Abstract
B-cell receptor (BCR) signaling is a critical pathway in the pathogenesis of several B-cell malignancies, including chronic lymphocytic leukemia (CLL), and can be targeted by inhibitors of BCR-associated kinases, such as Bruton tyrosine kinase (Btk). PCI-32765, a selective, irreversible Btk inhibitor, is a novel, molecularly targeted agent for patients with B-cell malignancies, and is particularly active in patients with CLL. In this study, we analyzed the mechanism of action of PCI-32765 in CLL, using in vitro and in vivo models, and performed correlative studies on specimens from patients receiving therapy with PCI-32765. PCI-32765 significantly inhibited CLL cell survival, DNA synthesis, and migration in response to tissue homing chemokines (CXCL12, CXCL13). PCI-32765 also down-regulated secretion of BCR-dependent chemokines (CCL3, CCL4) by the CLL cells, both in vitro and in vivo. In an adoptive transfer TCL1 mouse model of CLL, PCI-32765 affected disease progression. In this model, PCI-32765 caused a transient early lymphocytosis, and profoundly inhibited CLL progression, as assessed by weight, development, and extent of hepatospenomegaly, and survival. Our data demonstrate that PCI-32765 effectively inhibits CLL cell migration and survival, possibly explaining some of the characteristic clinical activity of this new targeted agent.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in western societies, is characterized by the accumulation of mature, CD5+CD23+ monoclonal B lymphocytes in the blood, secondary lymphatic tissues, and the bone marrow.1 Proliferating CLL cells, which account for approximately 0.1% to 1% of the CLL clone,2 are typically found within microanatomical structures called proliferation centers or pseudofollicles,3 where CLL cells interact with accessory cells (ie, stromal cells or T cells), thereby receiving survival and growth signals.4 Such external signals from the leukemia microenvironment can supplement intrinsic oncogenic lesions, thereby promoting maintenance and expansion of the CLL clone.3,5,6 Among the various external stimuli in the tissue microenvironments, B-cell receptor (BCR) activation and signaling, particularly in lymphatic tissues,6 is a central pathologic mechanism, even though the precise mechanism of BCR stimulation and the nature of the antigen(s) that activate the BCRs remain obscure.1,7 The most direct evidence for the importance of BCR signaling in CLL comes from recent comparative gene expression profiling (GEP) data that revealed BCR signaling as the most prominent pathway activated in CLL cells isolated from lymphatic tissues.6 These GEP changes displayed remarkable similarity to GEP changes of CLL cells cocultured with monocyte-derived nurselike cells (NLC),8 a system for studying the impact of the lymphatic tissue microenvironment in CLL in vitro. Additional evidence for the importance of BCR signaling in CLL comes from the observation that important CLL risk factors have functional links to the BCRs. The mutation status of the IgVH segments of the BCR distinguishes “mutated” (M-CLL) from “unmutated” CLL (U-CLL), with a low or high risk for disease progression, respectively, each accounting for approximately 50% of the patients. ZAP-70 is predominantly expressed in U-CLL cases,9 and ZAP-70 expression is associated with enhanced BCR signaling.10 Furthermore, CLL patients express restricted sets of BCRs, as determined by BCR sequencing. These BCRs have immunoglobulin (Ig) heavy-chain variable (V) gene sequences that are identical or stereotyped in subsets of patients,11,12 suggesting that these BCRs bind distinct antigens that are relevant to the pathogenesis of CLL. The correlation with prognosis of the amount of somatic mutations in the BCR and the remarkable similarity in amino acid structure of the BCR among unrelated patients suggests that antigen binding, and B-cell selection and stimulation play important roles in disease progression.1,7,13 Finally, cells from poor prognosis U-CLL patients display gene expression profiles suggesting the activation of genes downstream of the BCRs.9
The Bruton tyrosine kinase (Btk), a nonreceptor tyrosine kinase of the Tec kinase family, is a central player in BCR signaling. Btk is primarily expressed in hematopoietic cells, particularly in B cells, but not in T cells or plasma cells.14,15 Btk-deficiency because of mutations in the Btk gene causes X-linked agammaglobulinemia,16,17 which is characterized by low serum immunoglobulin levels and lack of peripheral B cells, manifesting with opportunistic infections in young boys after the normal decrease in protective maternal immunoglobulins occurs. Because of the B-cell restricted phenotype in humans and mice, Btk became an attractive target for developing therapeutics for B-cell lymphomas/leukemias and autoimmune diseases.18 On BCR activation, Btk becomes activated by other tyrosine kinases, such as Lyn and Syk, resulting in phospholipase C activation, intracellular calcium mobilization, and activation of transcription factors necessary for B-cell proliferation and differentiation.19 In addition to its role in antigen-mediated BCR signaling, Btk is also involved in signaling of other cell-surface receptors, such as the CXCR4 and CXCR5 chemokine receptors and adhesion molecules (integrins) that are essential for B-cell trafficking and tissue homing.20-22
PCI-32765 binds specifically and irreversibly to a cysteine residue in the Btk protein and inhibits Btk phosphorylation on Tyr223 and consequently its enzymatic activity.23 PCI-32765 shows encouraging clinical activity in patients with B-cell malignancies, particularly in CLL patients24,25 ; this response is characterized by a rapid resolution of lymphadenopathy and/or organomegaly, accompanied by a transient surge in lymphocyte counts, presumably because of “mobilization” of tissue-resident CLL cells into the blood. Herman et al recently reported that CLL cell apoptosis could be induced in the presence of various exogenous stimuli (CD40L, BAFF, IL-6, IL-4, TNF-α, fibronectin, and stromal cell contact) when Btk was inhibited by PCI-32765.26 To extend these studies and to model the characteristic clinical activity, we investigated the mechanism of action of this compound in systems that mimic CLL cell interactions with the microenvironment, in vitro and in vivo. In addition, we performed correlative studies on samples from patients undergoing therapy with PCI-32765.
Methods
Patient samples, CLL cell isolation, and reagents
After informed consent obtained in accordance with the Declaration of Helsinki on protocols reviewed and approved by the Institutional Review Board at the M. D. Anderson Cancer Center, peripheral blood samples were obtained from patients fulfilling clinical and immunophenotypic criteria for B-cell CLL at the Leukemia Department of the M. D. Anderson Cancer Center. PBMCs were isolated via density gradient centrifugation over Ficoll-Paque (GE Healthcare) and used freshly or viably frozen in fetal bovine serum (FBS: SAFC Biosciences) plus 10% dimethylsulfoxide (DMSO; Sigma-Aldrich) for storage in liquid nitrogen. PCI-32765, provided by Pharmacyclics Inc, was stored as stock solution of 10mM in 100% DMSO at −20°C. This stock solution was diluted in complete RPMI medium with 10% FBS, l-glutamine (HyClone Laboratories), and penicillin-streptomycin (Cellgro), and added to the assay medium to a final concentration of 1μM.
BCR triggering and NLC coculture
To determine the efficacy of the inhibitor to antagonize BCR-derived prosurvival signals after BCR triggering with anti-IgM, CLL samples (107 cells/mL) were preincubated in complete RPMI medium with or without PCI-32765 for 30 minutes at 37°C, and then stimulated by the addition of 10 μg/mL anti-IgM (polyclonal goat F(ab′)2 fragments to human IgM; MP Biomedicals). For coculture with NLCs, fresh CLL cells were suspended in medium to a concentration of 1.5 × 107 cells/mL and incubated for at least 14 days in 12-well plates (Techno Plastic Products), as previously described.27 After 14 days, CLL cells were harvested by thoroughly pipetting. CLL cells were divided into 2 fractions, one was placed back onto the NLCs, and the second fraction was placed into wells without NLCs. CLL cell viability and secretion of the chemokines CCL3 and CCL4 were evaluated at the indicated time points, and compared with respective controls.
Effects of PCI-32765 on TCL1 mouse CLL cell viability
CLL splenic cells isolated from 3 sick TCL1 mice were cultured at 2.5 × 105 cells per well in 96-well plates in medium (RPMI with 10% FBS and 0.1% DMSO) with or without PCI-32765. For BCR stimulation, 0.1 μg/mL F(ab′)2 fragment goat anti–mouse IgM, μ-chain specific antibody from Jackson ImmunoResearch Laboratories were applied at the same time cells were cultured and treated with PCI-32765. Seventy-two hours later, cell viability was determined by flow cytometry analysis on annexin-V and 7-AAD using LSRII (BD Biosciences).
Cell viability
CLL cell viability was measured by analysis of mitochondrial transmembrane potential by 3,3 dihexyloxocarbocyanine iodine (DiOC6; Invitrogen-Molecular Probes) and cell membrane permeability to propidium iodide (PI; Molecular Probes), as described.28 Briefly, 100 μL cell suspension aliquots were collected at the indicated time points and transferred to fluorescence-activated cell sorter tubes containing 300 μL of 60nM DiOC6 and 2 μg/mL PI in RPMI with 0.5% bovine serum albumin (BSA). Cells were incubated in the dark for 20 minutes at 37°C, 20 minutes at room temperature, and then finally analyzed by flow cytometry on a FACSCalibur (BD Biosciences).
3H-thymidine incorporation
CLL cells were plated in triplicates at 0.3 × 107 cells/well in 200 μL full medium in 96-well plates and incubated at 37°C for at least 14 days to achieve NLC cocultures. After NLCs were detectable by microscopy on the well bottom, cells were washed once with fresh medium and 0.2 × 107 CLL PBMC per well in 200 μL were put back into the wells on the NLCs. To test the effect of PCI-32765 on DNA replication, cells were either left untreated (control) or incubated at 37°C with 0.5μM or 1.0μM PCI-32765. After 8 or 32 hours 3H-thymidine (0.5 μCi/10μL; Amersham) was added, and cells were further incubated for 16 hours. Finally, CLL cells were harvested and thymidine incorporated into DNA was measured on a γ counter (Packard TopCount NXT).
Chemokine quantification
We measured CCL3 and CCL4 concentrations in patients' plasma samples before and during therapy with PCI-32765 and cell culture supernatants after CLL-cell activation via anti-IgM and NLCs in the presence or absence of PCI-32765, using Quantikine ELISA kits (R&D Systems) according to the manufacturer's protocol. The optical density at 450 nm (OD450) was read on a microplate reader (EL808; BioTek Instruments). A standard curve containing a blank was prepared for each experiment in the absence of chemokines, and its absorbance was subtracted from that obtained in the presence of the sample. Results were expressed as concentration in pg/mL for each sample.
Chemotaxis toward CXCL12 and CXCL13
Chemotaxis assays across polycarbonate Transwell inserts were performed as previously described.28 Briefly, 107 CLL cells/mL were incubated in complete RPMI medium with or without PCI-32765 at a concentration of 10, 100, or 1000nM for 30 minutes at 37°C. Then, cells were transferred into the top chambers of Transwell culture insert (Costar) with a diameter of 6.5 mm and a pore size of 5 μm. Filters were then placed onto wells containing medium (control) or medium with 200 ng/mL SDF-1α/CXCL12 (Upstate Biotechnology) or 1μg/mL BCA-1/CXCL13 (R&D Systems), and CLL cells were allowed to migrate for 3 hours at 37°C. Migrated cells in the lower chamber were then collected and counted on a FACSCalibur for 20 seconds at 60 μL/min in duplicates.
Actin-polymerization assay
Actin-polymerization assays were performed as previously described.28 Briefly, CLL cells were serum starved for at least 2 hours, then incubated without (control) or with PCI-32765 for 30 minutes. As positive control, cells were incubated with the CXCR4 chemokine receptor antagonist AMD3100 (40μM; Sigma-Aldrich). Cells then were stimulated with 200 ng/mL of CXCL12. To stop the actin polymerization and to fix, permeabilize, and stain the cells, a staining solution containing 1% paraformaldehyde in PBS, FITC-phalloidin, and L-α-Lysophosphatidylcholine was added to aliquots after 15, 60, 120, and 300 seconds, and cells were analyzed for changes in green fluorescence intensities on a FACSCalibur after 20 minutes of additional incubation at 37°C. The relative amount of intracellular F-actin compared with the mean relative fluorescence of the sample before addition of the chemokine was analyzed and plotted.
Immunoblotting
Cells were starved in RPMI + 0.5% BSA for 2 hours at 37°C, then incubated with the inhibitor for 30 minutes at 37°C and stimulated with anti-IgM, SDF-1/CXCL12 or BCA-1/CXCL13, respectively, for 10 minutes at 37°C. Cells were then washed once with ice-cold PBS and lysed for 30 minutes on ice with RIPA buffer containing 150mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50mM Tris-HCl, one mini Complete Protease Inhibitor (Roche Molecular Biochemicals) tablet, and one phosphostop tablet (Roche) per 10 mL of buffer. Then, cells were pelleted at 14 000 rpm for 30 minutes at 4°C and lysates stored at −80°C until further processed. Protein content was determined using the detergent-compatible protein assay kit, according to the manufacturer's instructions (Bio-Rad). Equivalent amounts of total cell protein were boiled with NuPAGE LDS sample buffer (Invitrogen), loaded onto 4% to 12% SDS-polyacrylamide gels (Invitrogen), and then transferred to nitrocellulose membranes (GE Osmonics Labstore). After blotting, membranes were blocked for at least 2 hours in PBS-tween containing 5% nonfat dried milk and then probed with primary antibodies either overnight at 4°C or for at least 2 hours at room temperature against the following proteins: AKT, phospho-AKT (Ser473), ERK/p44/42 MAP kinase, phospho-ERK (Thr202/Tyr204), Btk, phospho-Btk (Tyr223), PLCγ1, phospho-PLCγ1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. The phospho-Btk-antibody was purchased from Epitomics, anti-GAPDH from Abcam; all other antibodies were purchased from Cell Signaling Technology. Blots were incubated with species-specific HRP-conjugated secondary antibody (diluted 1/5000) for 1 hour at room temperature followed by incubation with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) for 5 minutes.
TCL1 CLL adoptive transfer mouse model
CB17 SCID mice and Eμ-TCL1 transgenic (Tg) mice on a C3H/BL6 background (provided by Dr Carlo Croce, The Ohio State University, Columbus, OH) were housed under conventional barrier protection. All animal procedures were performed in accordance with Federal and Institutional Animal Care and Use Committee requirements. We used a TCL1 adoptive transfer CLL mouse model29,30 that uses a TCL1 leukemic clone (TCL1-192), which expresses a BCR reactive with phosphatidylcholine, and that has been serially transferred in vivo, leading to more rapid growth and lethality (mice usually succumb from tumor in ∼ 6 weeks).31 In this study, 5 × 106 TCL1-192 cells were injected into 11 SCID mice. Three weeks after injection, 3 to 4 mice were given drinking water containing sterile control vehicle (1% HP-β-CD) or PCI-32765 in 1% HP-β-CD, at either 0.016 or 0.16 mg/mL. The volume of water consumed and the body weight of mice were recorded; on average, mice took 0, 2.5, or 25 mg/kg/d PCI-32765 for a total of 16 days. During treatment, blood was drawn weekly to track disease progression.
Histology and flow cytometry of mouse cells
Formalin-fixed, paraffin-embedded (FFPE) tissues were cut into 5-μm sections (AML Laboratories) and stained with hematoxylin and eosin. Tumors were classified using morphologic, flow cytometric, and immunohistochemical criteria in accordance with the Mouse Models of Human Cancer Consortium (MMHCC).32 Flow cytometry analysis was done on cell suspensions depleted of red blood cells by lysis and stained with PE-conjugated rat anti–mouse CD45R/B220 and PECy7-conjugated rat anti-CD5 antibodies (BD Pharmingen). Cells counts were performed using calibrated counting beads (Invitrogen) according to the manufacturer's instructions. Intracellular phospho-flow cytometry analysis was performed as previously described.33 In brief, CLL spleen cell populations were either left untreated or exposed to 25μM pervanadate for 4 minutes. Cells were then fixed with 16% paraformaldehyde (EM grade) for 10 minutes at room temperature, permeabilized with ice-cold methanol at 4°C for 10 minutes, and stained with Alexa647-conjugated pY-PLCγ2 antibody (BD Biosciences). Samples were acquired with LSRII (BD Biosciences), and data analyzed using with FlowJo Version 9.2 (TreeStar) or Cell Quest Pro (BD Biosciences) software.
Data analysis and statistics
All statistical analyses were performed using GraphPad Prism 5.0a for Mac. All results are expressed as mean ± SEM. Datasets deemed to be Gaussian or approximately Gaussian by normality testing were compared using 1-way ANOVA or paired Student t test. In the mouse experiments, a repeated measures analysis of variance (RMANOVA) was used to analyze the absolute lymphocyte counts (ALCs). Data were log-transformed to better conform to the model assumptions required in ANOVA. A Bonferroni-type adjustment (P < .01) was used to carry out pairwise comparisons within the ANOVA model.
Results
PCI-32765 interferes with antigen and NLC-mediated survival
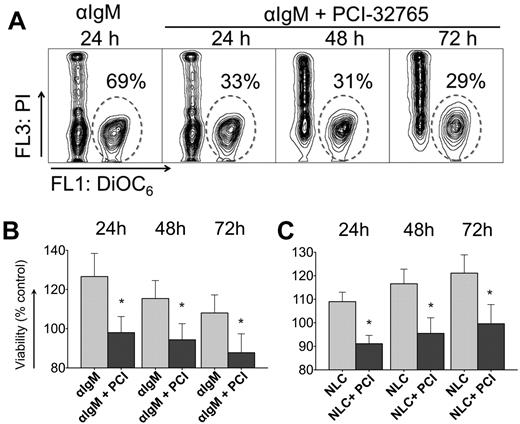
BCR-engagement with anti-IgM and coculture with NLCs leads to significant increase in CLL cell survival compared with control cells, so we wished to determine the effect of PCI-32765 on BCR and NLC-stimulated survival. Figure 1A displays a representative case, in which anti-IgM–supported CLL cell viability was reduced in the presence of PCI-32765 from 69% to 33% at 24 hours, and to 31% and 29% after 48 and 72 hours, respectively. Anti-IgM stimulation induced an average 27% ± 12% increase in viability after 24 hours (mean ± SEM, n = 11) compared with unstimulated controls (controls are normalized to 100%, Figure 1B). Preincubation with 1μM PCI-32765 before anti-IgM stimulation significantly reduced CLL cell viability to 98% ± 8% of unstimulated controls. Survival signals from NLCs were also effectively inhibited by PCI-32765 (Figure 1C). For example, after 24 hours of NLC coculture, CLL cell viability was increased to 109% ± 4% of controls without NLCs (100%, mean ± SEM, n = 15). At 24 hours (and subsequent time points, Figure 1C) PCI-32765 significantly reduced CLL cell viability in NLC coculture to 91% ± 4% (mean ± SEM, P = .0014).
PCI-32765 inhibits anti-IgM and NLC-mediated prosurvival signals in CLL cells. (A) Contour plots of a representative CLL sample, depicting CLL cell viabilities after 24, 48, and 72 hours of incubation with anti-IgM in the presence or absence of PCI-32765, as indicated above the plots. The gates in each of the plots highlight the viable cell populations. (B-C) Bar diagrams representing the mean relative CLL cell viabilities after 24, 48, and 72 hours of anti-IgM stimulation in the presence or absence of PCI-32765 (B, n = 11) or in NLC cocultures (C, n = 15). Viabilities were normalized to the relative viability of control samples with medium (100%) to account for differences in spontaneous apoptosis in samples from different patients. Displayed are means ± SEM. *P ≤ .05.
PCI-32765 inhibits anti-IgM and NLC-mediated prosurvival signals in CLL cells. (A) Contour plots of a representative CLL sample, depicting CLL cell viabilities after 24, 48, and 72 hours of incubation with anti-IgM in the presence or absence of PCI-32765, as indicated above the plots. The gates in each of the plots highlight the viable cell populations. (B-C) Bar diagrams representing the mean relative CLL cell viabilities after 24, 48, and 72 hours of anti-IgM stimulation in the presence or absence of PCI-32765 (B, n = 11) or in NLC cocultures (C, n = 15). Viabilities were normalized to the relative viability of control samples with medium (100%) to account for differences in spontaneous apoptosis in samples from different patients. Displayed are means ± SEM. *P ≤ .05.
PCI-32765 blocks anti-IgM–induced survival of TCL1 CLL cells in vitro
To determine whether murine CLL cells from the TCL1 mouse model display a similar response to anti-IgM stimulation and PCI-32765 in vitro, we cultured leukemia cell–infiltrated splenic cells from 3 TCL1 mice with or without μ-chain specific anti-mouse IgM antibodies and 0, 0.1, or 0.5μM PCI-32765. These concentrations of PCI-32765 did not alter TCL1 splenic CLL cell survival in vitro based on flow cytometry analysis using annexin-V and 7-AAD (supplemental Figure 2 left panel, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). When TCL1 splenic CLL cells were incubated with F(ab′)2 fragments of anti-μ pAbs for 72 hours, we noted a significant increase in viability (P = .005), consistent with that noted with human CLL cells in Figure 1. Treatment with 0.5μM PCI-32765, however, significantly blocked this increased survival (133.1 ± 15.8% vs 80.8 ± 6.7%, P < .05, supplemental Figure 2), suggesting that PCI-32765 represses survival signals delivered through the BCR-signaling pathway.
PCI-32765 inhibits DNA replication in CLL cells cocultured with NLC
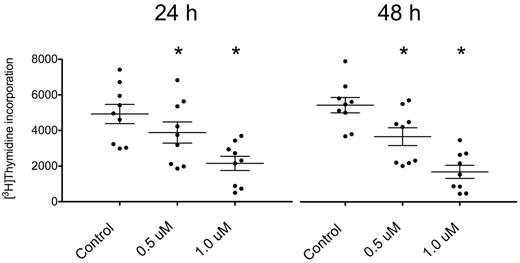
The uptake of 3H-thymidine by CLL cells cocultured with NLCs was significantly reduced by PCI-32765 in a dose-dependent manner (Figure 2, n = 9). After 24 hours, the incorporated thymidine decreased from 4926 ± 540 to 3884 ± 593 (mean ± SEM, P = .0004) with 0.5μM PCI-32765 (decrease of 21%) and to 2154 ± 397 (mean ± SEM, P < .0001) with 1μM PCI-32765 (decrease of 56%). After 48 hours, the values reached 5474 ± 434 in the untreated cells, 3693 ± 501 (mean ± SEM, P = .0007) with 0.5μM PCI-32765 (decrease of 33%), and 1702 ± 370 (mean ± SEM, P < .0001) with 1.0μM (decrease of 69%). Importantly, untreated CLL cells showed a slight increase of thymidine uptake (11% increase) from 24 to 48 hours, which was not observed after treatment with the inhibitor. This suggests that a subset of proliferating CLL cells in the coculture is inhibited by PCI-32765. These findings are consistent with the earlier report about inhibition of CpG-induced CLL cell proliferation by PCI-32765 by Herman et al.26
3H-thymidine incorporation by CLL cells in coculture with NLCs is decreased after treatment with PCI-32765.3H-thymidine uptake in CLL cells cocultured with NLC was measured with a scintillation counter. Cells were either left untreated (control) or incubated with 2 different concentrations of PCI-32765 (0.5 or 1.0μM) for 24 hours (left panel) or 48 hours (right panel), respectively. The uptake of the tritiated nucleoside was significantly decreased in cells treated with the inhibitor. In untreated CLL cells, a slight increase between 24 and 48 hours was observed, suggesting proliferation in a small subset of CLL cells cocultured with NLC. In CLL cells treated with PCI-32765 this proliferation was not observed. Shown are mean values ± SEM of 9 patients. *P ≤ .05.
3H-thymidine incorporation by CLL cells in coculture with NLCs is decreased after treatment with PCI-32765.3H-thymidine uptake in CLL cells cocultured with NLC was measured with a scintillation counter. Cells were either left untreated (control) or incubated with 2 different concentrations of PCI-32765 (0.5 or 1.0μM) for 24 hours (left panel) or 48 hours (right panel), respectively. The uptake of the tritiated nucleoside was significantly decreased in cells treated with the inhibitor. In untreated CLL cells, a slight increase between 24 and 48 hours was observed, suggesting proliferation in a small subset of CLL cells cocultured with NLC. In CLL cells treated with PCI-32765 this proliferation was not observed. Shown are mean values ± SEM of 9 patients. *P ≤ .05.
PCI-32765 down-regulates CCL3 and CCL4 levels in CLL-NLC cocultures and in patients receiving therapy with PCI-32765
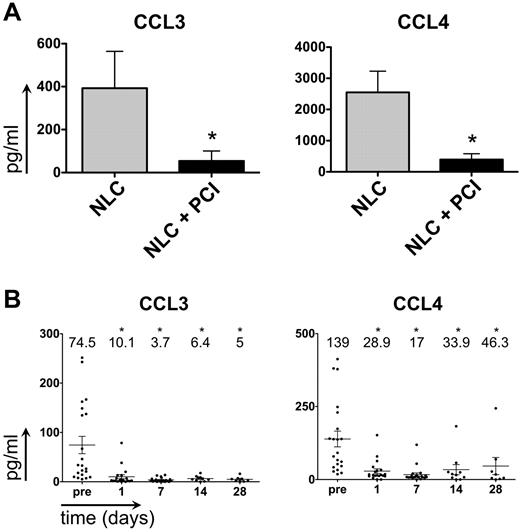
CCL3 and CCL4 are chemokines secreted by CLL cells in response to BCR activation and during coculture with NLCs.8,34 Moreover, plasma CCL3 levels are a robust prognostic marker for CLL disease progression.35 Consistent with previous results,8 we found that CLL-NLC coculture supernatants contained high CCL3 and CCL4 concentrations after 24 hours (Figure 3A; CCL3: 393 pg/mL ± 172 pg/mL; CCL4: 2550 pg/mL ± 678 pg/mL, mean ± SEM, n = 11). Secretion of both chemokines was significantly inhibited by PCI-32765 (CCL3: 54 pg/mL ± 46 pg/mL, P = .0417; CCL4: 394 pg/mL ± 188pg/mL, mean ± SEM, P = .0059). Plasma samples from CLL patients administered PCI-3276524 also contained relatively high levels of CCL3, and CCL4 (Figure 3B; CCL3: 75 pg/mL ± 18 pg/mL; CCL4: 139 pg/mL ± 27 pg/mL mean ± SEM, n = 20). After treatment with PCI-32765, CCL3 and CCL4 levels rapidly and significantly dropped, and remained low in most patients. For example, after 1 day of treatment with PCI-32765, significantly lower CCL3 and CCL4 concentrations were detected (CCL3: 10 pg/mL ± 4 pg/mL, mean ± SEM, P = .0008; CCL4: 29 pg/mL ± 8 pg/mL, mean ± SEM, P = .0002).
PCI-32765 down-regulates CCL3 and CCL4 secretion in vitro (in NLC cocultures) and in vivo in plasma samples of CLL patients undergoing therapy with PCI-32765. (A) Incubation with PCI-32765 significantly inhibits the secretion of CCL3 and CCL4 in CLL-NLC cocultures. The bars in this graph represent mean ± SEM CCL3 (left graph) and CCL4 (right graph) concentrations in supernatants of CLL-NLC cocultures from 11 different CLL samples in pg/mL. *P ≤ .05. (B) CCL3 (left graph) and CCL4 (right graph) plasma concentrations in CLL patients before and during therapy with PCI-32765. Displayed are individual plasma concentrations, indicated by the dots, and mean ± SD plasma concentrations, as indicated by the horizontal lines. CCL3 and CCL4 plasma concentrations were measured at the time points indicated on the horizontal axes. * indicates significant changes with P ≤ .05.
PCI-32765 down-regulates CCL3 and CCL4 secretion in vitro (in NLC cocultures) and in vivo in plasma samples of CLL patients undergoing therapy with PCI-32765. (A) Incubation with PCI-32765 significantly inhibits the secretion of CCL3 and CCL4 in CLL-NLC cocultures. The bars in this graph represent mean ± SEM CCL3 (left graph) and CCL4 (right graph) concentrations in supernatants of CLL-NLC cocultures from 11 different CLL samples in pg/mL. *P ≤ .05. (B) CCL3 (left graph) and CCL4 (right graph) plasma concentrations in CLL patients before and during therapy with PCI-32765. Displayed are individual plasma concentrations, indicated by the dots, and mean ± SD plasma concentrations, as indicated by the horizontal lines. CCL3 and CCL4 plasma concentrations were measured at the time points indicated on the horizontal axes. * indicates significant changes with P ≤ .05.
PCI-32765 inhibits CLL cell chemotaxis, actin-polymerization, and activation of BCR, CXCL12, and CXCL13 signaling
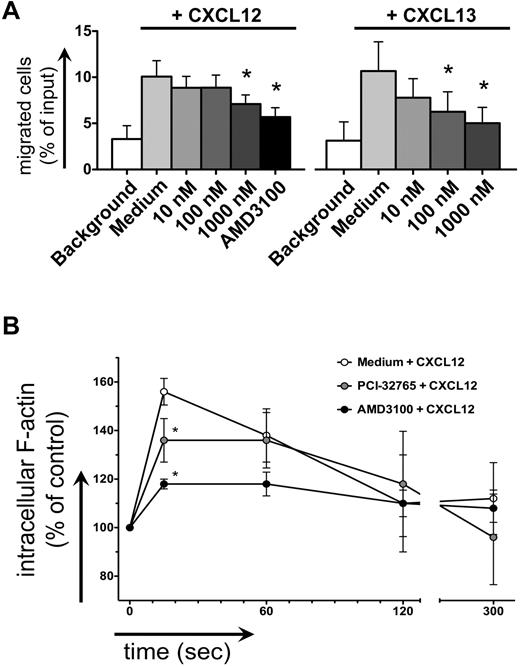
CLL cell chemotaxis toward CXCL12 and CXCL13 was inhibited after pretreatment with PCI-32765 (Figure 4A). One μM PCI-32765 significantly decreased CLL cell migration toward CXCL13 from 11% to 6% (mean ± SEM, n = 7, P = .04) and toward CXCL12 from 11% to 8% of input cells (mean ± SEM, n = 9, P = .02), compared with the CXCR4 antagonist AMD3100, which decreased chemotaxis to 6% ± 1%. Intracellular F-actin content after stimulation with 200 ng/mL CXCL12 in CLL cells increased by 56% ± 2% (mean ± SEM, n = 5, Figure 4B) compared with the baseline before chemokine stimulation. This increase was significantly inhibited by 1μM PCI-32765, where the increase in F-actin reached only 36% ± 4% (mean ± SEM, P = .011), compared with 18% ± 2% in the presence of AMD3100 (mean ± SEM, P = .0005).
Inhibition of CLL cell chemotaxis and actin polymerization. (A) Displayed is the mean ± SEM relative migration of CLL cells from 8 different patients toward CXCL12 (left graph) and CXCL13 (right graph) in the presence or absence (medium control) of different concentrations of PCI-32765, as indicated on the horizontal axes. White bars depict background migration toward wells without chemokine. (B) Displayed is the relative F actin content of CLL cells after stimulation with CXCL12 staining with FITC-labeled phalloidin in the presence or absence of PCI-32765 (or AMD3100 as control) at the time points indicated on the horizontal axis. * indicates P ≤ .05 compared with the control with chemokine; medium.
Inhibition of CLL cell chemotaxis and actin polymerization. (A) Displayed is the mean ± SEM relative migration of CLL cells from 8 different patients toward CXCL12 (left graph) and CXCL13 (right graph) in the presence or absence (medium control) of different concentrations of PCI-32765, as indicated on the horizontal axes. White bars depict background migration toward wells without chemokine. (B) Displayed is the relative F actin content of CLL cells after stimulation with CXCL12 staining with FITC-labeled phalloidin in the presence or absence of PCI-32765 (or AMD3100 as control) at the time points indicated on the horizontal axis. * indicates P ≤ .05 compared with the control with chemokine; medium.
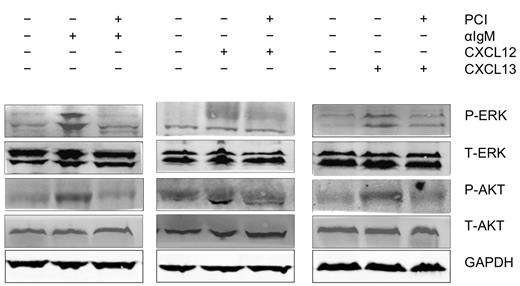
BCR engagement with anti-IgM, as well as stimulation with the chemokines CXCL12 and CXCL13 initiated activation of p44/42 MAP kinase (ERK) and AKT. As shown in Figure 5, activation of these signaling molecules was inhibited by preincubation of the CLL cells with PCI-32765.
Abrogation of signaling downstream of the BCRs and of CXCR4 and CXCR5 receptors. Displayed are immunoblots from CLL cells from 1 representative patient of 9 patients, that were either unstimulated or stimulated for 10 minutes with anti-IgM (αIgM, left row), CXCL12 (middle row), or CXCL13 (right row) in the presence or absence of PCI-32765 as indicated. P indicates immunoblotting for the active, phosphorylated form, whereas T represents the nonphosphorylated, total amount of the respective proteins.
Abrogation of signaling downstream of the BCRs and of CXCR4 and CXCR5 receptors. Displayed are immunoblots from CLL cells from 1 representative patient of 9 patients, that were either unstimulated or stimulated for 10 minutes with anti-IgM (αIgM, left row), CXCL12 (middle row), or CXCL13 (right row) in the presence or absence of PCI-32765 as indicated. P indicates immunoblotting for the active, phosphorylated form, whereas T represents the nonphosphorylated, total amount of the respective proteins.
PCI-32765 delays CLL disease progression in an adoptive transfer TCL1 mouse model
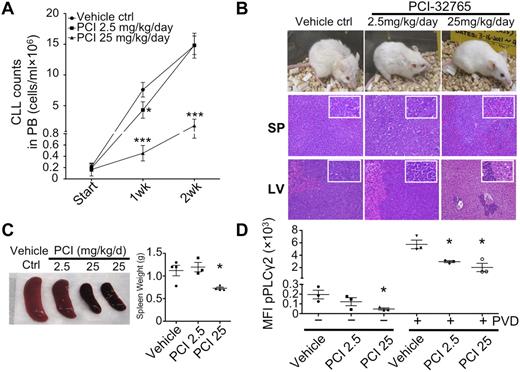
To model the effects of PCI-32765 on the number of circulating and tissue-based lymphocytes, PCI-32765 was examined in an EμTCL1 adoptive transfer mouse model that resembles CLL in patients. In this model, transfer of TCL1 leukemia cells in SCID mice leads to a dose-dependent, accelerated disease in which mice succumb 5 to 6 weeks after cell transfer. We used this approach, treating mice with suboptimal (2.5 mg/kg/d, n = 3) and optimal (25 mg/kg/d, n = 4) doses of PCI-32765 for 3 continuous weeks after cell transfer (untreated control mice n = 4). Interestingly, after 1 week of treatment, lymphocyte counts had fallen to a level lower than those of untreated animals (Figure 6A). After 2 weeks of treatment, animals receiving the optimal PCI32765 dose had reduced circulating ALCs, indicating that starting PCI-32765 treatment at 3 weeks postcell transfer delayed disease significantly under these conditions (Figure 6A).
Delayed CLL disease progression by PCI-32765 in TCL1 model. (A) Peripheral blood (100 μL) from mice treated with control vehicle (n = 4), 2.5 (n = 3), or 25 mg/kg/d (n = 4) PCI-32765 were incubated with B220 and CD5 antibodies. Numbers of CLL cells were determined using calibrated counting beads. Results shown are the mean CLL cell counts ± SD with RMANOVA statistic analysis on control versus treated mice. *P < .01, ***P < .0001. (B) All 11 mice were killed 16 days after treatment. Top: example of each group of mice after 16 days of treatment. Spleen (middle) and liver (bottom) tissue sections were assessed histologically after H&E staining. Less lymphocyte infiltration was observed in 25 mg/kg/d PCI-32765 treated mice compared with control mice. (C) Spleens from control or PCI-32765 treated mice were compared for size and weight. The right panel shows the mean spleen weight ± SD with the unpaired t test on control versus treated mice. *P < .05. (D) Spleen cells from mice treated for 16 days with vehicle or PCI-32765 were left untreated (−) or treated with 25μM pervanadate (PVD) for 4 minutes (+). Intracellular staining of phosphorylated PLCγ2 (pPLCγ2) was then performed and analyzed by flow cytometry on gated B220+CD5+ cells. Results are the mean fluorescence intensity (MFI) of phosphorylated-PLCγ2 ± SD with the unpaired t test on control versus treated mice. *P < .05.
Delayed CLL disease progression by PCI-32765 in TCL1 model. (A) Peripheral blood (100 μL) from mice treated with control vehicle (n = 4), 2.5 (n = 3), or 25 mg/kg/d (n = 4) PCI-32765 were incubated with B220 and CD5 antibodies. Numbers of CLL cells were determined using calibrated counting beads. Results shown are the mean CLL cell counts ± SD with RMANOVA statistic analysis on control versus treated mice. *P < .01, ***P < .0001. (B) All 11 mice were killed 16 days after treatment. Top: example of each group of mice after 16 days of treatment. Spleen (middle) and liver (bottom) tissue sections were assessed histologically after H&E staining. Less lymphocyte infiltration was observed in 25 mg/kg/d PCI-32765 treated mice compared with control mice. (C) Spleens from control or PCI-32765 treated mice were compared for size and weight. The right panel shows the mean spleen weight ± SD with the unpaired t test on control versus treated mice. *P < .05. (D) Spleen cells from mice treated for 16 days with vehicle or PCI-32765 were left untreated (−) or treated with 25μM pervanadate (PVD) for 4 minutes (+). Intracellular staining of phosphorylated PLCγ2 (pPLCγ2) was then performed and analyzed by flow cytometry on gated B220+CD5+ cells. Results are the mean fluorescence intensity (MFI) of phosphorylated-PLCγ2 ± SD with the unpaired t test on control versus treated mice. *P < .05.
Five weeks after cell transfer, control mice and mice treated for 2 weeks with the suboptimal dose of PCI-32765 exhibited lethargy, hunched posture, ruffled and lost fur, and weight loss (Figure 6B). Furthermore, these animals had massive lymphocytosis, hepatosplenomegaly, and lymphadenopathy. In contrast, mice receiving the optimal dose of PCI32765 appeared to be healthy, having significantly smaller livers and spleens with markedly reduced leukemic infiltration (Figure 6B-C). There were also significantly repressed levels of phosphor-PLCγ2 in spleen cells of optimally treated mice (Figure 6D). Overall, the data suggest that PCI32765 considerably delays CLL progression in vivo.
PCI-32765 causes an initial lymphocytosis in the adoptive transfer TCL1 mouse model
In patients, administration of PCI-32765 often results in a rapid and at times prolonged lymphocytosis that is probably because of exiting of CLL cells from solid lymphoid tissues. To determine whether PCI-32765 similarly affected the number of circulating lymphocytes in the TCL1 adoptive transfer mouse model, animals were treated with PCI-32765 2-weeks postcell transfer and then bled to track changes in blood lymphocyte counts. Indeed, animals treated at 2 weeks postcell transfer with the suboptimal (2.5 mg/kg/d) and optimal (25 mg/kg/d) doses exhibited a transient lymphocytosis at day 4, with an average of 7- and 10-fold increases in circulating TCL1 leukemia cells, respectively. However, by day 7 these levels had fallen to the same as those of untreated mice. Until weeks 5 to 6, animals receiving the optimal PCI-32765 dose had reduced ALCs (supplemental Figure 1).
Discussion
BCR signaling is a central factor in the pathogenesis of different B-cell malignancies, including CLL,6,36 where it promotes maintenance and expansion of the B-cell clone. PCI-32765, an inhibitor of the BCR-signaling kinase Btk, is clinically active in patients with B-cell lymphomas, particularly those with CLL, including high-risk CLL patients. In CLL patients, PCI-32765 is administered orally once-a-day, and characteristically causes a rapid shrinkage and normalization of lymphadenopathy and/or organomegaly within the first weeks of therapy, along with a transient surge in peripheral lymphocyte counts.24 This lymphocytosis typically improves after the first month of therapy, and then normalizes. Interestingly, an early clinical experience when PCI-32765 was administered for 28 days followed by 7 days off drug led to a saw-tooth–like pattern of peripheral lymphocyte counts, with transient increases in lymphocytosis during treatment, and rapid declines in lymphocyte counts during the days off treatment.24 These observations suggest that PCI-32765 treatment results in a compartment shift of CLL cells from infiltrated tissues into the blood. This process is accompanied by increased cell death or decreased cell birth, as inferred from the development of objective major clinical responses over time. The objective of the current study was to explicate the detailed mechanisms of these effects of PCI-32765 treatment.
We have studied PCI-32765 in a series of in vitro assays that model interactions of CLL cells with their microenvironment. PCI-32765 significantly inhibited CLL cell migration and survival in response to physiologically relevant stimuli. We further demonstrated that it blocks BCR and chemokine receptor-related intracellular signals, and secretion of CLL cell-derived chemokines (CCL3 and CCL4). These findings build on those of Herman et al, who recently reported that PCI-32765 blocks CLL cell proliferation and several external prosurvival pathways.26 The inhibition of CLL cell chemotaxis toward the tissue homing chemokines CXCL12 and CXCL13 by PCI-32765, as is here demonstrated for the first time, is likely to be fundamental to the early efflux of CLL cells from the tissues into the blood, most probably along with inhibition of adhesion molecule function/signaling.20,22
Our in vitro data are corroborated by in vivo data in the adoptive transfer TCL1 mouse model, which displays an early transient lymphocytosis (supplemental Figure 1), similar to the pattern observed in CLL patients. It remains to be determined whether PCI-32765 acts primarily through CLL cell mobilization, with subsequent CLL cell death because of deprivation from CLL cell growth-promoting tissue microenvironments. However, the effects of PCI-32765 on 3H-thymidine incorporation (Figure 2) and on ODN-induced proliferation,26 suggest that PCI-32765 has an additional, more direct effect on CLL cell survival and growth, which again is supported by the in vivo mouse model data. Unlike the original TCL1 mouse model,29 this adoptive transfer model causes relatively rapid disease progression, which allows for drug testing under predictable, controllable conditions. If PCI-32765 therapy is started at 2 weeks after CLL cell transfer, lymphocytosis can be observed, and then the antiproliferative effect of PCI-32765 dominates, as evidenced by concurrent marked reduction of circulating lymphocyte counts and hepatosplenomegaly (Figure 6). These in vitro and in vivo data demonstrate that PCI-32765 has a dual activity, inhibiting both leukemia cell migration and tissue retention/homing on one hand, and CLL cell survival and proliferation on the other.
Another interesting aspect of this study is related to the chemokines CCL3 and CCL4, which are secreted by normal37 and CLL8 B cells in response to BCR activation, presumably for promoting cognate interactions with accessory T cells. Untreated CLL patients have elevated plasma levels of both chemokines, and plasma levels correlate with prognostic markers and time to therapy,35 suggesting that the ability to signal through the BCR pathway of a CLL clone is critical for disease progression. PCI-32765 effectively and rapidly reduced and normalized CCL3 and CCL4 plasma levels in CLL patients treated with PCI-32765 (Figure 3B), consistent with inhibition of NLC-induced CCL3 and CCL4 secretion by CLL cells in lymphatic tissues. This is further supported by recent gene expression data of CLL cells isolated from different tissues, which demonstrate that CCL3 and CCL4, along with other BCR-regulated genes, were among the most up-regulated in CLL cells isolated from the secondary lymphatic tissues.6 Collectively, these data indicate that CCL3 and CCL4 could function as biomarkers for BCR activation of CLL cells and for response assessment after therapies targeting the BCR-signaling pathway.
In conclusion, our data demonstrate that treatment with PCI-32765 antagonizes BCR-related activation signals in vitro and in vivo. Furthermore PCI-32765 also affects CLL cell migration and tissue homing via inhibition of chemokine receptor function. These data provide mechanistic insight into the fascinating clinical activity of this novel, molecularly targeted drug in CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the clinical investigators as part of our PCI-32765 phase 1/2 study that provided CLL samples for analysis in this study. In addition, they thank Ms Ruth LaPushin who provided CLL samples and Mr Archie Tamayo and Mr Lan Pham of the Department of Hematopathology, M. D. Anderson Cancer Center, for their assistance with the thymidine assay procedure.
The study was supported by CLL Global Research Foundation grants (W.G.W., V.G., and J.A.B.), by Pharmacyclics Inc, and by a Cancer Prevention and Research Institute of Texas (CPRIT) grant (J.A.B.).
Authorship
Contribution: S.P. performed the in vitro experiments, analyzed the data, designed the figures, and wrote the paper; S.-S.C. and N.C. performed the mouse experiments, analyzed the data, designed the figures and revised the paper; J.J.B. helped with the study design, analyzed data, provided plasma samples, and revised the paper; K.B. performed immunoblots, and revised the paper; V.G. directed K.B. and reviewed the paper; S.O., W.G.W., and M.J.K. provided patient samples and data, and revised the paper; and J.A.B. designed the research, supervised the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: S.B. and J.A.B. are consultants for Pharmacyclics Inc and received research support from Pharmacyclics Inc. J.J.B. is an employee and shareholder of Pharmacyclics Inc. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, Dept of Leukemia, Unit 428, University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.
References
Author notes
S.P. and S.-S.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal