In this issue of Blood, Pleines and colleagues show how deletion of the GTPase family member RhoA in murine megakaryocytes/platelets induces macrothrombocytopenia but also protects against occlusive thrombosis or cerebral infarction, providing new insights into both RhoA function as well as platelet-related diseases.1
This multifaceted study of RhoA function in mouse platelets—by conditional gene knockout in megakaryocytes—is pertinent to the production of platelets in the marrow, their survival in the circulation, the hemostatic quality of the platelets in response to prothrombotic stimuli, and their effectiveness in thrombus formation in vivo. These experiments are topical in clinical hematology, because in certain congenital disorders, immune thrombocytopenia, or myeloproliferative disorders, all of which affect platelet number, size, shape and/or thrombotic propensity, the platelet count does not reflect the risk of bleeding, and there is no reliable means for distinguishing production versus destruction defects. Many of the molecular players involved in platelet function are known (see figure), but their precise role in the overlapping pathways leading to thrombus formation in response to different stimuli is uncertain. In addition, proteins such as RhoA can also regulate both platelet production and function.
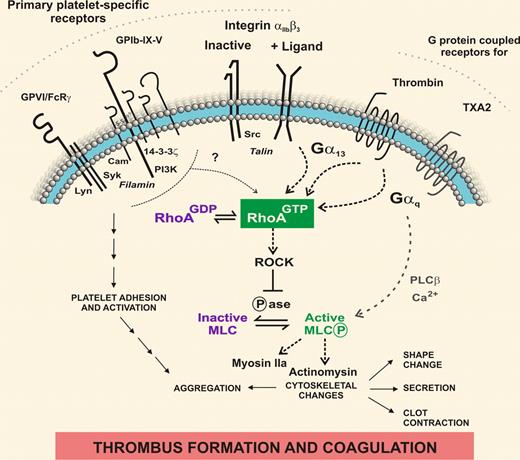
RhoA inside platelets. In response to vascular damage, shear stress, or other prothrombotic factors, the coordinated activation of intracellular signaling proteins by primary adhesion/activation and G protein–coupled receptors, as well as bidirectional signaling by unligated or ligand-bound αIIbβ3, exquisitely controls complex platelet functional responses and ultimately, prothrombotic and procoagulant activity. Pleines et al used megakaryocyte-targeted deletion in mice to identify functional roles for RhoA in platelets in vitro and in vivo.1 Defining how receptor-associated signaling/cytoskeletal proteins are localized and up- or down-regulated over time as platelets are activated to form a thrombus will increase understanding of platelet-related defects where dysregulation can affect platelet size, shape, number and/or quality and lead to bleeding or thrombosis.
RhoA inside platelets. In response to vascular damage, shear stress, or other prothrombotic factors, the coordinated activation of intracellular signaling proteins by primary adhesion/activation and G protein–coupled receptors, as well as bidirectional signaling by unligated or ligand-bound αIIbβ3, exquisitely controls complex platelet functional responses and ultimately, prothrombotic and procoagulant activity. Pleines et al used megakaryocyte-targeted deletion in mice to identify functional roles for RhoA in platelets in vitro and in vivo.1 Defining how receptor-associated signaling/cytoskeletal proteins are localized and up- or down-regulated over time as platelets are activated to form a thrombus will increase understanding of platelet-related defects where dysregulation can affect platelet size, shape, number and/or quality and lead to bleeding or thrombosis.
Rho GTPases are ubiquitously expressed and impact virtually all areas of cell biology.2 Members of this family are characteristically activated by conversion of bound GDP (inactive complex) to GTP (active complex). Abnormalities of Rho GTPases are implicated in human immunohematopoietic diseases and in leukemia/lymphoma.3 Expressly relevant to platelet signaling, activation of RhoA occurs downstream of G protein–coupled receptors associated with Gα13 or Gαq,4 in particular, platelet receptors for thrombin or thromboxane A2. In addition, the active form of the major platelet fibrinogen receptor αIIbβ3, also directly interacts with Gα13.5 Thus, in platelets activated by thrombin or other agonists, time-dependent activation of αIIbβ3 can lead to second-phase activation of RhoA as Gα13 becomes associated with ligand-bound αIIbβ3, with the potential to regulate αIIbβ3-dependent clot contraction and other downstream events. One pathway for propagation of RhoA signaling is to enable the effector protein ROCK to inhibit myosin light chain (MLC) phosphatase, promoting phosphorylation of MLC and MLC-mediated activation of actomyosin involved in regulating cytoskeletal changes; Gαq-coupled receptors can also activate MLC, independently of RhoA, via Ca2+-dependent activation of MLC kinase. Interestingly, MLC also regulates myosin IIa, a protein encoded by the myosin heavy chain 9 (MYH9) gene associated with May-Hegglin anomaly,6 an inherited condition typified by macrothrombocytopenia and with a variable bleeding risk in humans,7 and normal aggregation and secretion but impaired αIIbβ3-dependent contraction and a dramatic hemostatic defect in MYH9-inactivated mouse platelets.8
In the present study, RhoA depletion increased the number/ploidy of megakaryocytes; consistent with defective platelet production, the blood platelet count was about half the normal level, and platelets displayed an altered shape and demonstrably increased size (∼ 25% bigger platelets). However, the lifespan of RhoA-deficient platelets in the circulation was near-normal, indicating no increased clearance or fragility and at least partial nonredundancy among Rho family members.1 For agonists acting at Gα13/Gαq-coupled receptors, RhoA deficiency resulted in defective platelet shape change, and impaired secretion from both α-granules (that release von Willebrand factor and express P-selectin) and dense granules (that release ADP, procoagulant polyphosphates, and other factors). Activated αIIbβ3 was able to bind ligand, but αIIbβ3-mediated clot contraction was virtually absent.1 Notably, adhesion and activation pathways for primary platelet collagen (GPVI/FcRγ) and von Willebrand factor (GPIbα of the GPIb-IX-V complex) leading to αIIbβ3 activation were also at least partially dependent on functional RhoA, because platelet adhesion ex vivo to a collagen-coated surface or strength of interaction with immobilized von Willebrand factor at a high physiologic or pathologic shear rate (∼ 7700 s−1) was reduced. Previous studies using RhoA inhibitor similarly showed a requirement for RhoA in stabilizing αIIbβ3 contacts at high shear.9 Furthermore, RhoA-deficient platelets showed a hemostatic defect in vivo manifest as increased tail-bleeding times, and failure to form occlusive thrombi in vascular injury models.1 Significantly, mice with RhoA-deficient platelets were also protected from brain injury caused by thrombus formation in a model of cerebral stroke, while retaining higher neurologic competency in a postprocedure foot-grip test. What remains to be determined is the mechanistic link between the presence of functional RhoA and either the prevention of bleeding or the capacity to form stable occlusive thrombi under shear conditions. Determining how RhoA contributes to receptor signaling, αIIbβ3-dependent clot contraction, α- or dense-granule secretion, and/or thrombus stability under shear conditions will be important to relate RhoA activation to these functional consequences, and ultimately exploit these findings in human disease.
In light of the variability in clinical bleeding associated with macrothrombocytopenia,7 it is interesting to compare the platelet functional defect in RhoA-deficient macrothrombocytopenic mice with alternative models of thrombocytopenia. In humans or mice, the extent to which bleeding or thrombotic defects are attributable to aberrant platelet number, size, or function is a complex yet important issue. In this regard, to support a specific functional role for RhoA, and help rule out effects due to differences in platelet size or number compared with wild-type control mice, Pleines et al used platelets from thrombocytopenic mice generated using antiplatelet antibody, with similar platelet counts as RhoA-deficient mice (∼ 50% of normal).1 Surprisingly, the immune-depleted platelets were also larger than normal platelets, and actually comparable in size to the RhoA-deficient mice. These thrombocytopenic control mice, however, showed essentially normal thrombus occlusion times, not only providing ideal control platelets (matched for number and size) supporting a specific functional role for RhoA, but also raising additional mechanistic questions about the effect of immune-clearance on platelet size. What is clear is that despite no gross abnormalities in surface levels of GPIb-IX-V, GPVI, αIIbβ3 and other receptors, which could potentially explain the functional defects, RhoA-deficient platelets were severely hemostatically compromised.
In conclusion, these far-reaching findings suggesting that RhoA is required for normal hemostasis with deficiency resulting in increased bleeding time, as well as an impressive protective effect of RhoA deficiency on arterial thrombosis or the damage caused by ischemic stroke. Excitingly, these outcomes raise the possibility of therapeutically targeting RhoA without increasing bleeding risk. While new small molecule and other inhibitors of RhoA activity are being actively developed,10 further understanding of how RhoA/ROCK are localized within the receptor-proximal intracellular region of platelets, and identification of key interactive sites, could eventually allow selective targeting of aspects of the pathways relevant to thrombus stability at high shear rates. Further studies are needed to better define connections between platelet-specific receptors and signaling/cytoskeletal proteins linking various prothrombotic stimuli to functional outcomes in mice and humans.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal