Abstract
The normal accumulation of β-globin protein in terminally differentiating erythroid cells is critically dependent on the high stability of its encoding mRNA. The molecular basis for this property, though, is incompletely understood. Factors that regulate β-globin mRNA within the nucleus of early erythroid progenitors are unlikely to account for the constitutively high half-life of β-globin mRNA in the cytoplasm of their anucleate erythroid progeny. We conducted in vitro protein-RNA binding analyses that identified a cytoplasm-restricted β-globin messenger ribonucleoprotein (mRNP) complex in both cultured K562 cells and erythroid-differentiated human CD34+ cells. This novel mRNP targets a specific guanine-rich pentanucleotide in a region of the β-globin 3′untranslated region that has recently been implicated as a determinant of β-globin mRNA stability. Subsequent affinity-enrichment analyses identified AUF-1 and YB-1, 2 cytoplasmic proteins with well-established roles in RNA biology, as trans-acting components of the mRNP. Factor-depletion studies conducted in vivo demonstrated the importance of the mRNP to normal steady-state levels of β-globin mRNA in erythroid precursors. These data define a previously unrecognized mechanism for the posttranscriptional regulation of β-globin mRNA during normal erythropoiesis, providing new therapeutic targets for disorders of β-globin gene expression.
Introduction
The normal function of mature erythrocytes requires the efficient accumulation of hemoglobin in terminally differentiating progenitor cells. This process is critically dependent on the high stabilities of α- and β-globin mRNAs—and the reciprocal instabilities of heterologous mRNAs—during an interval when these cells are transcriptionally silent but translationally active. Estimates for the half-life of α-globin mRNA from studies of cultured murine erythroleukemia cells and human reticulocytes range from 24 to 60 hours,1,2 whereas similar measures of β-globin mRNA indicate a half-life value that exceeds 20 hours.3,4 In anucleate, transcriptionally silent erythroid precursors, the high half-life values for globin mRNAs provide both for their selective enrichment and for a proportional corresponding increase in the levels of the α- and β-globin proteins that they encode. The clear relevance of these processes to hemoglobinization has fostered intense interest in defining the mRNA determinants and corresponding trans-acting factors that mediate this critical aspect of normal erythropoiesis.
The posttranscriptional events that favor the accumulation of α-globin in erythroid precursors are largely mediated by members of the αCP-family of poly(C)–binding proteins. These ubiquitously expressed and multifunctional RNA-binding factors play several essential roles in regulating the levels of α-globin mRNA transcripts in erythroid cells. αCP assembles a messenger ribonucleoprotein (mRNP) complex on a 14-nt pyrimidine-rich element positioned within the α-globin 3′untranslated region (3′UTR)5,6 ; this mRNP “α-complex” is a critical determinant of both α-globin mRNA processing and stability. In erythroid cytoplasm, the α-complex prolongs the half-life of α-globin mRNA by enhancing the binding of poly(A)–binding protein (PABP) to its polyadenylate tail7 and/or by restricting access of a cell-specific endoribonuclease to the α-pyrimidine–rich element.8 Although α-complexes assemble on the 3′UTR of α-globin mRNA in extracts prepared from both erythroid and nonerythroid cells, their ability to stabilize α-globin mRNA appear to be erythroid specific,9 reflecting cell-restricted structures and/or functions that have not yet been identified. These studies illustrate both the diversity and the mechanistic complexity of posttranscriptional activities that contribute to the regulated expression of α-globin mRNA in differentiating erythroid precursors.
The posttranscriptional processes that regulate the levels of β-globin mRNA during erythropoiesis are thought to be mechanistically distinct from those affecting α-globin mRNA. At least 4 RNA-binding proteins (RBPs), each with known posttranscriptional regulatory properties, target β-globin mRNA in the erythroid nucleus. A ubiquitous RBP, nucleolin, binds to a specific 12-nt region of β-globin 3′UTR and may enhance αCP binding to a contiguous pyrimidine-rich sequence.10 Deletion of this αCP-binding motif reduces the accumulation of β-globin mRNA in vivo,11 raising the intriguing possibility that α- and β-globin mRNAs are posttranscriptionally coregulated through an αCP-dependent mechanism. A third nuclear factor, polypyrimidine tract binding protein 1 (PTBP1), separately binds to the 3′UTR of β-globin pre-mRNA, where it promotes efficient 3′-end cleavage by stimulating binding of hnRNP H to a distal β-globin gene 3′-flanking region. PTBP1 may additionally enhance polyadenylation by promoting hnRNP H binding to a proximal guanine-rich 3′UTR sequence.12 Whereas the importance of these factors to the processing and stability of β-globin mRNA in the erythroid nucleus is clear, their relationship to posttranscriptional regulatory events that transpire in the cytoplasm of anucleate erythroid precursors is less certain.
In contrast to the several RBPs that regulate β-globin mRNA in the nucleus, trans-acting factors that are anticipated to dictate the stability and translational efficiency of β-globin mRNA in erythroid cytoplasm have not been identified, nor have their corresponding mRNA-binding sites been characterized. It is possible that αCP, PTBP1, and nucleolin—each a nuclear-cytoplasmic shuttling protein13-15 —link the cytoplasmic characteristics of β-globin mRNA to nuclear processing events, as occurs with RNA splicing-coupled translation control and nonsense-mediated mRNA-decay mechanisms (for review, see Kuersten16 ). However, the high levels of β-globin mRNA in anucleate erythroid precursors testify to the existence of cytoplasmic regulatory programs that act independently of preceding nuclear events. Moreover, direct links between the previously identified nuclear RBPs and the cytoplasmic properties of β-globin mRNA have not been experimentally observed, implicating cytoplasm-restricted mechanisms (and corresponding trans-acting effector factors) that regulate biologically important properties of β-globin mRNA in that compartment.
The present work identifies both cis- and trans-acting determinants that appear to mediate compartment-specific posttranscriptional regulation of β-globin mRNA in erythroid cytoplasm. Our experiments demonstrate a novel cytoplasm-restricted mRNP that assembles on a regulatory region of the β-globin 3′UTR both in erythroid K562 cells and in human CD34+ cells induced along erythroid, but not granulocytic/monocytic (GM) differentiation. Both in vitro and in vivo assays identify the trans-acting components of this β-globin mRNP as AUF-1 and YB-1, 2 ubiquitous proteins with established regulatory functions in mRNA biology that have not been implicated previously in the posttranscriptional regulation of β-globin mRNA. Subsequent functional analyses demonstrate that a reduction in the levels of both factors reduces the accumulation of β-globin mRNA in cells that assemble the β-complex, but not in cells that lack the β-complex, validating the anticipated regulatory importance of AUF-1 and YB-1 during erythropoiesis. Our data identify previously unrecognized trans-acting factors that coordinate the accumulation of β-globin mRNA in erythroid precursors through cytoplasm-restricted activities and that appear to be critical determinants of normal erythrocyte function.
Methods
Plasmid constructions
The construction of pTRE-β-WT, which contains a 3.0-kb fragment of human genomic DNA encompassing the 1.5-kb full-length β-globin gene and its contiguous 3′-flanking region, has been described previously.10 Variant genes were generated by exchanging the 0.2-kb BstXI/SwaI fragment of pTRE-β-WT (containing the β-globin 3′UTR) with corresponding derivative DNAs (GenScript).
A template for in vitro transcription of the human β-globin 3′UTR, contiguous with a 20-nt poly(A) tail, was constructed by cloning the corresponding DNA into the XhoI/BglII-restriction sites of pSP72. Templates encoding 3′UTR-derivative RNAs were similarly generated. A DNA template encoding the α-globin 3′UTR was a kind gift of Dr Stephen A. Liebhaber (University of Pennsylvania).
pGEX-2TK-YB-1 was constructed by inserting the coding region of YB-1 in-frame into pGEX-2TK (GE Healthcare). pGEX–4T-1–AUF-1(p40) was generously provided by Dr Robert J. Schneider (New York University). The sequences of all recombinant DNAs were confirmed by automated sequencing.
EMSA
Template DNAs were linearized with BglII, and [32P]-labeled RNAs were transcribed in vitro using SP6 polymerase under conditions recommended by the manufacturer (Ambion). The structural integrities of all RNAs were verified by gel electrophoreses. Transcripts (2-3 × 104 cpm) were incubated in EMSA buffer (10mM Tris, pH 7.4, 150mM KCl, 1.5mM MgCl2, and 1mM DTT) supplemented with 5-10 μg of cellular extract or 1 μg of recombinant protein, for 30 minutes at room temperature. Reaction products were digested for 10 minutes at room temperature with RNase T1 (20 units; Roche), resolved on a native 5% polyacrylamide gel, and exposed to X-ray film (Kodak). Competitor DNAs (0.2 nmol) were added where indicated.
Cell culture
K562 cells expressing the tetracycline-regulated transactivator (tTA) fusion protein (K562/tTA17 ; a kind gift of Dr Ann-Bin Shyu, University of Texas) were maintained in RPMI 1640 + GlutaMAX, whereas tTA-expressing HeLa cells (HeLa/tTA; Clontech) were cultured in high-glucose DMEM. All media were amended with 10% FBS (Tet-System Approved; Clontech) and 1× AntiBiotic-AntiMycotic (Invitrogen). Cell lines were maintained at 37°C in a humidified 5% CO2 environment.
K562β cells, which stably express high levels of wild-type human β-globin mRNA, were generated by electroporating K562/tTA cells with pTRE-β-WT (Amaxa) and selecting for a single clone demonstrating resistance to hygromycin B (200 μg/mL; Roche). K562β cells were induced to megakaryocytic differentiation with 100 ng/mL of phorbol-myristate-acetate (PMA; Sigma-Aldrich) for 4 days18 ; lineage commitment was validated by RT-PCR analyses of CD10 mRNA (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
HeLaβ cells, which also stably express wild-type human β-globin mRNA, were similarly derived from HeLa/tTA cells, except that transfections with pTRE-β-WT were conducted with Superfect according to the manufacturer's recommendations (QIAGEN).
Human primary cell culture
Umbilical cord blood (UCB) was obtained from the Stem Cell Core Facility at the University of Pennsylvania under an existing Institutional Review Board–approved protocol. Mononuclear cells were isolated from fresh UCB on a Ficoll-Paque PLUS gradient (GE Healthcare), followed by immunomagnetic selection of CD34+ cells according to the manufacturer's protocol (Miltenyi Biotec). Cells were cultured in IMDM (Invitrogen) containing 20% BIT 9500 (StemCell Technologies) and 1× AntiBiotic-AntiMycotic, and induced to erythroid or GM differentiation as described previously (supplemental Figure 2).19,20 Morphology was monitored by light-microscopic examination of modified Wright-Giemsa–stained cytospins (Shandon). Flow cytometric analyses were conducted for erythroid markers (CD45 FITC, GlyA PE, and CD71 APC) and GM markers (CD15 FITC, CD11b PE, and CD45 APC).
Nuclear and cytoplasmic extracts
Cytoplasmic extract was prepared from PBS-washed cells that were resuspended in 10× packed-cell volume buffer A (10mM HEPES, pH 7.9, 1.5mM MgCl2, 10mM KCl, and 0.5mM DTT), incubated on ice for 5 minutes, and amended with 0.05% NP-40 for an additional 3 minutes. The cell suspension was centrifuged for 5 minutes (16 000g at 4°C), and the supernatant stored at −80°C. Nuclear extract was prepared from the pellet, which was resuspended in 5× packed-cell volume buffer C (20mM HEPES, pH 7.9, 25% vol/vol glycerol, 0.42M NaCl, 1.5mM MgCl2, 0.2mM EDTA, and 0.05mM DTT) and incubated for 40 minutes with intermittent vortexing at 10-minute intervals. The suspension was centrifuged for 10 minutes (16 000g, 4°C) and the supernatant stored at −80°C. Both buffers were supplemented with protease inhibitors (Complete Mini; Roche).
Western blotting
Protein samples were resolved on a precast 4%-12% gradient SDS-PAGE gel, and transferred to nitrocellulose using an XCell II Blot Module (Invitrogen). Membranes were sequentially incubated for 1 hour at room temperature in blocking buffer (Superblock T20 PBS; ThermoScientific), and for 1 hour in blocking buffer supplemented with primary Ab. Membranes were washed with PBS + Tween-20 (0.05%) and incubated for 1 hour in blocking buffer containing HRP-conjugated secondary Ab (GE Healthcare). Washed membranes were analyzed by chemiluminescence using a protocol recommended by the manufacturer (ECL Plus; GE Healthcare). Abs against hnRNP H, hnRNP F, HDAC-2, β-actin, and YB-1 were acquired from Santa Cruz Biotechnology and Abs against GAPDH and AUF-1 were purchased from Novus Biologicals and Millipore, respectively.
DNA-affinity analyses
Biotin-linked oligomers (100 pmol; IDT) were incubated for 1 hour at 4°C with avidin-agarose beads (ThermoScientific). Pelleted beads were resuspended with 250 μL of K562 cytoplasmic extract in EMSA-binding buffer and incubated for 2 hours at 4°C. The liganded beads were then washed twice with 0.05% PBS + Triton X-100 and twice with 1% PBS + Triton X-100. Retained proteins were eluted in NuPAGE LDS sample buffer and resolved on a precast 4%-12% gradient SDS-PAGE gel.
Purification of recombinant proteins
Competent BL-21 cells (CodonPlus DE3-RIPL; Stratagene) were transformed with pGEX plasmids encoding YB-1 or AUF-1(p40), and induced with 1mM IPTG for 3 hours at 30°C. Proteins were purified from lysozyme-treated, sonicated bacteria using GST-agarose as directed by the manufacturer (Sigma-Aldrich). Protein integrity was validated by Coomassie staining and protein concentration estimated by Bradford analysis (Sigma-Aldrich).
siRNA knockdown
K562β cells were subcultured for 2 days to a density of 0.5-1.0 × 106/mL, and 3-5 × 106 cells were then electroporated with 100 pmol of siRNA (Amaxa). After a 48-hour recovery, one-half of the cells were allocated to Western analysis, and one-half to RNA analysis. HeLaβ cells (4 × 105) were seeded in a 60-mm dish and transfected 2 hours later using HiPerFect according to the manufacturer's protocol (QIAGEN). siRNAs specific for AUF-1, YB-1, and lamin A/C were purchased from Dharmacon.
Quantitative RT-PCR
Total RNA was purified from cells using TRIzol reagent (Invitrogen) and incubated with RNase-free DNase I (Roche). mRNA was quantitated by Taqman quantitative RT-PCR (One-Step RT-PCR system; Applied Biosystems) with FAM-MGM–labeled β-globin (HBB; 00747223_g1) and control β-actin assays (ACTB; 4352935). Reactions were monitored on an SDS-7500 cycler (Applied Biosystems) and data analyzed with corresponding system software. Statistical significance was determined with the Student t test.
Results
The β-globin 3′UTR assembles a novel cytoplasm-restricted mRNP
The high stability of β-globin mRNA in anucleate erythroid precursors implicates the activities of cytoplasmic regulatory processes that are distinct from nuclear posttranscriptional events. We reasoned that these processes would require site-specific interactions between cytoplasmic factors and the 3′UTR of mature β-globin mRNA, which is thought to harbor one or more posttranscriptional regulatory elements.3 To identify candidate regulatory proteins, we conducted comparative EMSA analyses on a [32P]-labeled RNA corresponding to the full-length, polyadenylated human β-globin 3′UTR using validated subcellular extracts prepared from human erythroid K562 cells (Figure 1A and supplemental Figure 3). These analyses identified a robust β-globin mRNP β-complex that assembles readily in cytoplasmic extract (Figure 1A lane 5), but only faintly in nuclear extract (Figure 1A lane 2), which is consistent with a possible regulatory role in anucleate erythroid progenitor cells. β-complex assembly was inhibited by a 15-nt competitor DNA corresponding to a 3′UTR region that has been linked to posttranscriptional regulation of β-globin mRNA (WT15; Figure 1B)10 ; an antisense control DNA had no effect (Figure 1A compare lanes 6 and 7), confirming the β-complex mRNA-target sequence and predicting a potential cytoplasmic function. Parallel analyses conducted in extracts prepared from uninduced and erythroid-induced UCB CD34+ cells confirmed that the β-complex assembles in committed erythroid precursors, exhibits cytoplasm specificity, and targets the putative 3′UTR regulatory motif (Figure 1C-D). These analyses also validated K562 cells as an appropriate model for studying the structural and mechanistic properties of the β-complex.
A novel cytoplasm-specific mRNP assembles on the 3′UTR of β-globin mRNA. (A) Site-specific assembly of a cytoplasm-restricted β-complex in vitro. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with nuclear (Nuc) or cytoplasmic (Cyt) extract prepared from K562 cells, and RNase T1-resistant mRNPs resolved on a native polyacrylamide gel. Parallel reactions were supplemented with a 15-nt competitor DNA (WT15) corresponding to a previously identified regulatory region of the β-globin 3′UTR (panel B), either in the sense (S) or control antisense (AS) orientation. An arrow indicates the β-complex. (B) Composition of the 132-nt β-globin 3′UTR. The positions of the 15-nt regulatory region (WT15), the translation stop codon (TAA), and the poly(A) tail are indicated. (C) The β-complex assembles in erythroid-induced CD34+ cells. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from K562 cells, uninduced human CD34+ cells (U; day 5), or erythroid-induced CD34+ cells (I; day 17). Reactions were amended with WT15 competitor DNA in either the sense (S) or antisense (AS) orientation. An arrow indicates the β-complex. (D) The β-complex is restricted to the cytoplasm of erythroid precursor cells. EMSA analyses were conducted on a [32P]-labeled RNA using nuclear and cytoplasmic extracts prepared from erythroid-induced CD34+ cells after 12 days of culture. Sense (S) or antisense (AS) WT15 DNA competitor was added as indicated. An arrow denotes the β-complex.
A novel cytoplasm-specific mRNP assembles on the 3′UTR of β-globin mRNA. (A) Site-specific assembly of a cytoplasm-restricted β-complex in vitro. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with nuclear (Nuc) or cytoplasmic (Cyt) extract prepared from K562 cells, and RNase T1-resistant mRNPs resolved on a native polyacrylamide gel. Parallel reactions were supplemented with a 15-nt competitor DNA (WT15) corresponding to a previously identified regulatory region of the β-globin 3′UTR (panel B), either in the sense (S) or control antisense (AS) orientation. An arrow indicates the β-complex. (B) Composition of the 132-nt β-globin 3′UTR. The positions of the 15-nt regulatory region (WT15), the translation stop codon (TAA), and the poly(A) tail are indicated. (C) The β-complex assembles in erythroid-induced CD34+ cells. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from K562 cells, uninduced human CD34+ cells (U; day 5), or erythroid-induced CD34+ cells (I; day 17). Reactions were amended with WT15 competitor DNA in either the sense (S) or antisense (AS) orientation. An arrow indicates the β-complex. (D) The β-complex is restricted to the cytoplasm of erythroid precursor cells. EMSA analyses were conducted on a [32P]-labeled RNA using nuclear and cytoplasmic extracts prepared from erythroid-induced CD34+ cells after 12 days of culture. Sense (S) or antisense (AS) WT15 DNA competitor was added as indicated. An arrow denotes the β-complex.
Several nuclear factors that bind to the WT15 target sequence in the nucleus or that have previously been implicated in the posttranscriptional regulation of β-globin mRNA—including αCP11 and nucleolin10 —do not appear to be components of the cytoplasmic β-complex. The β-complex was not supershifted by an Ab that alters the migration of the αCP-containing α-globin mRNP (supplemental Figure 4) nor by 3 independent nucleolin Abs (data not shown). These data demonstrate that the mRNP β-complex is structurally distinct from β-globin mRNPs that assemble in the nucleus and encompasses trans-acting factors that have not been implicated previously in the regulation of β-globin mRNA.
The β-complex assembles in cells induced to erythroid differentiation but not in cells induced to hematopoietic nonerythroid differentiation
Posttranscriptional regulatory processes can be meditated by mRNPs that exhibit cell-restricted patterns of assembly.21,22 Because globin gene expression is unique to erythroid cells, we posited that critical posttranscriptional regulatory mechanisms might display similar lineage-specific restriction. We tested this possibility by inducing UCB CD34+ cells along either the erythroid or GM lineage (see supplemental Results) and assessing extracts for their abilities to form mRNP β-complexes. Whereas extracts of CD34+ cells induced to erythroid differentiation demonstrated strong β-complex assembly, similar extracts from cells induced to GM differentiation failed to support any β-complex formation (Figure 2A). These results were confirmed and extended in cultured cells: extracts prepared from untreated and control DMSO-treated K562β cells (engineered to express high levels of β-globin mRNA under a tetracycline-responsive promotor) assembled a robust β-complex. In contrast, extracts prepared from PMA-induced K562β cells induced to megakaryocytic differentiation with PMA (supplemental Figure 1) displayed an insignificant gel-mobility shift band (Figure 2B). Levels of β-globin mRNA in PMA-induced K562β cells were also significantly decreased compared with levels in untreated and DMSO-treated cells (Figure 2C), which is consistent with a lineage-specific regulatory role for the β-complex in erythroid cells. These experiments indicate a likely functional role for the β-complex during hematopoiesis.
β-complex assembly is restricted to cells induced to erythroid differentiation. (A) The β-complex assembles in CD34+ cells induced to erythroid, but not to granulocytic/monocytic differentiation. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from human UBC CD34+ cells that were induced along either the erythroid (Ery) or the GM lineages. GAPDH and β-globin immunoblots of extracts used in EMSA analyses control for extract integrity and erythroid differentiation, respectively. (B) β-complex assembly is reduced in K562 cells induced to megakaryocytic differentiation. β-complex formation was assessed in cytoplasmic extracts prepared from untreated, DMSO-treated, and PMA-induced K562 cells. The integrity of the extracts was validated by an immunoblot using control β-actin Ab. (C) Steady-state β-globin mRNA levels are reduced in PMA-induced K562 cells. Levels of β-globin mRNA were assessed in K562β cells relative to endogenous control β-actin mRNA using quantitative RT-PCR. The level of β-globin mRNA in untreated (−) cells was set as 100%. Bars depict the mean of 5 independent experiments; error bars indicate 1 SE.
β-complex assembly is restricted to cells induced to erythroid differentiation. (A) The β-complex assembles in CD34+ cells induced to erythroid, but not to granulocytic/monocytic differentiation. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from human UBC CD34+ cells that were induced along either the erythroid (Ery) or the GM lineages. GAPDH and β-globin immunoblots of extracts used in EMSA analyses control for extract integrity and erythroid differentiation, respectively. (B) β-complex assembly is reduced in K562 cells induced to megakaryocytic differentiation. β-complex formation was assessed in cytoplasmic extracts prepared from untreated, DMSO-treated, and PMA-induced K562 cells. The integrity of the extracts was validated by an immunoblot using control β-actin Ab. (C) Steady-state β-globin mRNA levels are reduced in PMA-induced K562 cells. Levels of β-globin mRNA were assessed in K562β cells relative to endogenous control β-actin mRNA using quantitative RT-PCR. The level of β-globin mRNA in untreated (−) cells was set as 100%. Bars depict the mean of 5 independent experiments; error bars indicate 1 SE.
The β-complex assembles on a defined pentanucleotide within the β-globin 3′UTR
The efficient ablation of the mRNP β-complex by the WT15 competitor DNA identifies a specific region of β-globin 3′UTR targeted by participating trans-acting factors. To map the mRNP-binding site with greater precision, we conducted survey EMSA analyses of β-globin 3′UTR transcripts using a library of WT15-derivative competitor DNAs containing site-specific hexanucleotide mutations (Figure 3A). DNAs that retained a native GGGGGA motif in the context of upstream (MB15) or downstream (MC15) mutations fully competed for the β-complex assembly, implicating this guanine-rich region of 3′UTR as a target for trans-acting factors. In contrast, DNAs that carried a mutated GGGGGA motif but retained the integrity of upstream (MA15) or downstream (MD15) sequence competed less efficiently, validating the importance of the guanine-rich region but suggesting additional, smaller contributions by nucleotides contiguous to this motif. We subsequently confirmed that the G-rich region of 3′UTR is indispensable to β-complex assembly by directly assessing the capacity of full-length β-globin 3′UTR RNAs containing informative G→C substitutions to assemble a β-complex (Figure 3B). RNAs containing wild-type (GGGGGA) and M10 (GGGGcA) sequences assembled strong β-complexes (lanes 2 and 6), validating the results from earlier, more extensive DNA competition experiments (see supplemental Results), whereas a β-globin 3′UTR RNA containing the M3 hexanucleotide (ccGGGA) formed a weak β-complex (lane 4). We also tested a full-length 3′UTR-derivative RNA that encompassed both the G-rich region and the immediate 5′ dinucleotide (CUGGGGGA→gacccGGA), reflecting an anticipated small contribution of this flanking region suggested by the results of competition with the MA15 DNA (Figure 3A). This mRNA failed to assemble any β-complex (Figure 3B lane 8), strongly implicating the CUGGG pentaribonucleotide as the 3′UTR target for trans-acting factors that comprise the β-complex.
The β-complex assembles on a site-specific sequence within the β-globin 3′UTR. (A) A guanine-rich region of RNA is critical for β-complex assembly. Top: EMSA analyses were conducted with K562 cytoplasmic extracts using [32P]-labeled RNA corresponding to the full-length β-globin 3′UTR. Reactions were supplemented with competitor DNAs derived from the WT15 sequence. The position of the β-complex is indicated by an arrow. Bottom: Sequences of competitor DNAs, with substituted nucleotides indicated in lower case. The corresponding region of the native β-globin 3′UTR is included for reference. (B) Site-specific mutations in the β-globin 3′UTR ablate β-complex assembly. EMSA analyses were conducted on [32P]-labeled RNAs, corresponding to full-length wild-type and derivative β-globin 3′UTRs, using K562 cytoplasmic extract. The G-rich motif is indicated, with the specific mutations in the full-length β-globin 3′UTR depicted in lower-case type. The position of the β-complex is indicated.
The β-complex assembles on a site-specific sequence within the β-globin 3′UTR. (A) A guanine-rich region of RNA is critical for β-complex assembly. Top: EMSA analyses were conducted with K562 cytoplasmic extracts using [32P]-labeled RNA corresponding to the full-length β-globin 3′UTR. Reactions were supplemented with competitor DNAs derived from the WT15 sequence. The position of the β-complex is indicated by an arrow. Bottom: Sequences of competitor DNAs, with substituted nucleotides indicated in lower case. The corresponding region of the native β-globin 3′UTR is included for reference. (B) Site-specific mutations in the β-globin 3′UTR ablate β-complex assembly. EMSA analyses were conducted on [32P]-labeled RNAs, corresponding to full-length wild-type and derivative β-globin 3′UTRs, using K562 cytoplasmic extract. The G-rich motif is indicated, with the specific mutations in the full-length β-globin 3′UTR depicted in lower-case type. The position of the β-complex is indicated.
The β-complex comprises the RNA-binding proteins AUF-1 and YB-1
We used 2 independent strategies to identify trans-acting members of the β-complex. Based on the observed effect of G→C substitutions on mRNP assembly, we used a candidate-factor approach to test the β-complex for the presence of hnRNP H family RBPs, which characteristically bind to G-rich RNA motifs.23 Supershift and siRNA analyses, however, each failed to indicate hnRNP H family members in the β-complex (see supplemental Results).
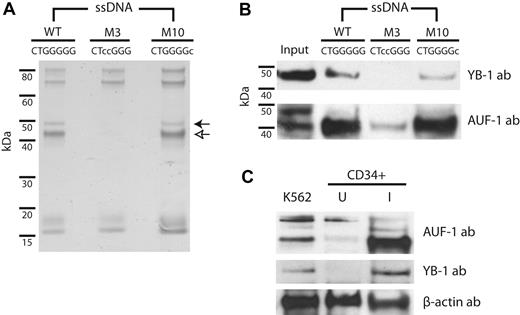
The apparent absence of hnRNP H family members compelled the use of a second, unbiased screening method to identify trans-acting components of the β-complex. We therefore performed affinity-capture analyses on K562 cytoplasmic extract using agarose-bound ssDNAs corresponding to the region of 3′UTR encompassing the β-complex RNA target sequence (Figure 4). Derivative ssDNAs containing mutations that do not (M3) or do (M10) support robust β-complex assembly (Figure 3B) were tested in parallel as controls. Whereas all 3 ssDNAs bound several proteins in common, the WT15 and M1015 variants additionally bound 42- and 47-kDa products that were not retained by the M315 probe (Figure 4A). Mass spectroscopic analyses of these 2 bands identified 3 RBPs (AUF-1, YB-1, and hnRNP A/B), each of which participates in the posttranscriptional regulation of nonglobin mRNAs.24-26 Importantly, the analyses did not detect factors that bind β-globin mRNA in the nucleus (nucleolin, PTBP1, and αCP), which is consistent with the previously demonstrated cytoplasmic specificity of the β-complex. The identities of YB-1 and AUF-1 as the 47- and 42-kDa products, respectively, were subsequently confirmed by immunoblot (Figure 4B); the presence of hnRNP A/B could not be validated, suggesting an artifactual identity (data not shown). We also demonstrated that AUF-1 and YB-1 are expressed at higher levels in erythroid-induced UBC CD34+ cells than in uninduced cells (Figure 4C), with a disproportional increase in AUF-1 (p40) levels, confirming that the trans-acting factors that comprise the β-complex are expressed in erythroid cells and assemble an erythroid stage-specific mRNP on the 3′UTR of β-globin mRNA.
The RNA-binding proteins AUF-1 and YB-1 participate in the β-complex. (A) The β-globin 3′UTR interacts with 2 trans-acting RBPs. K562 cytoplasmic extracts were incubated with agarose-linked ssDNAs encompassing the 15-nt WT15 sequence or with control ssDNAs containing derivative sequences that do not (M3) or do (M10) support β-complex assembly. Retained proteins were resolved by SDS-PAGE. Open and closed arrows indicate differentially bound 42- and 47-kDa bands, respectively. (B) AUF-1 and YB-1 bind specifically to β-globin 3′UTR DNAs. Immunoblots were conducted on retentate from WT15, M315, and M1015 ssDNAs using YB-1 and AUF-1 Abs. Input is 5%. (C) AUF-1 and YB-1 are highly expressed in erythroid-induced CD34+ cells. Cytoplasmic extracts prepared from K562, uninduced (U), and erythroid-induced (I) human CD34+ cells were analyzed by immunoblot using factor-specific or control β-actin Abs.
The RNA-binding proteins AUF-1 and YB-1 participate in the β-complex. (A) The β-globin 3′UTR interacts with 2 trans-acting RBPs. K562 cytoplasmic extracts were incubated with agarose-linked ssDNAs encompassing the 15-nt WT15 sequence or with control ssDNAs containing derivative sequences that do not (M3) or do (M10) support β-complex assembly. Retained proteins were resolved by SDS-PAGE. Open and closed arrows indicate differentially bound 42- and 47-kDa bands, respectively. (B) AUF-1 and YB-1 bind specifically to β-globin 3′UTR DNAs. Immunoblots were conducted on retentate from WT15, M315, and M1015 ssDNAs using YB-1 and AUF-1 Abs. Input is 5%. (C) AUF-1 and YB-1 are highly expressed in erythroid-induced CD34+ cells. Cytoplasmic extracts prepared from K562, uninduced (U), and erythroid-induced (I) human CD34+ cells were analyzed by immunoblot using factor-specific or control β-actin Abs.
YB-1 and AUF-1 exhibit sequence-specific binding to the β-globin 3′UTR
We conducted a series of experiments to validate that AUF-1 and YB-1 bind to the β-complex CUGGG target motif and that both factors are required components of the β-complex in vivo. Complementary analyses documented the formation of an mRNP in extract-free reactions containing either of the 2 recombinant factors, and demonstrated a reduction in β-complex assembly in extracts prepared from factor-depleted K562 cells. GST-fused YB-1 and AUF-1(p40) separately assembled complexes on the β-globin 3′UTR RNA that were distinct from β-complex and were effectively ablated by WT15 competitor DNA but not by its antisense control DNA (Figure 5A). Additional experiments confirmed the specific binding of AUF-1 to the G-rich motif, rather than a contiguous A/U-rich element (see supplemental Figure 8). Independent EMSA experiments using siRNA factor–depleted K562 extracts provided formal evidence that both AUF-1 and YB-1 participate in the β-complex (Figure 5B). Four replicate experiments confirmed that coordinated knockdown of both factors significantly reduced β-complex intensity, whereas independent knockdown of AUF-1 and YB-1 to < 20% of baseline only marginally affected β-complex assembly, suggesting the possibility of a redundant function. These 2 complementary analyses strongly implicate AUF-1 and YB-1 as RBPs that are integral participants of a functional β-globin mRNP complex in vivo.
AUF-1 and YB-1 assemble a β-complex in vivo. (A) AUF-1 and YB-1 bind to the β-complex target motif. EMSA analyses were conducted using a [32P]-labeled β-globin 3′UTR RNA in the presence of the GST-AUF-1(p40), GST-YB-1, control GST moiety, or K562 cytoplasmic extract. Select incubations were amended with sense (S) or antisense (AS) WT15 competitor DNA. The positions of the AUF-1 and YB-1 mRNPs are indicated by an open arrow and the β-complex by a closed arrow, respectively. (B) Double knockdown of AUF-1 and YB-1 abolishes β-complex assembly in vivo. Top: EMSA analyses were conducted on a [32P]-labeled β-globin 3′UTR RNA using cytoplasmic extracts prepared from siRNA-transfected K562β cells. An arrow indicates the β-complex. Bottom: K562β cells were transfected with specific siRNAs targeting AUF-1, YB-1, both AUF-1 and YB-1 (A/Y), or control lamin mRNAs. Cytoplasmic extracts were subsequently analyzed by immunoblot using factor-specific or control GAPDH Abs (ab).
AUF-1 and YB-1 assemble a β-complex in vivo. (A) AUF-1 and YB-1 bind to the β-complex target motif. EMSA analyses were conducted using a [32P]-labeled β-globin 3′UTR RNA in the presence of the GST-AUF-1(p40), GST-YB-1, control GST moiety, or K562 cytoplasmic extract. Select incubations were amended with sense (S) or antisense (AS) WT15 competitor DNA. The positions of the AUF-1 and YB-1 mRNPs are indicated by an open arrow and the β-complex by a closed arrow, respectively. (B) Double knockdown of AUF-1 and YB-1 abolishes β-complex assembly in vivo. Top: EMSA analyses were conducted on a [32P]-labeled β-globin 3′UTR RNA using cytoplasmic extracts prepared from siRNA-transfected K562β cells. An arrow indicates the β-complex. Bottom: K562β cells were transfected with specific siRNAs targeting AUF-1, YB-1, both AUF-1 and YB-1 (A/Y), or control lamin mRNAs. Cytoplasmic extracts were subsequently analyzed by immunoblot using factor-specific or control GAPDH Abs (ab).
YB-1 and AUF-1 are required for normal accumulation of β-globin mRNA in erythroid cells
The cytoplasmic- and differentiation-specific assembly of YB-1 and AUF-1 on the 3′UTR of β-globin mRNA suggested a likely role for the β-complex in biologically important posttranscriptional processes. To demonstrate a regulatory function, we assessed the effect of coordinate AUF-1 and YB-1 depletion on steady-state levels of β-globin mRNA in K562β cells. Whereas independent, siRNA-mediated reduction of either AUF-1 or YB-1 had little effect, coordinate knockdown of both factors produced a significant decrease in β-globin mRNA (Figure 6A). This activity appears to be specific for β-globin, because AUF-1/YB-1 double knockdown does not affect α-globin mRNA levels in the same cells (supplemental Figure 9). The parallel effects of single and coordinate factor depletion on β-complex assembly (Figure 5C) and β-globin mRNA accumulation (Figure 6A) link the structure of this mRNP to a likely posttranscriptional function.
β-globin mRNA levels are dictated by AUF-1 and YB-1 in erythroid cells, but not in nonerythroid cells that do not assemble the β-complex. (A) Coordinate depletion of AUF-1 and YB-1 decreases steady-state levels of β-globin mRNA in erythroid K562 cells. K562β cells were transfected with specific siRNAs targeting AUF-1, YB-1, both AUF-1/YB-1 (A/Y), or control lamin mRNAs. Cytoplasmic extracts were analyzed using factor-specific and control GAPDH Abs (Figure 5B). β-globin mRNA was assessed by quantitative RT-PCR relative to endogenous control β-actin mRNA. The level of β-globin mRNA in mock transfected cells was set at 100%. Bars depict the mean ± SE of 5 independent experiments. (B) β-globin mRNA levels are not significantly affected by coordinate depletion of AUF-1 and YB-1 in cells that express both factors but do not assemble a β-complex in vitro. Left: HeLaβ cells were transfected with siRNAs targeting both AUF-1 and YB-1 (A/Y) or control lamin mRNAs, and immunoblot conducted with Abs against AUF-1, YB-1, and GAPDH. Right: Levels of β-globin mRNA in siRNA-transfected HeLaβ cells were determined relative to internal control β-actin mRNA by quantitative RT-PCR. The level of β-globin mRNA in mock-transfected cells was set at 100%. Bars depict the mean ± SE of 3 independent experiments.
β-globin mRNA levels are dictated by AUF-1 and YB-1 in erythroid cells, but not in nonerythroid cells that do not assemble the β-complex. (A) Coordinate depletion of AUF-1 and YB-1 decreases steady-state levels of β-globin mRNA in erythroid K562 cells. K562β cells were transfected with specific siRNAs targeting AUF-1, YB-1, both AUF-1/YB-1 (A/Y), or control lamin mRNAs. Cytoplasmic extracts were analyzed using factor-specific and control GAPDH Abs (Figure 5B). β-globin mRNA was assessed by quantitative RT-PCR relative to endogenous control β-actin mRNA. The level of β-globin mRNA in mock transfected cells was set at 100%. Bars depict the mean ± SE of 5 independent experiments. (B) β-globin mRNA levels are not significantly affected by coordinate depletion of AUF-1 and YB-1 in cells that express both factors but do not assemble a β-complex in vitro. Left: HeLaβ cells were transfected with siRNAs targeting both AUF-1 and YB-1 (A/Y) or control lamin mRNAs, and immunoblot conducted with Abs against AUF-1, YB-1, and GAPDH. Right: Levels of β-globin mRNA in siRNA-transfected HeLaβ cells were determined relative to internal control β-actin mRNA by quantitative RT-PCR. The level of β-globin mRNA in mock-transfected cells was set at 100%. Bars depict the mean ± SE of 3 independent experiments.
We speculated that coordinate depletion of AUF-1 and YB-1 would have little effect on steady-state levels of β-globin mRNA in nonerythroid cells that fail to support assembly of a β-complex in vitro. This hypothesis was tested by siRNA knockdown analyses in HeLaβ cells (engineered to stably overexpress β-globin mRNA) that express both AUF-1 and YB-1 but do not support assembly of the β-complex (see supplemental Results). The successful depletion of AUF-1 and YB-1 in these cells (Figure 6B left panel) had no effect on the steady-state levels of β-globin RNA (Figure 6B right panel). The differential functional effects of coordinate AUF-1/YB-1 knockdown on the levels of β-globin mRNA in K562 and HeLa cell lines, which parallel the efficiency with which the 2 cell lines assemble the mRNP β-complex, demonstrate a clear posttranscriptional function for these trans-acting factors that is central to normal erythropoiesis.
Discussion
Although commonly cited as a paradigm for cell-specific posttranscriptional regulation, the molecular mechanism underlying the high stability of β-globin mRNA during normal erythropoiesis is poorly understood. Several RBPs that regulate β-globin mRNA in the erythroid nucleus10-12 cannot account for the protracted, high levels of β-globin mRNA observed in the cytoplasm of anucleate, but translationally active, erythroid precursors. The results of the present study identify a novel erythroid- and cytoplasm-restricted mRNP β-complex (Figures 1–2) that assembles on a region of 3′UTR that has been implicated previously as a determinant of β-globin mRNA stability (Figure 3)10 and specifies the mRNA-binding regulatory factors AUF-1 and YB-1 as its principal trans-acting components (Figures 4–5). Subsequent experiments validated the functional importance of the β-complex to the high-level accumulation of β-globin mRNA during normal erythropoiesis (Figures 2 and 6). Parallel analyses conducted in primary human cells demonstrated the appearance of the β-complex at a stage of differentiation when erythroid cells have been transcriptionally silenced (Figure 1), and indicate that assembly of the β-complex is restricted to hematopoietic stem cells that are committed to erythroid differentiation (Figure 2). These observations are consistent with the hypothesis that the β-complex is critical to posttranscriptional regulation of β-globin mRNA during the late stages of terminal erythroid differentiation.
Both AUF-1 and YB-1 exhibit properties that would be expected of RBPs participating in a β-globin mRNP regulatory complex. YB-1 contributes to the posttranscriptional regulation of heterogeneous genes through effects on the splicing, stability, and translation of their encoded pre-mRNAs.27,28 This 47-kDa factor preferentially binds to guanine-rich motifs,29 that resemble the CUGGG β-complex target sequence that we identified; moreover, YB-1 has been directly observed in mRNPs that assemble on globin mRNAs in rabbit reticulocyte lysate,30 which is consistent with its anticipated role in the cytoplasmic regulation of β-globin mRNA in human erythroid cells. YB-1 also decreases during in vitro differentiation of erythroid progenitor cells31 and is essential to normal erythroid development in embryonic mice,32 suggesting that the β-globin mRNA–regulating properties of YB-1 may be part of a more comprehensive program for managing gene expression during normal erythropoiesis.
AUF-1 plays a similarly critical role in regulating the expression of a large number of nonglobin mRNAs. Many studies describe the mRNA-destabilizing characteristics of AUF-1, which binds AU-rich elements in the 3′UTRs of short-lived mRNAs encoding cytokines, lymphokines, and other growth factors (reviewed in Khabar33 ). AUF-1, though, is also reported to bind with high affinity to UUAGGG motifs34 and has been specifically implicated in the posttranscriptional regulation of α-globin mRNA, where it may act coordinately with the mRNA-stabilizing factor αCP.35 These studies illustrate characteristics of YB-1 and AUF-1 that are consistent with their proposed roles in the posttranscriptional regulation of cytoplasmic β-globin mRNA after commitment of CD34+ cells to the erythroid lineage.
Our functional studies demonstrate that the β-complex is required for the constitutively high levels of β-globin mRNA in erythroid cells (Figures 2 and 6). Whereas we do not formally demonstrate that the β-complex affects the stability of cytoplasmic β-globin mRNA, the subcellular location of the β-complex combined with its demonstrated importance to the accumulation of β-globin mRNA in vivo would be most consistent with this regulatory property. This proposed activity would also agree with previous studies demonstrating increases in the stabilities of heterologous mRNAs that bind AUF-1 or YB-1 to their 3′UTRs.36,37 Considering the high constitutive stability of β-globin mRNA4 and the relatively short experimental interval in our siRNA-depletion analyses, the observed decrease in β-globin mRNA levels after coordinate knockdown of AUF-1 and YB-1 may significantly underestimate the true magnitude of this functionality.
We speculate that YB-1 and AUF-1 may increase levels of β-globin mRNA by facilitating the cytoplasmic interaction between PABP and its polyadenylate tail; both AUF-1 and YB-1 have been demonstrated to complex with PABP under defined cytoplasmic conditions.38,39 This mechanism would be anticipated to enhance the polyadenylation of β-globin mRNA and, consequently, prolong its cytoplasmic half-life. In fact, a similar process appears to ensure to the high stability of human α-globin mRNA in erythroid cells, where 3′UTR-bound αCP facilitates the binding of PABP, promoting a protective effect on the integrity of the α-globin mRNA polyadenylate tail.7,40 Similar events would be particularly important to prolonging the cytoplasmic half-life of β-globin mRNA, because de-adenylation appears to be the rate-limiting step in its decay.17
Alternatively, AUF-1 and YB-1 may simply enhance levels of β-globin mRNA by protecting a specific 3′UTR site from endonucleolytic attack. This mechanism would mirror one reported activity of αCP, in which site-specific binding obscures a defined cis-element in the related α-globin 3′UTR that is otherwise targeted by an erythroid-specific endoribonuclease.8 Earlier studies showing that β-globin mRNA can be cleaved at AU-rich sites in the 3′-terminal end of the mRNA41 are consistent with a model in which AUF-1 and/or YB-1 act as steric barriers to endoribonuclease access.
Whereas AUF-1 and YB-1 are expressed in a large number of tissues,42,43 their abilities to bind to the β-globin 3′UTR and to exert a corresponding functional effect during hematopoiesis appears to be restricted to cells that are committed to erythroid differentiation. This conclusion is consistent with previous analyses indicating that the half-life of β-globin mRNA is higher in erythroid cells than in nonerythroid cells.44 Moreover, the cell specificity of this process suggests that isoforms of AUF-1 and YB-1 that bind β-globin mRNA may be differentially distributed in cells that do or do not assemble the β-complex. Precedent studies have shown that AUF-1 and YB-1 exhibit significant structural heterogeneities resulting both from alternate splicing of pre-mRNA and from posttranslational modifications.45-47 In addition, patterns of protein phosphorylation appear to dictate the nucleic-acid binding characteristics of both factors,47,48 as well as the distribution of YB-1 among subcellular compartments.46 Our observations that the β-complex preferentially incorporates AUF-1(p40) (Figure 4B) and that expression of this isoform is up-regulated during erythroid differentiation (Figure 4C) are consistent with this hypothesis, suggesting a critical role for isoform specificity in the cell- and compartment-restricted properties of the β-complex that warrants future research.
Finally, the composition of the β-complex is distinct from the αCP-containing mRNA-stability complex that assembles in erythroid cytoplasm on the 3′UTR of α-globin mRNA. The derivation of both the α- and β-globin genes from a common ancestral gene,49 as well as reports of interactions between αCP and the β-globin mRNA,11 have both fed speculation that the 2 mRNAs might be posttranscriptionally coregulated. Our inability to identify αCP in the β-complex either by EMSA supershift analysis or by mass spectroscopy recapitulates seminal studies on the mRNA specificity of the α-complex,6 and strongly indicates that activities of posttranscriptional events for α- and β-globin mRNAs are mechanistically distinct. If confirmed, this arrangement would allow for separate manipulation of α- and β-globin levels through targeting of posttranscriptional regulatory events, which would be of clear therapeutic interest for managing thalassemias and other disorders of globin gene expression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. A. Liebhaber (University of Pennsylvania) and O. Abdulmalik (The Children's Hospital of Philadelphia) for critical reading of the manuscript; S. A. Liebhaber, A. B. Shyu (University of Texas), and R. J. Schneider (New York University) for sharing critical reagents; D. M. Frank (University of Pennsylvania) for morphologic analyses of differentiated CD34+ cells; and the University of Pennsylvania Proteomics Core Facility for mass spectroscopic analyses.
This work was supported in part by a research fellowship from the Cooley's Anemia Foundation (to S.v.Z.) and by National Institutes of Health grants HL082754 and HL61399 (both to J.E.R.).
National Institutes of Health
Authorship
Contribution: S.v.Z. designed the study, performed the experiments, and wrote the manuscript; G.R.J. and E.O.H. designed the study and performed the experiments; J.E.R. designed the study and wrote the manuscript; and all authors reviewed the manuscript and approved its content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Eric Russell, MD, The Children's Hospital of Philadelphia, Abramson Research Bldg, Rm 310-D, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: jeruss@mail.med.upenn.edu.

![Figure 1. A novel cytoplasm-specific mRNP assembles on the 3′UTR of β-globin mRNA. (A) Site-specific assembly of a cytoplasm-restricted β-complex in vitro. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with nuclear (Nuc) or cytoplasmic (Cyt) extract prepared from K562 cells, and RNase T1-resistant mRNPs resolved on a native polyacrylamide gel. Parallel reactions were supplemented with a 15-nt competitor DNA (WT15) corresponding to a previously identified regulatory region of the β-globin 3′UTR (panel B), either in the sense (S) or control antisense (AS) orientation. An arrow indicates the β-complex. (B) Composition of the 132-nt β-globin 3′UTR. The positions of the 15-nt regulatory region (WT15), the translation stop codon (TAA), and the poly(A) tail are indicated. (C) The β-complex assembles in erythroid-induced CD34+ cells. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from K562 cells, uninduced human CD34+ cells (U; day 5), or erythroid-induced CD34+ cells (I; day 17). Reactions were amended with WT15 competitor DNA in either the sense (S) or antisense (AS) orientation. An arrow indicates the β-complex. (D) The β-complex is restricted to the cytoplasm of erythroid precursor cells. EMSA analyses were conducted on a [32P]-labeled RNA using nuclear and cytoplasmic extracts prepared from erythroid-induced CD34+ cells after 12 days of culture. Sense (S) or antisense (AS) WT15 DNA competitor was added as indicated. An arrow denotes the β-complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/4/10.1182_blood-2011-10-387316/4/m_zh89991185090001.jpeg?Expires=1767698847&Signature=R~vnfnZJlqEjkaMN~ovS69rZRYwEKddkoAwFWa~LROATF-GoMBxqL2An~FsR2s-ypZvK5LERSuQEYVlM8jB4MbNt6D6BQ03v7m2qNRdyhmGBmCuaQeu~3FyH9Yd~RWYUge3h3InHYFjk35I~1APvQhZ5Sy08rafj1mbSdZHDKAwOnCMydhHSybPPTJeKXFxZTxkvNB~-uHlk6AQ~HiTLlV0BqG8Qm0tJCkK46-Y60TCrbjPLUuQWnS2k-h1s-X26eurRx709Rn88OMBiEcFRW~nWFpy832oXPklaHSjBSIfKkFTqgslxb3ZIf7LlrKYKZ9YC8rnQSRPQhLLE7alI3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. β-complex assembly is restricted to cells induced to erythroid differentiation. (A) The β-complex assembles in CD34+ cells induced to erythroid, but not to granulocytic/monocytic differentiation. A [32P]-labeled RNA, corresponding to the polyadenylated human β-globin 3′UTR, was incubated with cytoplasmic extract prepared from human UBC CD34+ cells that were induced along either the erythroid (Ery) or the GM lineages. GAPDH and β-globin immunoblots of extracts used in EMSA analyses control for extract integrity and erythroid differentiation, respectively. (B) β-complex assembly is reduced in K562 cells induced to megakaryocytic differentiation. β-complex formation was assessed in cytoplasmic extracts prepared from untreated, DMSO-treated, and PMA-induced K562 cells. The integrity of the extracts was validated by an immunoblot using control β-actin Ab. (C) Steady-state β-globin mRNA levels are reduced in PMA-induced K562 cells. Levels of β-globin mRNA were assessed in K562β cells relative to endogenous control β-actin mRNA using quantitative RT-PCR. The level of β-globin mRNA in untreated (−) cells was set as 100%. Bars depict the mean of 5 independent experiments; error bars indicate 1 SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/4/10.1182_blood-2011-10-387316/4/m_zh89991185090002.jpeg?Expires=1767698847&Signature=dpYYZLmfMXD8nB6ZuRuJ9LbrfJB4bzVPqB4LevjlFXRSzTHJV5F0uunel76r7Xr5M5QIEgISKLgs7Nsksto5vBcrIEHKev9i8iRF4D6cKEgJR-lEM37EvpRucgzWOb6Zhs8X4yzdGaO0HY44GfIzYJxmjF7vVJAyh2ra0~mxs-eWq-zNCbMHa9WRMNycblBntYNBQ32daMuFZ4p3Wwp8h5m7tXkk6mt7fH8iDuCE1EcJ31cnHBvxrBJX17H7JpJ5wHrnrwYjVJkWtbe-O30fvzxbNwUbqPoMsr0srxC~qaqS6dIT3~N2OzLxHAm6EuYCt0whcmb7-qRkwKtfLDZFBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. The β-complex assembles on a site-specific sequence within the β-globin 3′UTR. (A) A guanine-rich region of RNA is critical for β-complex assembly. Top: EMSA analyses were conducted with K562 cytoplasmic extracts using [32P]-labeled RNA corresponding to the full-length β-globin 3′UTR. Reactions were supplemented with competitor DNAs derived from the WT15 sequence. The position of the β-complex is indicated by an arrow. Bottom: Sequences of competitor DNAs, with substituted nucleotides indicated in lower case. The corresponding region of the native β-globin 3′UTR is included for reference. (B) Site-specific mutations in the β-globin 3′UTR ablate β-complex assembly. EMSA analyses were conducted on [32P]-labeled RNAs, corresponding to full-length wild-type and derivative β-globin 3′UTRs, using K562 cytoplasmic extract. The G-rich motif is indicated, with the specific mutations in the full-length β-globin 3′UTR depicted in lower-case type. The position of the β-complex is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/4/10.1182_blood-2011-10-387316/4/m_zh89991185090003.jpeg?Expires=1767698847&Signature=ohAy6f7ZP0OldiJDuGGSm1V1Bem8LGCD2YbKeee8UvU6XGZO72Arz5KoBxZbuLxEhXa0BrINLzD6FoNnMFZQ3JQgLdt5sLiygPD0QXqTyfreCaZRSMmmteZ58LyVvbiEMxVeHykUysHsVxccKmDuwl3aDo0l7LfFUZEaNFMuInc5Lbzsgj7e20b7Y2svBxcbAwu2G2pe4ncoyDN21FQfhtfD3i7bS4K3McGxihWW1~rfo-QT6j1QyjMqWxzpDLWZnRl6waGzMbCSF7aEbhVONc0yx5fh1nK01~s1dkT8uw68i1b20lG5ur6dN9GQRjWQSC8Qdo-0rvcae6X51-rRzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. AUF-1 and YB-1 assemble a β-complex in vivo. (A) AUF-1 and YB-1 bind to the β-complex target motif. EMSA analyses were conducted using a [32P]-labeled β-globin 3′UTR RNA in the presence of the GST-AUF-1(p40), GST-YB-1, control GST moiety, or K562 cytoplasmic extract. Select incubations were amended with sense (S) or antisense (AS) WT15 competitor DNA. The positions of the AUF-1 and YB-1 mRNPs are indicated by an open arrow and the β-complex by a closed arrow, respectively. (B) Double knockdown of AUF-1 and YB-1 abolishes β-complex assembly in vivo. Top: EMSA analyses were conducted on a [32P]-labeled β-globin 3′UTR RNA using cytoplasmic extracts prepared from siRNA-transfected K562β cells. An arrow indicates the β-complex. Bottom: K562β cells were transfected with specific siRNAs targeting AUF-1, YB-1, both AUF-1 and YB-1 (A/Y), or control lamin mRNAs. Cytoplasmic extracts were subsequently analyzed by immunoblot using factor-specific or control GAPDH Abs (ab).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/4/10.1182_blood-2011-10-387316/4/m_zh89991185090005.jpeg?Expires=1767698847&Signature=plOZncWV839sJqvIiOMIy5loo9VJSDw4pc2ep7rEikym~n~PTP8uSpS6lfgj9dB7YZ~Kvy2S0GLsfkkaUcuN2ZFfzyYg5FyxJ~zcyQB5Yt1Qza--IowwFaD2zFn-UqFf4hvMB61X5r47TubGXpMo1NrMTirYPmjLAmYElYllUtX8g-~Mt8iA~rhy3eWtjrL-J5DgHkk6AEcVzyQJLhj0xED2vBe1-Z4ElNPGGr3qrGGQAH3~CqFOGz-uj7VgX3ndIIND-ZDCgGycCEX~C~Ak7EfUG6wAMVX9dQZnPGvJHFsv8kfLEGdi-Tgsn2gv0kYZq6ehmTPbp7KXhJnKY6ykyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal