Abstract
Vascular injury initiates rapid platelet activation that is critical for hemostasis, but it also may cause thrombotic diseases, such as myocardial infarction or ischemic stroke. Reorganizations of the platelet cytoskeleton are crucial for platelet shape change and secretion and are thought to involve activation of the small GTPase RhoA. In this study, we analyzed the in vitro and in vivo consequences of megakaryocyte- and platelet-specific RhoA gene deletion in mice. We found a pronounced macrothrombocytopenia in RhoA-deficient mice, with platelet counts of approximately half that of wild-type controls. The mutant cells displayed an altered shape but only a moderately reduced life span. Shape change of RhoA-deficient platelets in response to G13-coupled agonists was abolished, and it was impaired in response to Gq stimulation. Similarly, RhoA was required for efficient secretion of α and dense granules downstream of G13 and Gq. Furthermore, RhoA was essential for integrin-mediated clot retraction but not for actomyosin rearrangements and spreading of activated platelets on fibrinogen. In vivo, RhoA deficiency resulted in markedly prolonged tail bleeding times but also significant protection in different models of arterial thrombosis and in a model of ischemic stroke. Together, these results establish RhoA as an important regulator of platelet function in thrombosis and hemostasis.
Introduction
Platelet activation on the injured vessel wall is induced by multiple signaling pathways and leads to extensive cytoskeletal rearrangements that are crucial for conversion from discoid to spheric shape, granule secretion, spreading, and clot retraction. Many of these processes are thought to involve proteins of the Rho family of small GTPases, such as RhoA, Rac1, and Cdc42.1
Like most Rho GTPases, RhoA (∼ 23 kDa) is active when bound to GTP, whereas the protein is inactive in the GDP-bound state. The active state enables RhoA to bind effector molecules such as Rho-associated, coiled-coil–containing protein kinase (ROCK) and mammalian diaphanous homologue (mDia). RhoA plays a central role in the organization of the actin cytoskeleton in various cell types, mainly through its ability to form stress fibers and to regulate actomyosin contractility.2 In addition, RhoA has been implicated in numerous further cellular processes, such as cytokinesis, regulation of microtubule dynamics, and the formation of focal adhesions.3,4
Platelet activation by soluble agonists, such as adenosine diphosphate (ADP), thromboxane A2 (TxA2), or thrombin, induces signaling through receptors coupled to heterotrimeric G proteins (Gq, Gi, and G13).5 Studies on mice deficient in the G protein subunits Gα136 and Gαq,7-9 as well as inhibitor studies using human platelets10-14 demonstrated a critical involvement of RhoA in the induction of platelet shape change downstream of Gα13 after stimulation with low doses of the TxA2 analog U46619 or thrombin. In detail, RhoA-mediated activation of ROCK and subsequent inhibition of myosin light chain (MLC) phosphatase induces MLC phosphorylation, leading to actomyosin modulation and conversion from discoid to spheric platelet shape. In addition to the G13-RhoA-ROCK pathway, MLC phosphorylation is thought to be achieved by stimulation of Gq and subsequent phospholipase (PL) Cβ activation, leading to Ca2+-mediated stimulation of MLC kinase.
In contrast to its function in platelet shape change, the role of RhoA for integrin regulation and degranulation is less well defined. Whereas most studies revealed unaltered inside-out activation of the main platelet integrin αIIbβ3 on inhibition of RhoA, several reports suggested a role of the GTPase for αIIbβ3-dependent outside-in signaling important for irreversible aggregation, clot retraction, and sustained platelet adhesion under high shear in vitro.6,15-17 Interestingly, a discrete spatiotemporal regulation of RhoA in platelets was recently proposed, involving RhoA activation during the initial phase of platelet activation, its inhibition during the spreading process, and its reactivation in the late phase of activation during clot retraction.18
Despite the numerous studies addressing the role of RhoA in platelets, the use of inhibitors acting on Rho and ROCK or of mice deficient in proteins upstream of the GTPase may cause misleading results.19 Here, we assessed the role of RhoA for platelet function in vitro and in vivo using mice in which RhoA deficiency is restricted to megakaryocytes and platelets. We show that RhoA is involved in different cellular responses downstream of G13- and Gq-coupled agonist receptors and integrin αIIbβ3 that critically contribute to platelet function in hemostasis and experimental thrombotic diseases.
Methods
Animals
Mice in which exon 3 of the RhoA gene is flanked by loxP sites (RhoAfl)20 were crossed with platelet factor (PF) 4 Cre mice21 resulting in RhoAfl/fl,PF4-Cre (hereafter named RhoA−/−) mice. Littermates (RhoAfl/fl, hereafter named wild type) served as controls. Mice were maintained on a SV/129/C57/Bl-6 background. If not stated otherwise, 8- to 12-week-old mice of mixed gender were used for all experiments. Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken).
Chemicals and reagents
The anesthetic drugs medetomidine (Pfizer), midazolam (Roche Pharma), and fentanyl (Janssen-Cilag) were used according to the regulation of the local authorities. ADP, phorbol 12-myristate 13-acetate, PAR-4 peptide (Thermo Fisher Scientific); high-molecular-weight heparin, phalloidin-FITC, human fibrinogen (Sigma-Aldrich); α-thrombin (Roche Diagnostics); anti-RhoA antibody (Cytoskeleton); anti-MLC, anti–phosphoSer19-MLC antibodies (Cell Signaling Technology); anti–mouse-IgG HRP, anti–rabbit-IgG HRP (Dako Deutschland); and apyrase type III (GE Healthcare) were purchased from the indicated sources. Collagen-related peptide was generated as described previously.22 The antibody against the activated form of integrin αIIbβ3 (JON/A-PE) was from Emfret Analytics. All other antibodies were generated and modified in our laboratory as described previously.23
Platelet preparation, spreading, and aggregometry
Platelet-rich plasma (prp) and washed platelets were prepared as described recently.24 For spreading experiments, coverslips were coated with 200 μg of human fibrinogen and blocked with 1% BSA. Washed platelets (100 μL with 0.03 × 106 platelets/μL) were added and incubated at room temperature for the indicated times. Platelets were visualized with an Axiovert 200 inverted microscope (×100/1.4 NA oil objective; Carl Zeiss). Platelet aggregation was measured on a Fibrintimer 4 channel aggregometer (APACT Laborgeräte und Analysensysteme) using prp or washed platelets as described previously.24
Electron microscopy
For transmission electron microscopy, platelets were fixed with 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.2, and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and examined under a CM120 transmission electron microscope (Carl Zeiss). For scanning electron microscopy, platelets were fixed with 2.5% glutaraldehyde. Platelets in suspension were allowed to adhere to poly-l-lysine–coated coverslips. Samples were dehydrated, air-dried, sputtered with gold, and examined under a scanning electron microscope (Carl Zeiss).
Flow cytometry and Western blot analysis
Flow cytometric measurements were performed as described previously.24 For Western blot analysis, blotted platelet lysates were probed with appropriate primary and secondary HRP-coupled antibodies. Proteins were visualized by ECL.
Platelet adhesion on collagen and VWF under flow conditions
Coverslips were coated with 0.25 mg/mL fibrillar type I collagen (Nycomed) and blocked with 1% BSA. Perfusion of heparinized whole blood was performed as described previously.25 To study platelet adhesion to VWF, coverslips were coated with anti–human VWF antibody (Dako Deutschland), followed by blocking with BSA and addition of murine platelet-poor plasma for 2 hours at 37°C. Perfusion of heparinized whole blood was carried out as described previously.26 Phase-contrast pictures were analyzed using MetaMorph software (Visitron).
Determination of platelet life span
Mice were injected intravenously with Dylight-488–conjugated anti-GPIX Ig derivative (0.5 μg/g body weight), and the platelet life span was determined as described previously.24
Bleeding time
Mice were anesthetized, 1 mm of the tail tip was cut off, and the tails were immersed in 0.9% isotonic saline at 37°C. The time until stop of bleeding (no blood flow for 1 minute) was determined.
Intravital microscopy of thrombus formation in FeCl3-injured mesenteric arterioles
Intravital microscopy was performed as described previously.27 In brief, injury of mesenteric arterioles was induced by topical application of 20% FeCl3. Adhesion and aggregation of fluorescently labeled platelets in arterioles was monitored for 40 minutes or until complete occlusion occurred (blood flow stopped for > 2 minutes).
Mechanical injury of the abdominal aorta
The abdominal cavity of anesthetized mice was opened to expose the abdominal aorta. An ultrasonic flowprobe (0.5PSB699; Transonic Systems) was placed around the vessel, and thrombus formation was induced by a single firm compression with a forceps upstream of the flowprobe. Blood flow was monitored for 30 minutes.
tMCAO model
Transient middle cerebral artery occlusion (tMCAO) was performed as described previously.25 In brief, a thread was advanced through the carotid artery into the middle cerebral artery to reduce cerebral blood flow and removed after 1 hour to allow reperfusion. The extent of infarction was quantitatively assessed 24 hours after reperfusion on 2,3,5-triphenyltetrazolium chloride–stained brain sections. Global neurologic status was scored according to Bederson et al. Motor function and coordination were graded using the grip test.
Data analysis
Results are shown as mean ± SD from 3 individual experiments per group, unless indicated otherwise. Statistical analysis between wild-type and RhoA−/− groups were assessed by the Welch t test. P values < .05 are considered statistically significant.
Results
RhoA−/− mice display macrothrombocytopenia
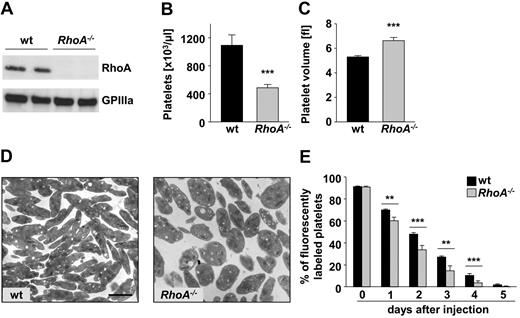
Mice in which exon 3 of the RhoA gene was flanked by loxP sites20 were crossed with PF 4 Cre mice,21 leading to efficient loss of RhoA protein in platelets of the resulting RhoA−/− mice (Figure 1A). RhoA deficiency resulted in pronounced macrothrombocytopenia, with a reduction of platelet counts by ∼ 50% (P < .001; Figure 1B) and an increase in platelet volume by ∼ 25% in RhoA−/− compared with wild-type mice (P < .001; Figure 1C). Transmission electron microscopy illustrated the increased size and revealed a more roundish shape of RhoA−/− platelets compared with the discoid-shaped wild-type platelets (Figure 1D).
RhoA−/− mice display a distinct macrothrombocytopenia but only moderately reduced platelet life span. (A) Analysis of RhoA expression in wild-type (wt) and RhoA−/− platelets by Western blot. Expression of GPIIIa was used as loading control. Peripheral platelet counts (B) and platelet volume (C) of wt and RhoA−/− mice measured with a blood cell counter are depicted. Data are presented as mean ± SD of 8 mice per group and are representative of 3 individual measurements. Fl indicates femtoliter. (D) Representative transmission electron microscopy pictures of resting wt and RhoA−/− platelets. Scale bar represents 2 μm. (E) Determination of the platelet life span in wt and RhoA−/− mice. Mice were injected with a DyLight 488–conjugated anti-GPIX Ig derivate (0.5 μg/g body weight) to label platelets in vivo. Results are percentage of fluorescently labeled platelets at the indicated days after injection as determined by flow cytometry. Values are mean ± SD of 5 mice per group (**P < .01; ***P < .001).
RhoA−/− mice display a distinct macrothrombocytopenia but only moderately reduced platelet life span. (A) Analysis of RhoA expression in wild-type (wt) and RhoA−/− platelets by Western blot. Expression of GPIIIa was used as loading control. Peripheral platelet counts (B) and platelet volume (C) of wt and RhoA−/− mice measured with a blood cell counter are depicted. Data are presented as mean ± SD of 8 mice per group and are representative of 3 individual measurements. Fl indicates femtoliter. (D) Representative transmission electron microscopy pictures of resting wt and RhoA−/− platelets. Scale bar represents 2 μm. (E) Determination of the platelet life span in wt and RhoA−/− mice. Mice were injected with a DyLight 488–conjugated anti-GPIX Ig derivate (0.5 μg/g body weight) to label platelets in vivo. Results are percentage of fluorescently labeled platelets at the indicated days after injection as determined by flow cytometry. Values are mean ± SD of 5 mice per group (**P < .01; ***P < .001).
Despite the altered shape, the subcellular morphology was similar in RhoA−/− and wild-type platelets (data not shown). Flow cytometric measurement of major platelet surface receptors revealed a significant (12%-30%) increase in mean fluorescence intensities for CLEC-2 and integrins β1 and αIIbβ3 in RhoA−/− compared with wild-type platelets (Table 1). This may be because of the increased size of the mutant platelets, whereas comparable mean fluorescence intensities in wild-type and RhoA−/− platelets for all other used glycoprotein antibodies indicated moderately reduced surface density of these glycoproteins in mutant platelets. Together, these results demonstrated markedly reduced platelet counts but only moderate changes in platelet morphology in the absence of RhoA in platelets and megakaryocytes.
Levels of platelet glycoproteins in wild-type and RhoA−/− mice
| . | Wild-type . | RhoA−/− . | P . |
|---|---|---|---|
| Mean FSC* | 326 ± 11 | 486 ± 32 | < .001 |
| GPIb | 353 ± 22 | 349 ± 14 | NS |
| GPV | 302 ± 8 | 329 ± 25 | NS |
| GPIX | 503 ± 16 | 530 ± 31 | NS |
| CD9 | 1433 ± 42 | 1441 ± 36 | NS |
| GPVI | 48 ± 5 | 56 ± 5 | NS |
| α2 | 61 ± 3 | 62 ± 1 | NS |
| β1 | 163 ± 4 | 183 ± 3 | < .001 |
| αIIbβ3 | 457 ± 10 | 590 ± 39 | < .01 |
| CLEC-2 | 155 ± 5 | 180 ± 12 | < .05 |
| . | Wild-type . | RhoA−/− . | P . |
|---|---|---|---|
| Mean FSC* | 326 ± 11 | 486 ± 32 | < .001 |
| GPIb | 353 ± 22 | 349 ± 14 | NS |
| GPV | 302 ± 8 | 329 ± 25 | NS |
| GPIX | 503 ± 16 | 530 ± 31 | NS |
| CD9 | 1433 ± 42 | 1441 ± 36 | NS |
| GPVI | 48 ± 5 | 56 ± 5 | NS |
| α2 | 61 ± 3 | 62 ± 1 | NS |
| β1 | 163 ± 4 | 183 ± 3 | < .001 |
| αIIbβ3 | 457 ± 10 | 590 ± 39 | < .01 |
| CLEC-2 | 155 ± 5 | 180 ± 12 | < .05 |
Expression of glycoproteins on the platelet surface was determined by flow cytometry. Diluted whole blood from the indicated mice was incubated with FITC-labeled antibodies at saturating concentrations for 15 minutes at room temperature, and platelets were analyzed directly. Data are expressed as mean fluorescence intensity ± SD (n = 4) and are representative of 3 individual experiments.
Mean platelet size (mean FSC) was determined by forward-scatter characteristics.
To determine whether the macrothrombocytopenia in RhoA−/− mice was caused by an increased platelet turnover, the life span of RhoA−/− platelets in vivo was determined (Figure 1E). Interestingly, however, RhoA−/− platelets displayed only a moderately reduced life span that alone does not explain the severely reduced platelet counts in the deficient animals. Analysis of bone marrow sections revealed that the number of megakaryocytes was markedly increased in RhoA−/− compared with wild-type mice, with a higher percentage of megakaryocytes displaying high ploidy (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This indicates that the macrothrombocytopenia in RhoA−/− mice represents a failure in the terminal stages of platelet production from the deficient megakaryocytes in vivo.
Defective shape change of RhoA−/− platelets after stimulation of G13 signaling
To clarify the role of RhoA for platelet shape change and aggregation, standard aggregometry was performed. At low agonist concentrations, the receptors of the TxA2 analog U46619 and thrombin mediate platelet activation predominantly by coupling to G13, whereas higher agonist concentrations induce additional signaling via Gq.9,29 Stimulation with very low doses of U46619, thrombin, and the thrombin receptor agonist PAR-4 peptide led to pronounced shape change and MLC phosphorylation in wild-type platelets, whereas these processes were abolished in RhoA−/− platelets (Figure 2A and supplemental Figure 1C-D). Notably, higher concentrations of U46619, thrombin or PAR-4 peptide induced shape change and MLC phosphorylation in RhoA−/− platelets but to a lesser extent than in the wild-type (Figure 2A and supplemental Figure 1C-D). Together, these results revealed that RhoA is indispensable for shape change induced by G13 signaling in platelets and also indicated a contribution of the GTPase in this process downstream of Gq stimulation. This hypothesis was supported by the finding that ADP also failed to mediate shape change in RhoA−/− platelets at a very low (0.025μM) concentration (Figure 2B top panel).
Defective shape change after G13 stimulation and partially reduced aggregation in RhoA−/− platelets. Washed platelets were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. ADP measurements were performed in prp. (A-B) Representative aggregation curves of 3 individual experiments and representative scanning electron microscopy pictures of wt and RhoA−/− platelets after stimulation with 0.01μM U46619 (right) are depicted. Scale bar indicates 2 μm. (C) Mean percentage of maximal aggregation ± SD of each group (**P < .01; ***P < .001). U46 indicates U46619; thr, thrombin; PAR, PAR-4 peptide; and coll, collagen.
Defective shape change after G13 stimulation and partially reduced aggregation in RhoA−/− platelets. Washed platelets were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. ADP measurements were performed in prp. (A-B) Representative aggregation curves of 3 individual experiments and representative scanning electron microscopy pictures of wt and RhoA−/− platelets after stimulation with 0.01μM U46619 (right) are depicted. Scale bar indicates 2 μm. (C) Mean percentage of maximal aggregation ± SD of each group (**P < .01; ***P < .001). U46 indicates U46619; thr, thrombin; PAR, PAR-4 peptide; and coll, collagen.
Remarkably, activation with the glycoprotein (GP) VI agonist collagen and to a lesser extent collagen-related peptide (CRP), which induce platelet activation via signaling of the immunoreceptor tyrosine-based activation motif (ITAM) of the GPVI-associated FcRγ chain,30 also resulted in reduced shape change in RhoA−/− compared with wild-type platelets (Figure 2B middle and bottom panels). In contrast, pronounced phosphorylation of MLC was evident after stimulation with high concentrations of CRP, indicating that GPVI signaling is principally able to induce MLC phosphorylation also in the absence of RhoA (supplemental Figure 1C).
Impaired aggregation of RhoA−/− platelets on stimulation with agonists coupling to G13 and Gq
Apart from partially reduced shape change, stimulation with the weak agonist ADP that signals via Gq- and Gi-coupled receptors and does not trigger granule release by itself caused normal aggregation of RhoA−/− platelets, indicating largely functional signaling by these G protein subunits in the absence of RhoA (Figure 2B top panel). Furthermore, aggregation responses to high concentrations of CRP and collagen were comparable between RhoA−/− and wild-type platelets and even enhanced at lower concentrations, confirming that GPVI-triggered activation is largely intact in RhoA−/− platelets (Figure 2B middle and bottom panels, C). The increased reactivity of the mutant cells to low doses of GPVI agonists may be explained by their increased size and the corresponding higher number of dense granules per platelet compared with wild-type platelets, which may lead to the release of higher amounts of ADP, ATP, or both on activation with threshold concentrations of these agonists (supplemental Figure 1E).
Remarkably, U46619- and PAR-4 peptide–induced aggregation was significantly reduced in RhoA−/− platelets at intermediate agonist concentrations but normal at high agonist concentrations (Figure 2A,C). In contrast, thrombin induced similar aggregation of RhoA−/− and wild-type platelets irrespective of the used concentration (Figure 2A,C).
Reduced integrin activation and granule release in RhoA−/− platelets on G13 and Gq stimulation
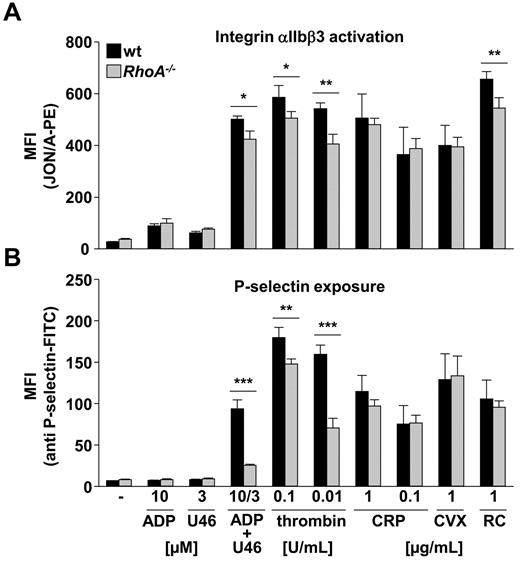
To address the effect of RhoA deficiency on integrin activation and degranulation in further detail, activation of the main platelet integrin αIIbβ3 and surface exposure of α granular P-selectin in response to different agonists were analyzed by flow cytometry in highly diluted platelet suspensions. Under these experimental conditions, the accumulation of secondary agonists is largely excluded.6 Stimulation with ADP or U46619 alone resulted in comparable integrin αIIbβ3 activation in RhoA−/− and wild-type platelets (Figure 3A). However, costimulation with these 2 agonists, as well as activation with thrombin or PAR-4 peptide (Figure 3A; data not shown) led to a small (∼ 15%) but significant reduction in integrin activation in RhoA−/− compared with wild-type platelets. In contrast, analysis of Gna13fl/fl, PF4-Cre (Gα13−/−) mice revealed that deficiency of this G protein subunit did not affect platelet count, size, and platelet surface receptor expression (supplemental Figure 2A). In accordance with earlier results,6 Gα13−/−platelets displayed unaltered integrin activation compared with the wild-type in response to all tested agonists (supplemental Figure 2B top panel). Interestingly, RhoA−/− platelets also showed decreased integrin activation levels in response to stimulation of the hemITAM receptor CLEC-227 by rhodocytin, although they reacted normally on activation of the GPVI-ITAM pathway by CRP and the snake venom toxin convulxin (Figure 3A).
Reduced integrin activation and α granule release in RhoA−/− platelets on G13 and Gq stimulation. Flow cytometric analysis of integrin αIIbβ3 activation (binding of JON/A-PE; A) and degranulation-dependent P-selectin exposure (B) in response to the indicated agonists in wt and RhoA−/− platelets. Data are mean fluorescence intensities (MFI) ± SD of 4 mice per group and representative of 4 individual experiments (*P < .05; **P < .01; ***P < .001). CVX indicates convulxin; and RC, rhodocytin.
Reduced integrin activation and α granule release in RhoA−/− platelets on G13 and Gq stimulation. Flow cytometric analysis of integrin αIIbβ3 activation (binding of JON/A-PE; A) and degranulation-dependent P-selectin exposure (B) in response to the indicated agonists in wt and RhoA−/− platelets. Data are mean fluorescence intensities (MFI) ± SD of 4 mice per group and representative of 4 individual experiments (*P < .05; **P < .01; ***P < .001). CVX indicates convulxin; and RC, rhodocytin.
In contrast to the moderate changes in integrin activation, RhoA−/− platelets displayed pronounced defects in α granule release after stimulation of G13 and Gq by thrombin and PAR-4 peptide (Figure 3B; data not shown). Furthermore, whereas activation with ADP or U46619 did not lead to detectable degranulation,29 a combination of both agonists induced strong P-selectin exposure in wild-type platelets but not RhoA−/− platelets (73% reduction; P < .001; Figure 3B). In contrast, RhoA−/− platelets displayed normal degranulation on stimulation with agonists inducing (hem)ITAM signaling, namely, CRP, convulxin, and rhodocytin. Consistently, lumi-aggregometry revealed that the release of dense granules was also decreased in RhoA−/− platelets after stimulation with high and intermediate concentrations of U46619 and PAR-4 peptide, but comparable with the wild-type after stimulation with thrombin, as well as on induction of GPVI signaling by collagen or CRP (supplemental Figure 3A).
Slightly different results were obtained with Gα13−/− platelets that displayed decreased P-selectin exposure only after activation with PAR-4 peptide (data not shown) or ADP plus U46619 (43% reduction; P < .01; supplemental Figure 2B bottom panel; Moers et al6 ). Thus, RhoA is critically involved in α and dense granule release on G13 and, to a lesser extent, Gq stimulation, whereas the GPVI-ITAM pathway is not affected by RhoA deficiency.
RhoA is dispensable for agonist-induced Ca2+ mobilization in platelets
The above-mentioned results revealed an involvement of RhoA in signaling by Gq. To assess whether RhoA deficiency influenced PLCβ activation and subsequent Ca2+ mobilization downstream of this G protein subunit,29 changes in intracellular calcium concentrations [Ca2+]i in response to U46619 (3μM) and thrombin (0.1 U/mL) were assessed using CRP (10 μg/mL) as a control (supplemental Figure 3B-C). Notably, Ca2+ release from intracellular stores was unaltered in RhoA−/− compared with wild-type platelets in response to all tested agonists, indicating that RhoA is dispensable for activation of PLCβ, as well as PLCγ2 in platelets. Furthermore, direct activation of PKC with phorbol 12-myristate-13-acetate resulted in similar integrin αIIbβ3 activation and P-selectin expression levels in RhoA−/− and wild-type platelets (data not shown). These results demonstrated that RhoA is not required for Ca2+ mobilization and PKC-mediated responses downstream of Gq and GPVI-ITAM signaling in platelets.
RhoA−/− platelets spread normally on fibrinogen but exhibit abolished integrin-dependent clot retraction
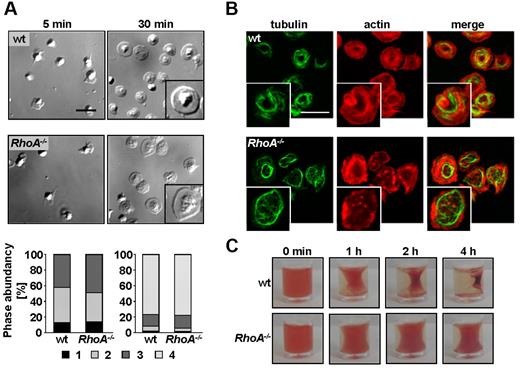
Ligand binding to integrin αIIbβ3 induces outside-in signaling, leading to cytoskeletal reorganizations and platelet spreading. The role of RhoA in these processes has been controversially debated with reports suggesting that RhoA is either required,31 dispensable,15 or has to be inhibited18 for spreading. To test this directly, mutant and wild-type platelets were allowed to spread on a fibrinogen-coated surface in the presence of thrombin (Figure 4). Differential interference contrast microscopy and scanning electron microscopy demonstrated that RhoA−/− platelets formed filopodia and lamellipodia to the same extent and with similar kinetics as wild-type platelets, resulting in full spreading after 30 minutes (Figure 4A; supplemental Figure 4A; supplemental Videos 1-2). Interestingly, although RhoA deficiency did not affect the ability of the mutant platelets to assemble filamentous (F)–actin in response to activation (supplemental Figure 4D), the morphology of spread RhoA−/− platelets was slightly altered. This was evident in a less condensed cell body, a broader distribution of α granules, and a partially more diffuse F-actin pattern in the mutant platelets (Figure 4A right panel inset; supplemental Figure 4B-C). Furthermore, microtubule coils seemed less pronounced and partially fragmented in the majority of spread RhoA−/− platelets compared with wild- type (Figure 4B). Remarkably, Gα13−/− platelets also spread normally on fibrinogen on activation, standing in clear contrast to a recent study using Gα13 knockdown platelets18 (supplemental Figure 4E).
RhoA−/− platelets spread normally on fibrinogen but fail to mediate clot retraction. (A-B) Washed platelets of wt and RhoA−/− mice were allowed to spread on fibrinogen (200 μg/mL) after stimulation with 0.01 U/mL thrombin. (A) Representative differential interference contrast images of 3 individual experiments from the indicated time points (top) and statistical evaluation of the percentage of spread platelets at different spreading stages (bottom). 1, roundish; 2, only filopodia; 3, filopodia and lamellipodia; and 4, full spreading. Scale bar represents 5 μm. (B) Analysis of filamentous actin (red) and tubulin (green) structure in spread (30 minutes) RhoA−/− and wt platelets by confocal microscopy. Scale bar represents 5 μm. (C) Clot retraction of prp on activation with 5 U/mL thrombin in the presence of 20mM CaCl2 at the indicated times. Representative images of 2 different experiments are depicted.
RhoA−/− platelets spread normally on fibrinogen but fail to mediate clot retraction. (A-B) Washed platelets of wt and RhoA−/− mice were allowed to spread on fibrinogen (200 μg/mL) after stimulation with 0.01 U/mL thrombin. (A) Representative differential interference contrast images of 3 individual experiments from the indicated time points (top) and statistical evaluation of the percentage of spread platelets at different spreading stages (bottom). 1, roundish; 2, only filopodia; 3, filopodia and lamellipodia; and 4, full spreading. Scale bar represents 5 μm. (B) Analysis of filamentous actin (red) and tubulin (green) structure in spread (30 minutes) RhoA−/− and wt platelets by confocal microscopy. Scale bar represents 5 μm. (C) Clot retraction of prp on activation with 5 U/mL thrombin in the presence of 20mM CaCl2 at the indicated times. Representative images of 2 different experiments are depicted.
Integrin αIIbβ3 outside-in signaling also regulates clot retraction,32,33 and the role of RhoA in this process is controversial.15,18 To address this directly, clot formation was induced in prp from wild-type and RhoA−/− mice by the addition of thrombin (5 U/mL, 20mM Ca2+). Although clot retraction started already after 30 minutes in wild-type prp and proceeded to the maximum after 4 hours, the process was virtually abolished in RhoA−/− prp (Figure 4C).
Together, these data demonstrate that RhoA is not required for spreading of activated platelets on fibrinogen but that it is indispensable for integrin-mediated clot retraction.
RhoA−/− platelets display moderately reduced aggregate formation on collagen and increased rolling velocity on VWF at high shear
Thrombus formation at the site of vascular injury requires stable platelet adhesion on the extracellular matrix as well as auto- and paracrine platelet activation by locally released secondary mediators.29 To address the effect of RhoA deficiency on these processes, platelet adhesion to collagen was studied in a whole blood perfusion system. In these experiments, blood from RhoA−/− mice was reconstituted with isolated RhoA−/− platelets to normalize platelet numbers.
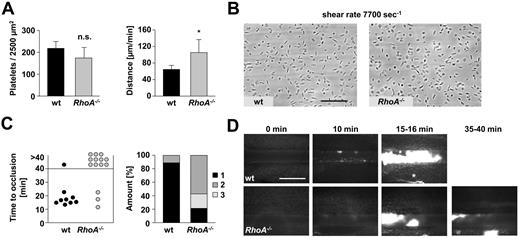
Perfusion of blood over immobilized collagen at a shear rate of 1700 seconds−1 resulted in large platelet aggregates of comparable size in RhoA−/− and wild-type blood (mean surface coverage, 52.3% ± 4.3% vs 53.7% ± 6.0%; P = .68; supplemental Figure 5A). This was not because of side effects caused by platelet reconstitution, because experiments using nonreconstituted blood also revealed similar aggregate formation in RhoA−/− and wild-type blood when determining the surface coverage relative to the platelet count (supplemental Figure 5B right panel). However, at higher shear rates (7700 seconds−1), resembling flow conditions in small arterioles or stenosed arteries, aggregate formation of RhoA−/− platelets was moderately but significantly reduced compared with the wild-type (mean surface coverage, 3.2% ± 0.9% vs 5.2% ± 1.8%; P < .05; supplemental Figure 5C), indicating that RhoA signaling contributes to stable platelet adhesion and aggregate formation at high shear.
At lower shear rates, platelet adhesion and aggregation is primarily dependent on integrin αIIbβ3. In contrast, at very high shear, the interaction of platelet GPIb with VWF becomes increasingly important for platelet adhesion.34 To test whether reduced aggregate formation in RhoA-deficient blood at high shear was caused by altered GPIb-VWF interaction, platelet adhesion to immobilized murine VWF was analyzed (Figure 5A-B). Whereas the number of attaching platelets at 7700 seconds−1 was similar in RhoA−/− and wild-type blood (174.2 ± 48.0/2500 μm2 vs 218.7 ± 30.8/2500 μm2; P = .09; Figure 5A left panel, B), the interaction of the mutant platelets with VWF was less stable, resulting in increased velocity of rolling RhoA−/− compared with wild-type platelets (mean distance/minute, 26.4 ± 7.8 μm vs 16.1 ± 2.3 μm; P < .05; Figure 5A right panel).
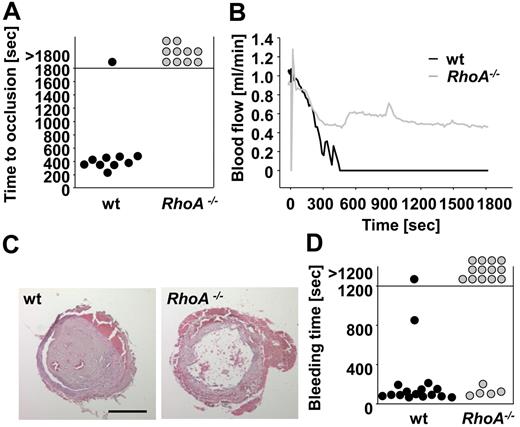
RhoA−/− platelets display increased rolling velocity on VWF ex vivo and form only instable thrombi after FeCl3 injury in vivo. (A-B) Unaltered adhesion but accelerated rolling of RhoA−/− platelets on VWF ex vivo. Heparinized whole blood was perfused over immobilized murine VWF at a shear rate of 7700 seconds−1. (A) Mean distance per minute ± SD (left) and mean number of adherent platelets per 2500 μm2 ± SD (right) are shown (n = 6; *P < .05). (B) Representative phase contrast images after 1 minute of washing with Tyrode HEPES buffer are illustrated. Scale bar represents 50 μm. (C-D) RhoA−/− mice display instable thrombus formation after FeCl3-induced vascular injury. Small mesenteric arterioles were injured by topical application of FeCl3, and thrombus formation of fluorescently labeled platelets was monitored using intravital microscopy. (C) Time to stable vessel occlusion (left) and amount of stably occluded (1), recanalized (2), and nonoccluded vessels (3) after injury of wt and RhoA−/− mice (right) are shown. Each symbol represents 1 arteriole. (D) Representative pictures at the indicated time. Scale bar represents 100 μm. Asterisk indicates stable occlusion of the vessel.
RhoA−/− platelets display increased rolling velocity on VWF ex vivo and form only instable thrombi after FeCl3 injury in vivo. (A-B) Unaltered adhesion but accelerated rolling of RhoA−/− platelets on VWF ex vivo. Heparinized whole blood was perfused over immobilized murine VWF at a shear rate of 7700 seconds−1. (A) Mean distance per minute ± SD (left) and mean number of adherent platelets per 2500 μm2 ± SD (right) are shown (n = 6; *P < .05). (B) Representative phase contrast images after 1 minute of washing with Tyrode HEPES buffer are illustrated. Scale bar represents 50 μm. (C-D) RhoA−/− mice display instable thrombus formation after FeCl3-induced vascular injury. Small mesenteric arterioles were injured by topical application of FeCl3, and thrombus formation of fluorescently labeled platelets was monitored using intravital microscopy. (C) Time to stable vessel occlusion (left) and amount of stably occluded (1), recanalized (2), and nonoccluded vessels (3) after injury of wt and RhoA−/− mice (right) are shown. Each symbol represents 1 arteriole. (D) Representative pictures at the indicated time. Scale bar represents 100 μm. Asterisk indicates stable occlusion of the vessel.
RhoA is required for the formation of stable thrombi in vivo
To examine to which extent the observed defects of RhoA−/− platelets in vitro influenced thrombotic events in vivo, thrombus formation after injury of small mesenteric arterioles by FeCl3 was assessed using intravital fluorescence microscopy (Figure 5C-D). Although initial aggregate formation was comparable between wild-type and RhoA−/− mice (data not shown), subsequent formation of stable thrombi was strongly impaired in RhoA−/− mice compared with the wild-type, resulting in complete vessel occlusion in 89% (8 of 9) of wild-type arterioles (mean time to occlusion, 16.9 ± 2.9 minutes; Figure 5C left panel, D), but only 21.5% of RhoA−/− vessels (P < .01). In 57% of arterioles of RhoA−/− mice, no occlusion occurred during the observation period of 40 minutes and further 21.5% of the vessels recanalized after short (< 2 minutes) occlusion (Figure 5C right panel, D). The defective thrombus formation in RhoA−/− mice was mainly characterized by embolization of fragments after the thrombus had reached a certain size, demonstrating a critical role of RhoA for thrombus stabilization in vivo. Similar results were obtained when the mice were tested in a second thrombosis model in which thrombus formation was induced by mechanical injury of the abdominal aorta (Figure 6A-C). Although stable occlusion occurred in 9 of 10 wild-type mice within 8 minutes (mean time to occlusion, 384 ± 79 seconds; Figure 6A-C), blood flow in RhoA−/− mice only decelerated to ∼ 50% of the initial value after injury and, apart from some fluctuations indicating thrombus embolization, remained rather constant until the end of the observation period of 30 minutes (P < .001; Figure 6A-C). Importantly, the thrombus formation defect in RhoA−/− mice was not because of the macrothrombocytopenia in the deficient animals: Wild-type mice in which the platelet count was reduced to ∼ 50% of normal and that displayed platelet sizes comparable with RhoA−/− mice formed stable occlusive thrombus formation within a similar time frame as the controls (supplemental Figure 5D).
Defective mechanically induced thrombus formation and impaired hemostasis in RhoA−/− mice. (A-C) The abdominal aorta of wt and RhoA−/− mice was injured by tight compression with a forceps, and blood flow was monitored for 30 minutes. Time to stable vessel occlusion (A) and representative blood flow curves (B) are shown. Each symbol represents 1 individual. (C) Representative H&E–stained cross sections of the abdominal aorta of wt and RhoA−/− mice 30 minutes after injury. Scale bar represents 200 μm. (D) Defective hemostasis in RhoA−/− mice. Tail bleeding times of wt and RhoA−/− mice in saline at 37°C. Each symbol represents 1 individual.
Defective mechanically induced thrombus formation and impaired hemostasis in RhoA−/− mice. (A-C) The abdominal aorta of wt and RhoA−/− mice was injured by tight compression with a forceps, and blood flow was monitored for 30 minutes. Time to stable vessel occlusion (A) and representative blood flow curves (B) are shown. Each symbol represents 1 individual. (C) Representative H&E–stained cross sections of the abdominal aorta of wt and RhoA−/− mice 30 minutes after injury. Scale bar represents 200 μm. (D) Defective hemostasis in RhoA−/− mice. Tail bleeding times of wt and RhoA−/− mice in saline at 37°C. Each symbol represents 1 individual.
Together, these results demonstrate that RhoA is required for thrombus stabilization in small, as well as in large arteries irrespective of the type of injury.
Defective hemostasis in RhoA−/− mice
To assess the impact of RhoA deficiency on hemostasis, tail bleeding times were determined (Figure 6D). Whereas bleeding stopped in 15 of 16 (94%) wild-type mice (mean bleeding time, 179 ± 196 seconds), bleeding times were dramatically increased in RhoA−/− mice (13 of 18 bled for > 20 minutes; P < .001; Figure 6D). Interestingly, in many of the RhoA−/− mice, blood loss temporarily decreased or even transiently stopped for several seconds but then increased again (data not shown). This indicates that RhoA is required for stabilization of clots not only in intravascular thrombus formation but also in normal hemostasis.
RhoA−/− mice are protected from ischemic brain infarction
Cerebral ischemia is a complex disease in which the microvascular integrity is disturbed, resulting in acute inflammation and neuronal cell death. Platelets contribute to this pathology by mechanisms involving GPIb-VWF interactions but obviously not integrin αIIbβ3-dependent aggregation. However, the exact underlying mechanisms have not been identified.35-37
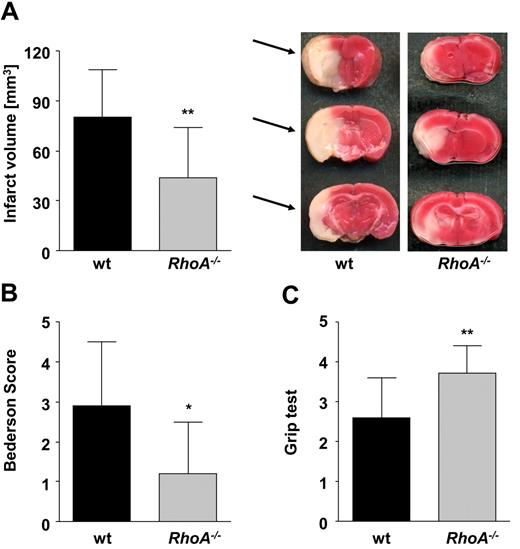
To investigate the impact of RhoA deficiency in this pathology, mice were challenged in a model of experimental cerebral ischemia using the tMCAO model36 (Figure 7). Strikingly, in RhoA−/− mice, brain infarct volumes were reduced to ∼ 50% of the infarct volumes in wild-type mice (43.7 ± 30.5 mm3 vs 80.1 ± 28.8 mm3; P < .01; Figure 7A). This translated into significantly less severe neurologic deficits compared with wild-type mice, determined by Bederson score assessing global neurologic function (1.2 ± 1.3 vs 2.9 ± 1.6; P < .05; Figure 7B) and grip test that indicates motor function and coordination of the mice (3.7 ± 0.7 vs 2.6 ± 1.0; P < .01; Figure 7C). These data revealed an important involvement of RhoA-dependent signaling in platelets for the propagation of ischemic stroke.
RhoA−/− mice are protected from cerebral ischemia. Formation of cerebral brain infarction and consequential neurologic defects were investigated in a murine stroke model of tMCAO. (A) Brain infarct volumes in wt (n = 24) and RhoA−/− mice (n = 14) presented as mean ± SD (left). Representative images of 3 corresponding coronal sections from wt and RhoA−/− mice stained with 2,3,5-triphenyltetrazolium chloride 24 hours after tMCAO. Infarcted areas are marked with arrows (right). Bederson score (B) and grip test (C) determined 24 hours after tMCAO (*P < .05; **P < .01).
RhoA−/− mice are protected from cerebral ischemia. Formation of cerebral brain infarction and consequential neurologic defects were investigated in a murine stroke model of tMCAO. (A) Brain infarct volumes in wt (n = 24) and RhoA−/− mice (n = 14) presented as mean ± SD (left). Representative images of 3 corresponding coronal sections from wt and RhoA−/− mice stained with 2,3,5-triphenyltetrazolium chloride 24 hours after tMCAO. Infarcted areas are marked with arrows (right). Bederson score (B) and grip test (C) determined 24 hours after tMCAO (*P < .05; **P < .01).
Discussion
In this study, megakaryocyte- and platelet-specific knockout mice for RhoA were used to assess the role of the GTPase for platelet function in vitro and in vivo. We show that RhoA deficiency leads to macrothrombocytopenia and signaling defects downstream of G13- and Gq-coupled receptors, resulting in severely impaired hemostasis and marked protection in models of arterial thrombus formation and ischemic stroke.
The pronounced macrothrombocytopenia in RhoA-deficient animals was associated with largely normal platelet morphology, only moderately increased platelet turnover (Figure 1B-E), and increased numbers of mature megakaryocytes in the bone marrow of the mutant mice (supplemental Figure 1A-B). These findings strongly indicate a critical involvement of RhoA in the terminal stages of platelet production, as suggested previously by in vitro studies.38,39 The defects in platelet formation in the mutant mice are currently under investigation.
In line with indirect studies, our results confirm the essential role of RhoA signaling for platelet shape change and MLC phosphorylation on induction of G13 signaling6-14 (Figure 2 and supplemental Figure 1C-D). However, our findings clearly (Figure 2) suggest an involvement of RhoA signaling in these processes also downstream of Gq. This hypothesis is in line with studies using Gαq/13 double-deficient platelets40 and mouse embryonic fibroblasts that show that receptors coupled to G13 and Gq/G11 can activate RhoA via Gq/G11 at high agonist concentrations.9 In addition, our data emphasize that RhoA/ROCK-mediated inhibition of MLC phosphatase is the primary pathway for MLC phosphorylation in response to agonists coupling to G13 and Gq, as suggested by studies using human platelets.13
Interestingly, we observed a shape change defect in RhoA−/− platelets in aggregometry on stimulation with GPVI agonists (Figure 2) despite pronounced MLC phosphorylation after activation with high concentrations of CRP (supplemental Figure 1C). This finding demonstrates that GPVI signaling efficiently induces MLC phosphorylation via the Ca2+/MLC kinase pathway independently of RhoA, as shown for human platelets.41 Conversely, these results emphasize that fibrous collagen (and to a lesser extent CRP) are largely dependent on generated TxA2 for induction of shape change under conditions as present in the aggregometry cuvette.6,29
Besides shape change, our results using RhoA−/− and Gα13−/− platelets reveal an important role of RhoA for secretion of α and dense granules by G13 but to a lesser extent also by Gq signaling (Figure 3 and supplemental Figures 2B and 3A), in agreement with previous indirect studies.6,9,40,42 Little is known about how Rho GTPases mechanistically facilitate exocytosis (reviewed in Ory and Gasman43 ). In platelets, cytoskeletal rearrangements are known to be critical for degranulation,44 and observations in different cell types indicated that RhoA associates to secretory granules and proteins of the exocyst complex.43 However, these studies suggested an inhibitory rather than activatory role for the GTPase during exocytosis. Thus, the exact mechanisms by which RhoA participates in platelet granule release remain to be determined.
Likewise, the mechanism by which RhoA contributes to Gq-mediated platelet activation requires further investigation. Notably, RhoA was not involved in the classic signaling cascade downstream of Gq in platelets leading to PLC activation, Ca2+ mobilization, and protein kinase C (PKC) activation (supplemental Figure 3B-C). This indicates that Gq may directly regulate RhoA activity by activating Rho-guanine nucleotide exchange factors, such as LARG, as indicated by indirect studies.40,45
RhoA has been shown to regulate inside-out activation, as well as outside-in signaling of the main integrin αIIbβ3 in platelets.9,29,46 We show that RhoA deficiency results in partially reduced platelet integrin activation (Figure 3). Thus, RhoA may to some part contribute to inside-out activation of integrin αIIbβ3 in platelets, although it is unlikely that the mild defect significantly affected in vitro and in vivo function of the mutant platelets. In contrast, RhoA−/− platelets displayed discrete defects in integrin outside-in signaling. RhoA was clearly dispensable for platelet spreading on fibrinogen after activation (Figure 4A and supplemental Figure 4A) but essential for clot retraction (Figure 4C). These observations stand in contrast to a previous study using C3 exoenzyme,15 but they reflect recent findings by Gong et al.18 Importantly, however, we also found that Gα13-deficient platelets spread normally on fibrinogen after activation, demonstrating that the underlying mechanisms are more complex than assumed in that study (supplemental Figure 4E).
Although modulation of actinomyosin is thought to be one major event of RhoA signaling,2 our data indicate that RhoA is not required for F-actin assembly and that its deficiency only moderately affects stress fiber formation in platelets (Figure 4B and supplemental Figure 4C-D). These results are in agreement with findings made in recently generated RhoA-deficient keratinocytes20 and mouse embryonic fibroblasts.47 In the latter studies, unchanged actomyosin regulation may be explained by a functional redundancy of RhoA with RhoB and RhoC. In contrast, RhoA was shown to be the only Rho isoform expressed in platelets.47 However, redundancies of RhoA and other GTPases may exist in platelets. Cdc42 and RhoF represent potential candidates in this context because they share the downstream effector mDia with RhoA.19,49,50
Interestingly, RhoA deficiency affected the structure of microtubules in spread platelets (Figure 4B). This finding indicates that RhoA may be involved in microtubule reorganization after platelet activation, as suggested in a study indicating a role for RhoA/ROCK-mediated regulation of microtubules during platelet shape change.51 Besides ROCK, RhoA also may regulate microtubules via mDia, as shown for other cell types.3
Our flow adhesion experiments indicated that RhoA deficiency may selectively affect platelet function at very high shear rates (Figure 5 and supplemental Figure 5C). Notably, the increased rolling velocity of the mutant platelets on immobilized VWF under these in vitro conditions also may partially be explained by their increased size. Importantly, however, RhoA deficiency resulted in protection from arterial thrombosis in 2 different injury models (Figures 5 and 6), and this phenotype was neither caused by the decreased platelet counts nor by the increase in platelet size in these animals (Figure 1B and supplemental Figure 5D). These results indicate that the observed protection is caused by the loss of RhoA function and that signaling of the GTPase is critical for the stabilization of formed thrombi in vivo. Remarkably, RhoA−/− mice were also significantly protected from neuronal damage in the tMCAO model of ischemic stroke, which is known to be strictly dependent on GPIb and VWF but not integrin αIIbβ3-dependent aggregation.35-37 Interestingly, this protection was not associated with an increased incidence of intracranial hemorrhage, although the mutant mice displayed severely prolonged tail bleeding times caused by frequent rebleeding (Figure 6D), a phenotype that is similar to that seen in mice treated with GPIb-blocking antibodies.35
Little is known about the potential association of RhoA deregulation in human hematologic diseases. Previously, RhoA was implicated as an upstream regulator of the gene encoding nonmuscle myosin heavy chain (Myh9) in indirect studies.38 Interestingly, the phenotype of RhoA deficiency in mice in many aspects mirrors the clinical manifestation of patients carrying mutations in the Myh9 gene. Like RhoA-deficient mice, patients with Myh9-related disorders suffer from macrothrombocytopenia and a mild bleeding diathesis. The residual platelets display a shape change defect and are unable to facilitate clot retraction (reviewed in Althaus and Greinacher52 ). Thus, our study provides direct evidence for the importance of RhoA signaling leading to Myh9 activation and thus to the development of this complex disease. It is unlikely that loss-of-function mutations of RhoA exist in humans as the GTPase is ubiquitously expressed and its dysfunction would most probably lead to embryonic lethality. However, further studies are required to assess how a potential involvement of altered RhoA regulation or activity may contribute to defects in platelet biogenesis and function in humans. Taken together, our studies reveal distinct signaling defects in RhoA−/− platelets that have a major impact on hemostasis and thrombotic diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jonas Müller and Sylvia Hengst for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688) and the Rudolf Virchow Center. S.G. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Science, University of Würzburg.
Authorship
Contribution: I.P. and I.H. performed experiments, analyzed data, and contributed to the writing of the paper; S.G., F.M., L.C., and C.K. performed experiments and analyzed data; J.v.H., S.O., G.K., and C.B. provided vital new reagents and contributed to the writing of the paper; and B.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University Hospital Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.
References
Author notes
I.P. and I.H. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal