dose according to the International Warfarin Pharmacogenetics Consortium (IWPC) algorithm (r = 0.76, P < .001). However, the algorithm consistently overestimated warfarin dose in our cohort of children by, on average, 1.5 ± 1.4 mg/day (Figure 3).
Relationship between actual daily warfarin dose (milligrams) and IWPC predicted daily warfarin dose (milligrams).
Relationship between actual daily warfarin dose (milligrams) and IWPC predicted daily warfarin dose (milligrams).
Discussion
This study is the largest cross-sectional study of the effect of genetic, clinical, and demographic factors on warfarin dose requirement in children reported to date. The results show that a major proportion (72.4%) of the interindividual variability in warfarin dose requirement in children is attributed to by VKORC1, CYP2C9, height, and indication for warfarin therapy. A recent study published by Nowak-Gottl et al identified only a minor effect of VKORC1 (−1639G > A) and CYP2C9 (*2, *3) polymorphisms on maintenance warfarin dose in children, 3.7% and 0.4%, respectively.16 However, only 59 children were included in the analysis, not all were anticoagulated with the same oral coumarin antagonist, there were few children identified as having CYP2C9 *2 and *3 alleles, and a less robust criterion was used to establish stable anticoagulation status (requiring only 3 consecutive days of stable warfarin dose before subject study participation)16 than that adopted for our study. A further study by Kato et al in 48 children of Japanese origin identified a 28% lower warfarin dose requirement in children with VKORC1 −1173TT genotype compared with those with −1173CT or −1173CC genotype.17 The investigators were unable to assess the impact of CYP2C9 genotype on warfarin dose requirement as they found only 1 child to be of CYP2C9*3 genotype. This study was also limited by the small sample size. Neither Nowak-Gottel et al16 nor Kato et al17 evaluated the effect of CYP4F2 polymorphism on pediatric warfarin dose requirement.
In keeping with the studies by Nowak-Gottl et al16 and Kato et al,17 body size, in this case measured by height, remains the primary determinant of warfarin dose requirement in a pediatric cohort, contributing 29.8% to interindividual variability. Age and weight were highly correlated with height, but there was a closer correlation between height and warfarin dose. This may be explained by the presence of a positive correlation between liver size and height, resulting in greater warfarin dose requirement because of increased warfarin hepatic clearance18,19 and possibly greater hepatic availability of coagulation proteins and stored vitamin K with increasing height. The positive correlation of dose with height overrides the smaller effect of younger children requiring a higher warfarin dose per kilogram body weight than older children and adolescents, shown by this (Figure 1) and previous studies.1,3
Studies in adults anticoagulated with warfarin have shown that carriers of the common allelic variants (*2 or *3) of the CYP2C9 are associated with a lower warfarin dose requirement accompanied by a greater tendency to experience hemorrhagic complications during warfarin initiation.6,7 Studies have shown that the CYP2C9 polymorphism accounts for 5.7% to 17.5% of interindividual variability in warfarin dose requirement in adults.14,20,21 The influence of CYP2C9 on maintenance warfarin dose requirement was similar in our pediatric cohort (in which it accounted for 12.8% of variability) to adults. Our findings are more in keeping with the adult literature than either those of Nowak-Gottl et al16 or of an earlier study by Ruud et al22 who found no association between CYP2C9 genotype and warfarin dose in 29 anticoagulated children, although the latter noted that children with a CYP2C9 variant allele reached target INR sooner and were more likely to be overanticoagulated than those without. In the Ruud et al study, warfarin doses were not corrected for either body size or age.22
Vitamin K epoxide reductase (target enzyme for warfarin) is responsible for the recycling of vitamin K and is encoded by the vitamin K epoxide reductase subunit complex (VKORC1). Adults with VKORC1 (−1639) GG genotype require higher warfarin doses than those with GA or AA genotype.4,5 VKORC1 genotype accounts for 15% to 30% of interindividual variability in warfarin dose requirement in adults,4,14,20 consistent with that seen in our pediatric population in which it accounted for 26.6%.
Indication for warfarin (Fontan procedure) contributed to 3.2% of interindividual variability. A lower warfarin dose requirement in children after a Fontan procedure was previously reported by Streif et al,1 showing a 25% reduction in dose compared with patients with the same target INR range. The mechanism for the lower dose requirement may be related to abnormal liver function, resulting in reduced warfarin metabolism.23 Although all of the children in our cohort who had a Fontan procedure were anticoagulated with the lower target INR range of 2.0 to 3.0, target INR range was not an independent variable in terms of its effect on warfarin dose requirement.
Recent studies have identified that the rs2108622 SNP in CYP4F2 accounts for 2% to 7% of interindividual variability in warfarin dose requirements in adults.8,9 Although there appears to be no direct role for CYP4F2 in warfarin metabolism, it has been suggested that it influences vitamin K oxidase activity and therefore hepatic levels of vitamin K.24 The lack of effect of CYP4F2 on warfarin dose in our cohort of children may relate to differences in dietary vitamin K intake compared with their adult counterparts. This requires further study of the impact of vitamin K status on warfarin dose requirement in children.
In this study, we found that children of Indian/Pakistan origin tended to require a higher daily warfarin dose than other ethnic groups, which was accounted for by the higher frequency of VKORC1 (−1639) GG genotype in this ethnic subgroup. This is consistent with data previously reported in adults.25 Our study was not designed to explore the effect of ethnicity on warfarin dose requirement, which should be evaluated in a larger population.
Based on the study data, we have developed a regression equation for predicting warfarin maintenance dose (Table 3). However, because of the lack of additional eligible patients across the participating clinics, we were unable to test the equation for its accuracy to predict warfarin maintenance dose in an unrelated patient population. Most recently, the IWPC published a pharmacogenetic-based warfarin dosing algorithm based on genetic and clinical data derived from a large cohort (n = 4043) of geographically and ethnically diverse adult patients.10 We found that, despite a good correlation between the predicted maintenance dose according to the IWPC algorithm and the actual dose, the algorithm consistently overestimated warfarin dose in our cohort of children (Figure 3). The overestimation of warfarin dose by the IWPC algorithm can be explained by a greater influence of age on the variability in warfarin dose requirement in children than in adults. This indicates that pharmacogenetic-based dosing algorithms for use in children requiring coumarin therapy should be based on data derived from such patients if the potential benefit of a pharmacogenetic-guided warfarin dosing in children is to be realized. Our dosing equation should now be validated and prospectively evaluated to determine whether it will aid the efficacy and safety of anticoagulant therapy with warfarin in children.
The remaining currently unexplained 27.4% of the interindividual variability in warfarin dose requirement in children may relate to differences in dietary vitamin K status. This is known to influence warfarin dose requirement in adult populations, those with a lower vitamin K status requiring a lower warfarin maintenance dose,26 an effect that has not yet been studied in a pediatric population.27 The impact of frequent intercurrent illness was also unaccounted for in the present study. Genome-wide association studies have been applied to adult populations in an attempt to explain some of the remaining interindividual variability in warfarin dose requirement through the contribution of other genes but have failed to identify a significant genetic influence on warfarin dose outside of the CYP2C9 and VKORC1 genes.28 A genome-wide association study is probably even less applicable to a pediatric population in whom a greater proportion of interindividual variability is already explained by the factors included in our algorithm. It is possible that dietary vitamin K status is responsible for a significant proportion of the unexplained variance in warfarin dose requirement in children.
Presented in abstract form at the XXIII Congress of the International Society on Thrombosis and Haemostasis, Kyoto, Japan, July 26, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following, who were responsible for study coordination and the recruitment of children at each of the study sites: Patricia Walsh, RN, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle on Tyne, United Kingdom; Darlene Castle, RN, Dewi Clark, BSc, and Margaret Rand, PhD, The Hospital for Sick Children, Toronto, ON; Aileen Gibson, RN, Royal Hospital for Sick Children, Glasgow, United Kingdom; Gillian Taylor, RN, Birmingham Children's Hospital, Birmingham, United Kingdom; and Anne Littley, RN, Royal Manchester Children's Hospital, Manchester, United Kingdom. The authors also thank the children and young adults and their parents/caregivers who participated in the study.
T.T.B. was supported by Baxter and the Royal College of Pathologists (research training fellowship).
Authorship
Contribution: T.T.B., P.J.A., and F.K. designed the study; T.T.B., L.R.B., E.A.C., M.D.W., J.D.G., J.P.H., and F.K. conducted the trial; T.T.B., J.B.S.L., and A.K.D. performed genotyping experiments; T.T.B. and F.K. analyzed and interpreted data; T.T.B. and P.J.A. performed statistical analysis; and all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farhad Kamali, Institute of Cellular Medicine, William Leech Building, 4th Floor, Newcastle University, Newcastle upon Tyne, NE2 4HH United Kingdom; e-mail: farhad.kamali@ncl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal