Abstract
Dasatinib is a highly potent BCR-ABL inhibitor with established efficacy and safety in imatinib-resistant/-intolerant patients with chronic myeloid leukemia (CML). In the phase 3 DASISION trial, patients with newly diagnosed chronic-phase (CP) CML were randomized to receive dasatinib 100 mg (n = 259) or imatinib 400 mg (n = 260) once daily. Primary data showed superior efficacy for dasatinib compared with imatinib after 12 months, including significantly higher rates of complete cytogenetic response (CCyR), confirmed CCyR (primary end point), and major molecular response (MMR). Here, 24-month data are presented. Cumulative response rates by 24 months in dasatinib and imatinib arms were: CCyR in 86% versus 82%, MMR in 64% versus 46%, and BCR-ABL reduction to ≤ 0.0032% (4.5-log reduction) in 17% versus 8%. Transformation to accelerated-/ blast-phase CML on study occurred in 2.3% with dasatinib versus 5.0% with imatinib. BCR-ABL mutations, assessed after discontinuation, were detected in 10 patients in each arm. In safety analyses, fluid retention, superficial edema, myalgia, vomiting, and rash were less frequent with dasatinib compared with imatinib, whereas pleural effusion and grade 3/4 thrombocytopenia were more frequent with dasatinib. Overall, dasatinib continues to show faster and deeper responses compared with imatinib, supporting first-line use of dasatinib in patients with newly diagnosed CML-CP. This study was registered at ClinicalTrials.gov: NCT00481247.
Introduction
The BCR-ABL oncoprotein is the cause of chronic myeloid leukemia (CML) and the dominant mechanism behind disease progression.1,2 Imatinib, a BCR-ABL tyrosine kinase inhibitor, was approved for first-line therapy for newly diagnosed CML in chronic phase (CP) based on efficacy and safety results from the IRIS (International Randomized Study of Interferon and STI571) trial.3,4 However, an intention-to-treat analysis suggested that approximately one-third of imatinib-treated patients with newly diagnosed CML-CP have inadequate responses or do not experience long-term benefit.5
Dasatinib is a second-generation BCR-ABL inhibitor that has 325-fold higher potency in vitro compared with imatinib against engineered cell lines expressing nonmutated BCR-ABL, and inhibitory activity against the majority of imatinib-resistant BCR-ABL mutants.6 In the CA180-034 trial of patients with imatinib-resistant or imatinib-intolerant CML-CP, dasatinib 100 mg once daily (QD) showed durable efficacy and safety, including a 5-year progression-free survival (PFS) rate of 57%, overall survival (OS) rate of 78%, and cumulative major molecular response (MMR) rate by 5 years of 44%.7 With the aim of improving on responses achieved with first-line imatinib, dasatinib was initially evaluated in patients with newly diagnosed CML-CP in a single-institution trial, and showed promising results compared with historical data for imatinib.8
DASISION (DASatinib versus Imatinib Study In treatment-Naive CML patients) is a randomized phase 3 trial comparing treatment with dasatinib 100 mg QD or imatinib 400 mg QD in patients with newly diagnosed CML-CP. Initial results showed that dasatinib had superior efficacy over imatinib during the first year of therapy, including significantly higher rates of complete cytogenetic response (CCyR; 83% vs 72%, P = .0011), confirmed CCyR (cCCyR [primary end point]; 77% vs 66%, P = .007), and MMR (46% vs 28%, P < .0001) by 12 months, and an acceptable safety and tolerability profile.9 Dasatinib was subsequently approved as first-line therapy for newly diagnosed CML-CP by various health authorities worldwide. Here, results from DASISION after a minimum follow-up of 24 months are reported.
Methods
Patients
Patients and eligibility criteria have been described in detail previously.9 Briefly, adults with cytogenetically confirmed Philadelphia chromosome (Ph)–positive CML-CP diagnosed within 3 months, who had adequate hepatic and renal function, and no serious medical conditions, were eligible. No prior CML therapy was permitted except for anagrelide or hydroxyurea. The trial was approved by all institutional review boards and ethics committees and all patients gave written informed consent before randomization in accordance with the Declaration of Helsinki.
Study design and treatment
DASISION (CA180-056; ClinicalTrials.gov: NCT00481247) is an ongoing multinational, open-label, phase 3 trial. Patients were stratified by Hasford risk score10 and randomized to receive either oral dasatinib (administered as 100 mg QD with or without food) or imatinib (administered as 400 mg QD with food). Patients were randomized in a 1:1 ratio and stratified by the study sponsor using a central automated telephone system, which dynamically minimized imbalance between treatment arms. Treatment allocation was not masked. Data were collected at participating institutions, analyzed using the sponsor's data-management systems, and interpreted by the sponsor's statistical team and the study steering committee. Access to primary clinical data were available to all authors.
Study treatment was discontinued for protocol-defined disease progression, unacceptable toxicity, patient/investigator decision, or pregnancy. Treatment interruptions and dose reductions were permitted for managing adverse events (AEs). Dose escalations to dasatinib 140 mg QD or imatinib 600-800 mg/d were permitted for suboptimal response (European LeukemiaNet [ELN] 2006 definitions) at 3-18 months.11 The primary end point was cCCyR rate by 12 months. Key secondary end points included time in cCCyR, rates of MMR at any time, times to cCCyR or MMR, and durations of PFS and OS.
Evaluations
CCyR was defined as lack of Ph in ≥ 20 bone marrow (BM) metaphases. Confirmed CCyR was defined as CCyR in consecutive cytogenetic assessments. MMR was defined as BCR-ABL transcript level of ≤ 0.1% on the International Scale (3-log reduction compared with the standardized baseline). Missing samples and atypical transcripts were counted as no response. BCR-ABL mutational analysis by direct sequencing was required after discontinuation of study therapy for any reason (samples obtained within 45 days before or after discontinuation were tested). Molecular and mutational assessments were performed at a centralized laboratory (MolecularMD).12 Transformation to accelerated-phase/blast-phase (AP/BP) CML was defined using European LeukemiaNet 2006 criteria, and did not include clonal evolution.11 Patients who discontinued first-line dasatinib or imatinib who had not died and agreed to follow-up continued to be followed for transformation and survival on a yearly basis for up to 5 years. Investigators were also permitted to provide additional information on patient status after discontinuation at other times (ie, after shorter intervals). The occurrence of transformation was based on the judgment of the treating physician using protocol-defined criteria. AEs were assessed continuously and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0. To assess patients for pleural effusion, chest x-ray was stipulated by the protocol in all patients at baseline and after 6 months of treatment, or more frequently if indicated clinically.
Statistical considerations
The sample size was based on a minimum of 518 patients estimated to show a statistically significant difference in the primary end point. Efficacy analyses were performed in all randomized patients (intention-to-treat population). Two-sided exact 95% confidence intervals (CIs) were calculated using the Clopper and Pearson method. Cumulative response rates (ie, not adjusted for loss of response) and differences between study arms were assessed by competing risk analysis using the Gray test. Comparisons of response rates in the current manuscript are posthoc analyses; P values provided are descriptive and have not been adjusted for multiple comparisons. Rates of PFS, failure-free survival (FFS), and OS were estimated using Kaplan-Meier analysis. To calculate PFS, progression was defined per protocol as any of the following: doubling of white cell count to > 20 × 109/L in the absence of complete hematologic response (CHR); loss of CHR; increase in Ph-positive BM metaphases to > 35%; transformation to AP/BP; or death from any cause. Failure was defined as no hematologic response by 3 months, no CHR or cytogenetic response by 6 months, no partial cytogenetic response by 12 months, or no CCyR by 18 months. For FFS, protocol-defined progression was also counted as failure. For PFS and FFS analyses, patients who discontinued without progression/failure were censored at the time of the last on-study hematologic or cytogenetic evaluation. Information relating to the occurrence of protocol-defined progression or failure after study discontinuation was not obtained from investigators (unlike information relating to transformation or survival). For OS analysis, patients who had not died were censored on the last date the patient was known to be alive, which included dates after study discontinuation if provided by investigators. Safety analyses were performed in all treated patients (received at least 1 dose of study drug).
Results
Patients and treatment
Between September 2007 and December 2008, 519 patients with newly diagnosed CML-CP from 108 centers in 26 countries were randomized to receive dasatinib or imatinib (Figure 1). Baseline characteristics have been published previously9 and were well balanced between the dasatinib and imatinib arms, including median age at baseline (46 and 49 years), and the proportions of patients with low (33% and 33%), intermediate (48% and 47%), or high (19% and 19%) risk according to Hasford score. In the dasatinib and imatinib arms, 5 and 3 patients, respectively, had atypical BCR-ABL transcripts and were counted as nonresponders in molecular response analyses. In both arms, the median time from diagnosis to randomization was 1 month.
CONSORT diagram for the DASISION trial after a minimum follow-up of 24 months. *Reasons for discontinuation are provided in Table 1.
CONSORT diagram for the DASISION trial after a minimum follow-up of 24 months. *Reasons for discontinuation are provided in Table 1.
At the time of analysis, 199 (77%) dasatinib-treated patients and 194 (75%) imatinib-treated patients remained on study and had a minimum follow-up of 24 months. Reasons for discontinuation are shown in Table 1. Of 59 and 64 patients who discontinued dasatinib or imatinib, additional data on transformation and survival status were provided by investigators after discontinuation from 45 (76%) and 50 (78%) patients. In the dasatinib and imatinib arms, median durations of follow-up from randomization to last assessment (including after study discontinuation) were 26.5 months (range, 0.03-37.0) and 26.7 months (0.03-38.4), and median durations of treatment were 24.9 months (0.03-37.0) and 24.9 months (0.3-44.1). During dasatinib or imatinib treatment, 152 (59%) and 111 (43%) patients had a treatment interruption, 71 (28%) and 39 (15%) had a dose reduction, and 19 (7%) and 52 (20%) had a dose escalation. Median dose intensities were 99.5 mg/d for dasatinib and 400 mg/d for imatinib.
Treatment status and reasons for discontinuation
| Status . | Patients, n (%) . | |
|---|---|---|
| Dasatinib, 100 mg QD, N = 258 . | Imatinib, 400 mg QD, N = 258 . | |
| Received treatment | 258 (100) | 258 (100) |
| Still on treatment | 199 (77) | 194 (75) |
| Discontinued treatment | 59 (23) | 64 (25) |
| Progression* | 14 (5) | 17 (7) |
| Treatment failure† | 8 (3) | 11 (4) |
| Drug-related adverse event | 18 (7) | 12 (5) |
| Nonhematologic | 12 (5) | 8 (3) |
| Hematologic | 6 (2) | 4 (1) |
| Unrelated adverse event | 5 (2) | 1 (< 1) |
| Death‡ | 4 (2) | 1 (< 1) |
| Withdrew consent | 4 (2) | 3 (1) |
| Became pregnant | 2 (1) | 1 (< 1) |
| Did not adhere to therapy | 0 | 2 (1) |
| Lost to follow-up | 0 | 3 (1) |
| Requested to discontinue§ | 2 (1) | 3 (1) |
| Other reason | 2 (1) | 10 (4) |
| Status . | Patients, n (%) . | |
|---|---|---|
| Dasatinib, 100 mg QD, N = 258 . | Imatinib, 400 mg QD, N = 258 . | |
| Received treatment | 258 (100) | 258 (100) |
| Still on treatment | 199 (77) | 194 (75) |
| Discontinued treatment | 59 (23) | 64 (25) |
| Progression* | 14 (5) | 17 (7) |
| Treatment failure† | 8 (3) | 11 (4) |
| Drug-related adverse event | 18 (7) | 12 (5) |
| Nonhematologic | 12 (5) | 8 (3) |
| Hematologic | 6 (2) | 4 (1) |
| Unrelated adverse event | 5 (2) | 1 (< 1) |
| Death‡ | 4 (2) | 1 (< 1) |
| Withdrew consent | 4 (2) | 3 (1) |
| Became pregnant | 2 (1) | 1 (< 1) |
| Did not adhere to therapy | 0 | 2 (1) |
| Lost to follow-up | 0 | 3 (1) |
| Requested to discontinue§ | 2 (1) | 3 (1) |
| Other reason | 2 (1) | 10 (4) |
QD indicates once daily; and CHR, complete hematologic response.
Progression was defined per protocol as any of the following: doubling of white cell count to > 20 × 109/L in the absence of CHR, loss of CHR, increase in Philadelphia chromosome–positive BM metaphases to > 35%, transformation to accelerated/blast phase, or death from any cause.
Treatment failure was defined per protocol as no hematologic response by 3 months, no CHR or cytogenetic response by 6 months, no partial cytogenetic response by 12 months, or no complete cytogenetic response by 18 months.
Patients who discontinued due to death; total deaths and causes in patients on treatment or after discontinuation are shown in Table 6.
Patients who requested to discontinue but agreed to long-term follow-up.
Efficacy
Cumulative cytogenetic response rates by 24 months for dasatinib versus imatinib, respectively, were CCyR in 86% versus 82% and cCCyR in 80% versus 74% (Table 2). Median time to CCyR calculated by competing risks analysis was 3.2 months with dasatinib and 6.0 months with imatinib. The cumulative CCyR rate was higher for dasatinib versus imatinib across the period analyzed (P = .0002; Figure 2A). CCyR rates at 24 months, in subjects with cytogenetic assessments (dasatinib arm: n = 154, imatinib arm: n = 147), for dasatinib versus imatinib were 91% versus 90%, respectively; corresponding rates at 12 months in assessable patients (dasatinib arm: n = 217, imatinib arm: n = 215) for dasatinib versus imatinib were 86% versus 74%, respectively. The cumulative MMR rate by 24 months was 64% for dasatinib versus 46% for imatinib, and median time to MMR was 15 versus 36 months. The cumulative rate of BCR-ABL transcript reduction to ≤ 0.0032% (4.5-log reduction from standardized baseline) by 24 months was 17% for dasatinib versus 8% for imatinib (Table 2). Cumulative rates of MMR and BCR-ABL ≤ 0.0032% were higher with dasatinib compared with imatinib across the period analyzed (P < .0001 and P = .002, respectively; Figure 2B-C). MMR rates at 24 months, in subjects with PCR assessments (dasatinib arm: n = 154, imatinib arm: n = 161), for dasatinib versus imatinib were 69% versus 52%, respectively; corresponding rates at 12 months in assessable patients (dasatinib arm: n = 222, imatinib arm: n = 219) for dasatinib versus imatinib were 45% versus 30%, respectively. By Hasford score, MMR was achieved in dasatinib and imatinib arms by 63 (73%) of 86 versus 49 (56%) of 87 patients with low risk, 76 (61%) of 124 versus 62 (50%) of 123 with intermediate risk, and 28 (57%) of 49 versus 19 (38%) of 50 with high risk scores. At last follow-up for the 199 and 194 patients who were still receiving study treatment with dasatinib or imatinib (including patients who received treatment for more than 24 months), 194 versus 188 patients had achieved CCyR, 152 versus 126 had achieved MMR, and 46 versus 23 had achieved a BCR-ABL transcript level of ≤ 0.0032%.
Response rates by 24 months
| Responses achieved by 24 mo . | Dasatinib, N = 259 . | Imatinib, N = 260 . | ||
|---|---|---|---|---|
| n . | % (95% CI) . | n . | % (95% CI) . | |
| Complete cytogenetic response | 223 | 86 (81-90) | 213 | 82 (77-86) |
| Confirmed complete cytogenetic response | 208 | 80 (75-85) | 193 | 74 (68-79) |
| Major molecular response | 165 | 64 (58-70) | 120 | 46 (40-52) |
| BCR-ABL transcript level reduction to ≤ 0.0032% | 44 | 17 (13-22) | 22 | 8 (5-13) |
| Responses achieved by 24 mo . | Dasatinib, N = 259 . | Imatinib, N = 260 . | ||
|---|---|---|---|---|
| n . | % (95% CI) . | n . | % (95% CI) . | |
| Complete cytogenetic response | 223 | 86 (81-90) | 213 | 82 (77-86) |
| Confirmed complete cytogenetic response | 208 | 80 (75-85) | 193 | 74 (68-79) |
| Major molecular response | 165 | 64 (58-70) | 120 | 46 (40-52) |
| BCR-ABL transcript level reduction to ≤ 0.0032% | 44 | 17 (13-22) | 22 | 8 (5-13) |
CI indicates confidence interval.
Cumulative incidences of response in dasatinib and imatinib arms. (A) Complete cytogenetic response. (B) Major molecular response. (C) BCR-ABL transcript level reduction to ≤ 0.0032%. CCyR indicates complete cytogenetic response; and MMR, major molecular response.
Cumulative incidences of response in dasatinib and imatinib arms. (A) Complete cytogenetic response. (B) Major molecular response. (C) BCR-ABL transcript level reduction to ≤ 0.0032%. CCyR indicates complete cytogenetic response; and MMR, major molecular response.
Transformation to AP/BP during study treatment occurred in fewer than half the number of patients from the dasatinib arm (6 patients, 2.3%) compared with the imatinib arm (13 patients, 5.0%). A similar trend was seen for the total number of transformations to AP/BP that occurred throughout study follow-up (including during and after study treatment): 9 (3.5%) patients from the dasatinib arm versus 15 (5.8%) patients from the imatinib arm (includes 5 additional patients who transformed to AP/BP after discontinuing from first-line dasatinib or imatinib). Transformation during treatment occurred in 9 patients who achieved CCyR (3 with dasatinib, 6 with imatinib), including 1 patient who achieved MMR (dasatinib arm). At 24 months, PFS rates (defined as survival without loss of response or transformation to AP/BP) for dasatinib versus imatinib were 93.7% versus 92.1%, and FFS rates were 91.2% versus 87.8%. OS rates, including follow-up beyond first-line treatment, for dasatinib versus imatinib, were 95.3% versus 95.2%. Survival data remain immature; all patients, including those who discontinue, will be followed for 5 years.
BCR-ABL mutational analysis
Mutational analysis was stipulated by the protocol in patients who discontinued study treatment (Table 3). A BCR-ABL mutation was detected in 10 patients in each arm, affecting 3 amino acids in dasatinib-treated patients (T315I in 7 patients, F317L in 2 patients, and F317I/ V299L in 1 patient), and 9 amino acids in imatinib-treated patients (M244V, G250E, E255K, F359V, L387M, H396P, and E450G in 1 patient each, E355G in 2 patients, and D276G/F359C in 1 patient). Dasatinib-treated patients who developed a mutation had discontinued after protocol-defined progression in 8 cases, treatment failure in 1 case, and loss of CCyR (not defined as progression or failure) in 1 case. Imatinib-treated patients with a mutation had discontinued after protocol-defined progression in 7 cases and treatment failure in 3 cases. Among patients who developed a mutation, transformation to AP/BP occurred in 4 dasatinib- treated patients (3 with T315I, 1 with F317L), 3 of whom died (1 patient with T315I was alive at last assessment), and 3 imatinib-treated patients (with E255K, F359V, and D276G/F359C), who all died. Median dose intensities in patients who did or did not develop a mutation were 92.5 and 100.0 mg/d dasatinib, and 404.0 and 400.0 mg/d imatinib. Of 37 patients who discontinued therapy but in whom a mutational assessment was not performed or insufficient cDNA was amplified, 3 of 21 patients in the dasatinib arm and 3 of 16 patients in the imatinib arm had discontinued for treatment failure or protocol-defined progression.
BCR-ABL mutations detected in DASISION participants
| . | Dasatinib . | Imatinib . |
|---|---|---|
| Discontinued study treatment, n (%) | 59 (23) | 64 (25) |
| Mutation assay performed, n | 44 | 49 |
| BCR-ABL mutation detected | 10 | 10 |
| No BCR-ABL mutation detected | 28 | 38 |
| Insufficient BCR-ABL cDNA amplified | 6 | 1 |
| . | Dasatinib . | Imatinib . |
|---|---|---|
| Discontinued study treatment, n (%) | 59 (23) | 64 (25) |
| Mutation assay performed, n | 44 | 49 |
| BCR-ABL mutation detected | 10 | 10 |
| No BCR-ABL mutation detected | 28 | 38 |
| Insufficient BCR-ABL cDNA amplified | 6 | 1 |
Evaluation of BCR-ABL mutations was stipulated by the protocol in patients who discontinued study treatment for any reason.
DASISION indicates DASatinib versus Imatinib Study In treatment-Naive CML patients.
Safety
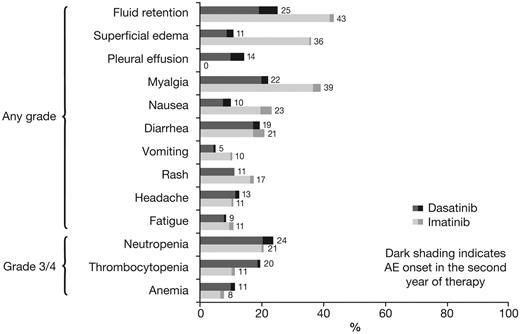
Rates of AEs and biochemical abnormalities are shown in Tables 4 and 5. For all types of drug-related nonhematologic AEs, rates of grade 3/4 AEs were 0%-1% in both arms. Rates of nonhematologic and hematologic AEs after 12 and 24 months of minimum follow-up were comparable, both in the dasatinib and imatinib arms (Figure 3).
Rates of drug-related nonhematologic adverse events that occurred in ≥ 10% of patients
| . | Treated patients, n (%) . | |||
|---|---|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |||
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Fluid retention | 65 (25) | 4 (2) | 112 (43) | 2 (1) |
| Superficial edema | 28 (11) | 0 | 93 (36) | 1 (< 1) |
| Pleural effusion | 37 (14) | 2 (1) | 0 | 0 |
| Myalgia* | 57 (22) | 0 | 101 (39) | 0 |
| Nausea | 26 (10) | 0 | 60 (23) | 0 |
| Diarrhea | 50 (19) | 1 (< 1) | 54 (21) | 3 (1) |
| Vomiting | 13 (5) | 0 | 27 (10) | 0 |
| Rash | 29 (11) | 0 | 45 (17) | 3 (1) |
| Headache | 33 (13) | 0 | 28 (11) | 0 |
| Fatigue | 22 (9) | 1 (< 1) | 28 (11) | 0 |
| . | Treated patients, n (%) . | |||
|---|---|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |||
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Fluid retention | 65 (25) | 4 (2) | 112 (43) | 2 (1) |
| Superficial edema | 28 (11) | 0 | 93 (36) | 1 (< 1) |
| Pleural effusion | 37 (14) | 2 (1) | 0 | 0 |
| Myalgia* | 57 (22) | 0 | 101 (39) | 0 |
| Nausea | 26 (10) | 0 | 60 (23) | 0 |
| Diarrhea | 50 (19) | 1 (< 1) | 54 (21) | 3 (1) |
| Vomiting | 13 (5) | 0 | 27 (10) | 0 |
| Rash | 29 (11) | 0 | 45 (17) | 3 (1) |
| Headache | 33 (13) | 0 | 28 (11) | 0 |
| Fatigue | 22 (9) | 1 (< 1) | 28 (11) | 0 |
Myalgia includes myalgia, muscle inflammation, or musculoskeletal pain.
Rates of grade 3/4 hematologic adverse events and biochemical abnormalities
| . | Treated patients, n (%) . | |
|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |
| Hematologic adverse events | ||
| Neutropenia | 61 (24) | 53 (21) |
| Thrombocytopenia | 50 (19) | 29 (11) |
| Anemia | 29 (11) | 20 (8) |
| Biochemical abnormalities | ||
| Hypophosphatemia | 17 (7) | 64 (25) |
| Hypocalcemia | 8 (3) | 5 (2) |
| Hypokalemia | 0 | 6 (2) |
| Elevated alanine aminotransferase | 1 (< 1) | 4 (2) |
| Elevated aspartate aminotransferase | 1 (< 1) | 2 (1) |
| Elevated total bilirubin | 3 (1) | 0 |
| Elevated creatinine | 3 (1) | 2 (1) |
| . | Treated patients, n (%) . | |
|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |
| Hematologic adverse events | ||
| Neutropenia | 61 (24) | 53 (21) |
| Thrombocytopenia | 50 (19) | 29 (11) |
| Anemia | 29 (11) | 20 (8) |
| Biochemical abnormalities | ||
| Hypophosphatemia | 17 (7) | 64 (25) |
| Hypocalcemia | 8 (3) | 5 (2) |
| Hypokalemia | 0 | 6 (2) |
| Elevated alanine aminotransferase | 1 (< 1) | 4 (2) |
| Elevated aspartate aminotransferase | 1 (< 1) | 2 (1) |
| Elevated total bilirubin | 3 (1) | 0 |
| Elevated creatinine | 3 (1) | 2 (1) |
Change in rates of drug-related nonhematologic and grade 3/4 hematologic adverse events after 12 and 24 months of minimum follow-up. AE indicates adverse event.
Change in rates of drug-related nonhematologic and grade 3/4 hematologic adverse events after 12 and 24 months of minimum follow-up. AE indicates adverse event.
The relative proportion of patients who had different types of AEs was analyzed using a Forest plot to determine whether the rate difference favored dasatinib or imatinib (ie, rates were lower; Figure 4). Of nonhematologic AEs that occurred in ≥ 10% of patients, rates of fluid retention, superficial edema, myalgia, vomiting, and rash were lower for dasatinib compared with imatinib, whereas only pleural effusion was more common with dasatinib; rates of diarrhea, fatigue, and headache were similar in both arms. Of grade 3/4 hematologic AEs, the rate of thrombocytopenia was higher for dasatinib compared with imatinib; rates of neutropenia and anemia were similar. Grade 3/4 bleeding of any type occurred in 2 (1%) dasatinib-treated patients and 3 (1%) imatinib-treated patients. Pleural effusion occurred in 37 (14.3%) dasatinib-treated patients (grade 1 in 9 [3.5%], grade 2 in 26 [10.1%], and grade 3 in 2 patients [0.8%]), and was managed by treatment interruption (n = 30), dose reduction (n = 19), diuretics (n = 17), corticosteroids (n = 15), and therapeutic thoracocentesis (n = 4). In dasatinib-treated patients with or without pleural effusion, responses by 24 months were CCyR in 95% versus 85% and MMR in 68% versus 64%. Five patients (1.9%) discontinued dasatinib because of pleural effusion. Drug-related pulmonary hypertension, indicated by elevated pulmonary artery systolic pressure measured with Doppler echocardiography, occurred in 3 dasatinib-treated patients (1.2%; grade 1 in 2 patients, grade 2 in 1 patient). In 1 case, right heart catheterization was performed but found no evidence of pulmonary arterial hypertension. In the other 2 cases, no further investigations were performed. A diagnosis of pulmonary hypertension was recorded in all 3 cases. No patient discontinued therapy because of pulmonary hypertension. Drug-related cardiac AEs of any type or grade occurred in a similar proportion of patients in the dasatinib and imatinib arms (17 [6.6%] vs 14 [5.4%]). Infections of any type or grade were recorded as a drug-related AE in 27 (10.5%) and 18 (7.0%) patients, respectively.
Forest plot comparing differences in rates of drug-related nonhematologic and grade 3/4 hematologic adverse events for patients treated with dasatinib or imatinib.
Forest plot comparing differences in rates of drug-related nonhematologic and grade 3/4 hematologic adverse events for patients treated with dasatinib or imatinib.
Except for grade 3/4 hypophosphatemia (dasatinib, 7%; imatinib, 25%), other types of grade 3/4 biochemical abnormality occurred in 3% or less and with a similar frequency in the dasatinib and imatinib arms (Table 5). No patient had a grade 3/4 elevation in serum lipase, amylase, or glucose. Five patients discontinued treatment because of biochemical abnormalities: 1 with dasatinib (elevated skeletal creatine phosphokinase with no evidence of myocardial ischemia), and 4 with imatinib (hypophosphatemia and hypocalcemia in 1 patient each, hepatic toxicity in 2 patients).
At last follow-up, 16 dasatinib-treated patients and 14 imatinib-treated patients had died, of which 9 and 10 patients, respectively, died more than 30 days after discontinuing from the trial. Causes of death are shown in Table 6. Five patients from the dasatinib arm died after an infection (2 more than 30 days after discontinuation), because of sepsis/infection (no organism identified; 3 patients), Klebsiella meningoencephalitis, and pneumonia (no organism identified); in 4 patients with available data, there was no evidence of cytopenia at the time of infection. One patient from the imatinib arm died as a result of pneumonia (no organism identified). Of patients who died after infection, the treating investigator concluded that the infection was unrelated to study therapy in 3 of the 5 dasatinib-treated patients and in the single imatinib-treated patient.
Causes of death
| . | Treated patients, n (%) . | |
|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |
| Total deaths | 16 (6) | 14 (5) |
| Death on study treatment or within 30 days of last dose | 7 (3) | 4 (2) |
| Death > 30 days after discontinuing study treatment | 9 (3) | 10 (3) |
| Causes of death | ||
| CML progression | 8 (3) | 10 (4) |
| Cardiovascular disease | 2 (1) | 1 (< 1) |
| Infection | 5 (2) | 1 (< 1) |
| Bleeding | 0 | 1 (< 1) |
| Other neoplasm | 1 (< 1) | 0 |
| Clinical deterioration* | 0 | 1 (< 1) |
| . | Treated patients, n (%) . | |
|---|---|---|
| Dasatinib, N = 258 . | Imatinib, N = 258 . | |
| Total deaths | 16 (6) | 14 (5) |
| Death on study treatment or within 30 days of last dose | 7 (3) | 4 (2) |
| Death > 30 days after discontinuing study treatment | 9 (3) | 10 (3) |
| Causes of death | ||
| CML progression | 8 (3) | 10 (4) |
| Cardiovascular disease | 2 (1) | 1 (< 1) |
| Infection | 5 (2) | 1 (< 1) |
| Bleeding | 0 | 1 (< 1) |
| Other neoplasm | 1 (< 1) | 0 |
| Clinical deterioration* | 0 | 1 (< 1) |
The patient who died after clinical deterioration while receiving imatinib therapy had progressive severe grade 4 peripheral edema despite diuretics, with acute renal failure and rapid decline in performance status ultimately leading to cardiorespiratory arrest. The leading cause of death was unknown.
Discussion
In the initial report describing results from the DASISION trial of patients with newly diagnosed CML-CP, dasatinib 100 mg QD was associated with superior efficacy compared with imatinib 400 mg QD by 12 months of minimum follow-up, thereby meeting the primary end point of the trial.9 After 24 months of minimum follow-up, dasatinib continues to show higher and faster response rates and deeper responses compared with imatinib, supporting earlier findings. Although the difference in cumulative CCyR rates for dasatinib versus imatinib has narrowed from 12% by 12 months (85% vs 73%) to 4% by 24 months (86% vs 82%), the difference in cumulative MMR rates seen by 12 months of 18% (46% vs 28%) has remained consistent by 24 months (64% vs 46%). The median time to MMR was nearly 2 years shorter for dasatinib versus imatinib (15 vs 36 months). Furthermore, a BCR-ABL transcript level of ≤ 0.0032% (4.5-log reduction) was achieved by 17% versus 8% by 24 months. Based on recent findings that ∼ 40% of imatinib-treated patients who had undetectable BCR-ABL transcripts for at least 2 years could stop imatinib therapy without molecular relapse,13 achieving the deepest levels of molecular response is likely to become an increasingly important clinical end point. At present, successful BCR-ABL inhibitor therapy should only be stopped within a clinical trial. Further studies are warranted to determine whether rates of molecular remission after stopping therapy are similar for dasatinib and imatinib.
During the study, a smaller number of patients transformed to AP/BP with dasatinib compared with imatinib (6 vs 13); a similar trend was seen for transformations occurring either on study or after discontinuation. Analyses of several cohorts of patients with CML-CP receiving first-line imatinib have shown that those who respond earlier, for example, CCyR by 6-12 months, have a reduced rate of events, transformation to AP/BP, and death,4,5,14 whereas delayed cytogenetic and molecular response is associated with a progressively increasing risk of events.15 Because responses are achieved faster with dasatinib compared with imatinib, a difference in long-term outcomes between the treatment arms may be observed with longer follow-up. Although PFS, FFS, and OS rates are similar for dasatinib versus imatinib after 2 years, follow-up will continue for 5 years. Detailed analyses will be needed to determine whether future treatment recommendations should take account of faster responses achieved with second-generation agents.
The long-term predictive value of achieving MMR in patients already in CCyR has been debated.3,14 Recent data from the IRIS trial suggested that patients who achieved MMR at 12 or 18 months had an improved rate of transformation-free survival or event-free survival, respectively, compared with patients who had a BCR-ABL transcript level of 0.1% to ≤ 1% at these time points.16 In addition, several analyses have found that MMR increases the stability of CCyR.16-20 Here, only 1 patient who achieved MMR transformed to AP/BP, whereas 8 patients with CCyR but no MMR transformed to AP/BP. This finding underlines importance of achieving MMR to reduce the risk of transformation.
BCR-ABL mutations were detected in a similar proportion of patients who discontinued dasatinib or imatinib (10 per arm, 4% of patients), and outcomes to date were similar in patients who developed mutations in the 2 arms. However, the spectrum of mutations was narrower for dasatinib versus imatinib, with 3 versus 9 different amino acids affected, respectively. The small number of mutations associated with resistance to dasatinib is consistent with in vitro studies6,21 and previous findings in the second-line setting.22-27 The apparent imbalance in T315I mutations with dasatinib (n = 7) and imatinib (n = 0) may reflect the different activity profiles of the 2 agents against BCR-ABL mutants and/or limited follow-up to date. A wider analysis of BCR-ABL mutations from DASISION and/or other studies of first-line dasatinib is needed.
Dasatinib 100 mg QD has an acceptable safety and tolerability profile in patients with CML-CP, and safety findings reported here are consistent with earlier findings.9 In both study arms, few patients discontinued after drug-related toxicity (5%-7%). The frequency of the majority of nonhematologic AEs for dasatinib was comparable or lower than imatinib. Pleural effusion was reported with dasatinib but not imatinib, but was manageable in the majority of cases with dose interruption and/or reduction, and diuretics and/or corticosteroids, and did not impact efficacy. Few patients experienced onset of a new hematologic or nonhematologic AE during the second year of therapy, as shown by similar rates after 12 and 24 months.
In this study, 5 patients died because of infection in the dasatinib arm compared with 1 patient in the imatinib arm. In 3 of the dasatinib-treated patients, the investigator concluded that the infection was unrelated to the study therapy. No predisposing factors were identified in patients who died after infection. Previous analyses of patients with CML-CP treated in the second-line setting have concluded that there is a low risk of infectious events during dasatinib therapy and opportunistic infections are uncommon.28,29 In the current study, rates of infections recorded as a drug-related AE of any grade were 10.5% for dasatinib compared with 7.0% for imatinib. Causes of death among DASISION participants will continue to be closely examined.
Overall, longer follow-up continues to support the use of dasatinib 100 mg QD as first-line treatment for newly diagnosed CML-CP. Efficacy and safety trends in the DASISON trial have been supported by preliminary data from a randomized phase 2b study performed by a US/Canadian intergroup trial (S0325).30 The approval of dasatinib in the first-line CML-CP setting provides an important therapeutic option that enables patients to achieve faster and deeper treatment responses compared with imatinib.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Professional medical writing support was provided by Jeremy Gardner of StemScientific.
This work was supported by research funding from Bristol-Myers Squibb. Funding for medical writing support was provided by Bristol-Myers Squibb.
Authorship
Contribution: H.M.K., M.B., M.B.B.-G., C.Z., and A.H. wrote the manuscript; H.M.K., N.P.S., J.E.C., M.B.B.-G., C.Z., and A.H. participated in data analysis and interpretation; C.Z. was responsible for statistical analysis; M.B., M.B.A., M.S.U., J.W., J.J.K.I., D.-W.K., M.O., C.P., C.J., J.H.M., F.E.N., T.R., J.V.D., and E.V. enrolled patients, collected data, and critically reviewed the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: H.M.K. has provided consultancy for Novartis and received research funding from Bristol-Myers Squibb (BMS), Novartis, and Pfizer. N.P.S. has provided consultancy for BMS, Novartis, and Ariad. J.E.C. has provided consultancy for and received research funding from BMS, Novartis, and Pfizer, and also received research funding from Ariad, Deciphera, and Chemgenex. M.B. has provided consultancy for BMS, Novartis, Pfizer, and Ariad, and received research funding from Novartis. M.S.U. has acted as a speaker for BMS and Novartis. J.W. has provided consultancy for Novartis. J.J.K.I. has provided consultancy for and received research funding from BMS and Novartis, and acted as a speaker for Novartis. D.-W.K. has provided consultancy for and received research funding from BMS, Novartis, and Pfizer, and has acted as a speaker for and received travel grants from BMS and Novartis. C.P. has acted as a speaker for BMS and Novartis. F.E.N. has provided consultancy and acted as a speaker for BMS and Novartis. T.R. has received honoraria and research funding from BMS. J.V.D. has provided consultancy for BMS and Novartis. M.B.B.-G. and C.Z. are employees of BMS. A.H. has provided consultancy for and received research funding from BMS, Novartis, Pfizer, and Ariad. The remaining authors declare no competing financial interests.
Lead investigators of participating centers in the DASISION trial are as follows: Argentina: E. Bullorsky, J. Milone, B. Moiraghi, S. Pavlovsky; Austria: G. Gastl, P. Valent; Australia: S. Durrant, R. Herrmann, A. Nicol, P. Rowlings; Belgium: J. Van Droogenbroeck, A. Ferrant; Brazil: A. Moellmann Coelho, V. Colturato, P. E. Dorlhiac Llacer, R. Pasquini, C. De Souza, M. A. Zanichelli; Chile: M. S. Undurraga; China: J. Hu, X. Huang, Z. Shen, J. Wang; Colombia: L. Enciso, C. Ramirez; Czech Republic: E. Faber, H. Klamova, J. Mayer, J. Voglova; Denmark: J. Stentoft; France: C. Berthou, D. Bordessoule, A. Buzyn, V. Dubruille, M. Escoffre-Barbe, T. Facon, A. Guerci-Bresler, F. Guilhot-Gaudeffroy, R. Herbrecht, F. Huguet, M. Michallet, D. Rea, J.-F. Rossi; Germany: P. Le Coutre, C. Junghanss, M. Soekler, F. Stegelmann; Greece: A. Fassas; Hungary: S. Fekete, M. Udvardy; India: U. Agarwal, V. P. Gangadharan, V. Mathew, G. Narayan, K. Prabhash, T. Saikia, S. Shah; Italy: E. Abruzzese, G. Alimena, C. Gambacorti-Passerini, M. Lazzarino, F. Di Raimondo, G. Saglio; Japan: H. Akiyama, S. Fujisawa, M. Hino, Y. Ishida, K. Ishizawa, K. Matsue, M. Ogura, K. Tamura, M. Tanimoto, M. Taniwaki, K. Usuki, A. Utsunomiya; Mexico: D. G. Almaguer, J. L. Ayala, A. A. Gonzalez, J. J. Kassack Ipiña, R. R. Llamas; The Netherlands: A. V. M. B. Schattenberg, E. Vellenga; Peru: L. Casanova, J. Navarro, J. M. Zenteno; Poland: J. Holowiecki, M. Komarnicki, T. Robak, A. Skotnicki, K. Warzocha; Russia: N. Khoroshko, Y. Shatokhin, A. Zaritsky; Singapore: C. T. H. Chuah; South Korea: D.-W. Kim, K.-H. Lee; Spain: A. Alvarez, C. Boqué, C. Del Canizo, F. Cervantes, A. Jimenez-Velasco, J. Martinez-Dominguez, A. R. Payer, R. De Paz, M. Perez, J. L. Steegmann; Turkey: M. Cetin, I. C. Haznedaroglu; Study Steering Committee: M. Baccarani, J. E. Cortes, A. Hochhaus, H. M. Kantarjian, N. P. Shah.
Dr Santiago Pavlovsky, noted above as a lead investigator of the Argentina center of the DASISION trial, died on September 19, 2010.
Correspondence: Prof Hagop M. Kantarjian, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.