Abstract
The JAK2V617F mutation is present in the majority of patients with polycythemia vera and one-half of those with essential thrombocythemia and primary myelofibrosis. JAK2V617F is a gain-of-function mutation resulting in constitutive JAK2 signaling involved in the pathogenesis of these diseases. JAK2V617F has been shown to promote S-phase entry. Here, we demonstrate that the CDC25A phosphatase, a key regulator of the G1/S cell-cycle transition, is constitutively overexpressed in JAK2V617F-positive cell lines, JAK2-mutated patient CD36+ progenitors, and in vitro–differentiated proerythroblasts. Accordingly, CDC25A is overexpressed in BM and spleen of Jak2V617F knock-in mice compared with wild-type littermates. By using murine FDC-P1–EPOR and human HEL and SET-2 cell lines, we found that JAK2V617F-induced CDC25A up-regulation was caused neither by increased CDC25A transcription or stability nor by the involvement of its upstream regulators Akt and MAPK. Instead, our results suggest that CDC25A is regulated at the translational level through STAT5 and the translational initiation factor eIF2α. CDC25A inhibition reduces the clonogenic and proliferative potential of JAK2V617F-expressing cell lines and erythroid progenitors while moderately affecting normal erythroid differentiation. These results suggest that CDC25A deregulation may be involved in hematopoietic cells expansion in JAK2V617F patients, making this protein an attracting potential therapeutic target.
Introduction
A unique somatic mutation, JAK2V617F, recently was described in myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1-4 This mutation promotes cytokine independence in cell lines and is sufficient to induce MPNs in mouse models, suggesting that it represents a major molecular event in the pathogenesis of these diseases.5,6 At the molecular level, JAK2V617F leads to JAK2 tyrosine kinase autophosphorylation and constitutive activation of downstream cell signaling pathways, including STAT, MAPK/Erk, and PI3K.1,4 Although these pathways are known to be involved in cell proliferation and survival, the link between JAK2V617F and hematopoietic cell expansion remains not entirely understood.
JAK2V617F has been shown to promote G1/S cell cycle transition in parallel with p27kip1 down-regulation and cyclin D2 induction in HEL and Ba/F3-EPOR cell lines.7,8 Cell cycle progression is controlled by cyclin/cyclin-dependent kinase (CDK) complexes that are inactive when phosphorylated. One of the key regulators of the G1/S transition is CDC25A, a member of the CDC25 dual (Tyr/Thr) specificity phosphatase family. The CDC25 enzymes stimulate cell proliferation by dephosphorylating the 2 inhibitory residues of CDK1 and CDK2.9 The main target of CDC25A is the cyclin E– or cyclin A–associated CDK2, whose activation is necessary for both the full completion of the G1 phase and DNA synthesis activation. It has been shown that CDC25A expression can be regulated by transcriptional factors such as E2F-1,10 c-myc,11 and STAT3.12 Moreover, the posttranslational down-regulation of CDC25A in response to genotoxic stress by the ubiquitin-dependent proteasomal degradation pathway has been greatly documented.13 This degradation involves CDC25A phosphorylation by different kinases, including CHK1, CHK2, p38 MAPK,14 and GSK3β,15 but can be counteracted by deubiquitylation by Dub3.16
Up-regulation of CDC25A has been reported in various solid cancers, in which it is frequently correlated with a poor prognosis.9 Moreover, the authors of recent studies confirmed a role of CDC25A in oncogene-dependent tumorigenesis in mice.17,18 Previously, we have established that CDC25A is up-regulated downstream of integrins in acute myeloid leukemia19 and thus participates in leukemic cell proliferation induced by their adhesion to fibronectin. More recently, we described CDC25A overexpression in cells expressing different tyrosine kinase oncogenic products such as NPM-ALK (present in 70% of anaplastic large cell lymphomas), BCR-ABL (responsible for chronic myeloid leukemia), and FLT3-ITD (a mutated form of the FLT3 receptor found in 30% of patients experiencing acute myeloid leukemia).20 As far as NPM-ALK is concerned, we have reported that CDC25A regulation involves both transcriptional and posttranslational mechanisms downstream of the PI3K/Akt pathway.20
Whether CDC25A is a target for JAK2V617F had never been documented. This question could be of high interest in the light of the preparation of potent CDC25 inhibitors.9,21,22 One of them, IRC-083864, is entered in clinical trial phase 2 under the name of Debio 0931.22 In this study, we show that JAK2V617F expression results in enhanced CDC25A expression and that CDC25 inhibition leads to a dramatic reduction of JAK2V617F erythroid progenitor proliferation.
Methods
Cell lines culture and treatment
Murine JAK2WT- and JAK2V617F-expressing FDC-P1-EPOR, human erythroleukemia HEL (expressing JAK2V617F; ATCC), and SET-2 (expressing both JAK2V617F and JAK2WT) cell lines were cultured in RPMI medium supplemented with 10% fetal FBS (Invitrogen) and 1 IU/mL human recombinant erythropoietin (EPO) for JAK2WT-expressing cells. Murine JAK2WT- and JAK2V617F-expressing BA/F3-EPOR cells were cultured in DMEM supplemented with 10% FBS and 1 IU/mL EPO for JAK2WT-expressing cells.
For inhibition of signaling pathways, cells were preincubated with AG490, JAK2 inhibitor II, STAT5 inhibitor, salubrinal, or cycloheximide (Calbiochem). IRC-083864 was synthesized by IPSEN (Biomeasure), as described previously.21
Patient samples
BM samples from JAK2V617F-positive and JAK2WT patients were obtained after informed consent in accordance with the Declaration of Helsinki and stored at the HIMIP collection, at the Tumorothèque Cancer-Est, and at the hematologic malignancies tissue bank, an institutional review board–approved protocol at the University of Pennsylvania. According to the French law, HIMIP collection has been declared to the Ministry of Higher Education and Research (DC 2008-307 collection 1) and obtained a transfer agreement (AC 2008-129) after approbation by ethical committees (Comité de Protection des Personnes Sud-Ouest et Outremer II and APHP ethical committee). Clinical and biologic annotations of the samples from French laboratories have been declared to the CNIL (Comité National Informatique et Libertés).
Purification of CD36+ cells and CD34+ cells
Mononuclear cells were purified from BM by Ficoll-Hypaque density gradient centrifugation. Next, CD36+ cells were obtained by positive selection on magnetic beads (Miltenyi Biotec) after incubation with an anti-CD36 antibody (Beckman Coulter). The purity of recovered cells was always > 97% as determined by cytometry.
Purification of CD34+ cells was performed by magnetic positive selection (StemCell Technologies) in accordance to the manufacturer's recommendation. The purity of recovered cells was always > 95% as determined by flow cytometry. The presence of JAK2V617F mutation was determined by allelic discrimination as previously described.23
Mouse BM and spleen lysates
Mouse BM and spleen cells were isolated from Jak2V617F knock-in (KI) animals and wild-type (WT) littermates, as previously described,6 counted, and lysed in Laemmli buffer.
Immunohistochemistry
BM samples were fixed in 10% buffered formalin or in Duboscq-Brazil (alcohol-based Bouin), embedded in paraffin, and processed for routine histopathologic examination. Then, 3-μm-thick sections were stained with H&E. For immunohistochemical examination, paraffin sections (3-γm-thick sections) were rehydrated and subjected to microwaving (750W at 6 minutes) with the use of 1 mmol/L EDTA buffer, pH 8, as the antigen retrieval solution. Then, 3-μm-thick sections were tested with a TechMate (Glostrup). The panel included antibodies directed against CDC25A (polyclonal; Santa Cruz Biotechnology). Staining was performed on CDC25A and detected with a Leica DMR microscope (Rueil-Malmaison and IM50 software; Leica).
Western blot
Western blotting was performed via the use of the following primary antibodies: anti-CDC25A (F6), anti-CDC25C (C20), and anti-Cyclin A (C19 and BF683; Santa Cruz Biotechnology); anti-p27 (BD Biosciences Pharmingen); anti–phospho-Akt (Ser 473), anti-Akt, anti–phospho-STAT5 (Tyr694), anti-STAT5, anti–phospho-JAK2 (Tyr1007/1008), anti-JAK2, anti-Erk, anti–phospho-eIF-2α (ser 51), and anti–eIF-2α (Cell Signaling Technologies); anti–phospho-ERK and anti-α tubulin (Sigma-Aldrich); or anti–β-actin (Lab Vision). Quantifications were performed with the use of Quantity One Software.
Cell cycle analysis
Cells were labeled with 10μM BrdU for 1 hour, washed with PBS, and fixed in cold 70% ethanol for 20 minutes. BrdU detection was performed with the BrdU staining kit from BD Pharmingen and cell cycle distribution with a 30-minute propidium iodide staining (Invitrogen). Fluorescence was analyzed on a BDLSRII cytometer (BD Biosciences).
SiRNA transduction
The FDC-P1-EPOR-JAK2V617F cell line was transfected with the Amaxa nucleofection technology (Amaxa). Cells (2 × 106) were resuspended in 100 μL of Amaxa solution L. Specific STAT5A and STAT5B siRNA (2 × 30 pmol; ON-TARGETplus SMARTpool, mouse STAT5A and STAT5B; Dharmacon) or negative control (60 pmol; AllStars Negative Control siRNA; QIAGEN) were added, and cells were transfected with the nucleofector device (program D-17). Cells were subsequently resuspended in normal culture medium at a concentration of 3 × 105 cells/mL. Twelve hours after transfection, cells were counted (trypan blue staining), and Western blotting was performed.
CD34+ cells were sorted as previously described, cultured overnight in erythroid differentiation culture medium previously described, and nucleofected with the use of Amaxa nucleofection technology. Cells (3.4 × 105 to 2.1 × 106) were resuspended in 100 μL of Amaxa Human CD34+ Cell Nucleofector solution. Specific CDC25A-siRNA (Hs_CDC25A_9 FlexiTube siRNA; QIAGEN) or a siRNA-negative control (30 pmol; AllStars Negative Control siRNA; QIAGEN) were added and cells were transfected by the use of program U-08. CD34+ cells were then resuspended in IMDM 10% FBS. Cells (3 × 104 per dish) were immediately plated in duplicate in methylcellulose. The other cells were cultured in erythroid differentiation culture medium, counted (trypan blue staining) 24 hours after transfection, and controlled for transfer efficiency.
Clonogenic assays
Fresh human BM CD34+ cells were plated in duplicate between 1500 and 3000 cells per dish culture in H4431 StemCell Technologies methylcellulose medium. IRC-083864 was initially added at the correct concentration in the culture medium. Erythroid colonies were scored at day 14.
Amplification of erythroid progenitors
CD34+ cells were cultured in IMDM supplemented with 10% FBS, 50 ng/mL recombinant human SCF, 10 ng/mL IL-3 (R&D systems), and 1 U/mL EPO. Erythroid differentiation was followed by May Grunwald Giemsa staining and analysis of CD34, CD36, and GPA expression by flow cytometry (BDLSRII cytometer; BD Biosciences).
Statistical analysis
Results are expressed as mean value ± SD. Statistical analysis of the data was performed by the Student t test or the Mann-Whitney U test with the SigmaStat 3.0 software (SPSS). Differences were considered as significant for P values < .05, *P < .05, **P < .01.
Results
CDC25A is over- and constitutively expressed in JAK2V617F-positive cell lines
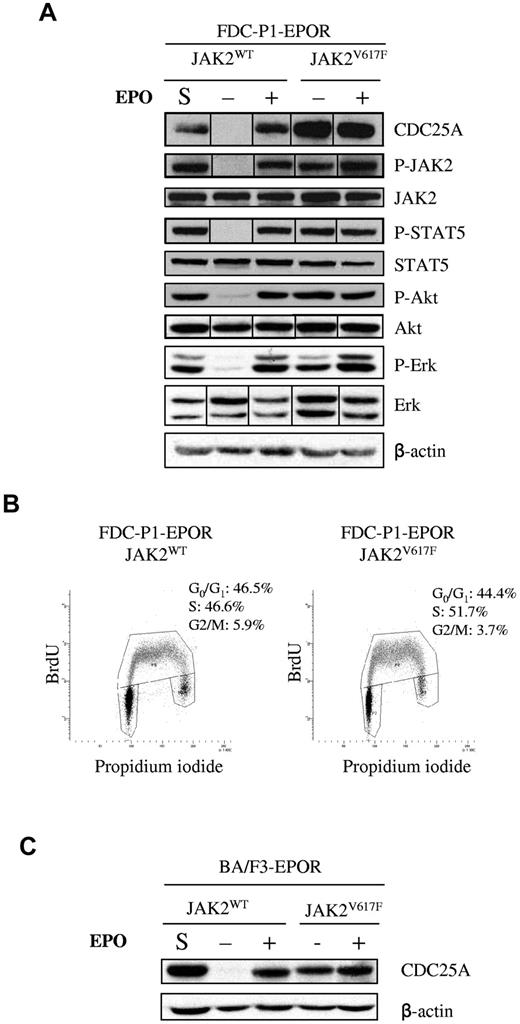
The FDC-P1 cell line was transduced to coexpress the EPOR with either JAK2V617F or JAK2WT. JAK2V617F cells were grown in the absence of EPO, whereas EPO was added in the culture medium of JAK2WT cells. Although JAK2 expression was similar in the 2 cell lines, CDC25A protein level was significantly greater in JAK2V617F cells than in JAK2WT (Figure 1A). In contrast, protein and phosphorylation levels of Akt, STAT5, or Erk were not influenced by JAK2V617F in these culture conditions. Because CDC25A expression is cell cycle dependent, we compared cell cycle distribution and BrdU incorporation rates in the 2 cell lines (Figure 1B). No significant difference in JAK2V617F or JAK2WT cells was found, suggesting that modified CDC25A protein levels was cell cycle–independent.
CDC25A is overexpressedand constitutively expressed in JAK2V617F-positive cell lines. (A) JAK2, STAT5, Akt, and Erk phosphorylation and protein levels were analyzed by Western blotting in FDC-P1–EPOR-JAK2WT and FDC-P1–EPOR-JAK2V617F cells. S indicates steady-state culture conditions; −, 12 hours of EPO deprivation; +, 12 hours of EPO restimulation (10 IU/mL). β-actin was used as a loading control. Western blots are representative of 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Cell cycle distribution of JAK2WT and JAK2V617F-expressing FDC-P1–EPOR cells was analyzed by BrdU and propidium iodide costaining under normal culture conditions. Results are representative of 3 independent experiments. (C) Western blot analysis of CDC25A protein level in JAK2WT- and JAK2V617F- expressing BA/F3-EPOR cells. S indicates steady-state culture conditions; −, 12 hours of EPO deprivation; +, 12 hours of EPO restimulation (10 IU/mL). β-actin was used as a loading control. Western blots are representative of 3 independent experiments.
CDC25A is overexpressedand constitutively expressed in JAK2V617F-positive cell lines. (A) JAK2, STAT5, Akt, and Erk phosphorylation and protein levels were analyzed by Western blotting in FDC-P1–EPOR-JAK2WT and FDC-P1–EPOR-JAK2V617F cells. S indicates steady-state culture conditions; −, 12 hours of EPO deprivation; +, 12 hours of EPO restimulation (10 IU/mL). β-actin was used as a loading control. Western blots are representative of 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Cell cycle distribution of JAK2WT and JAK2V617F-expressing FDC-P1–EPOR cells was analyzed by BrdU and propidium iodide costaining under normal culture conditions. Results are representative of 3 independent experiments. (C) Western blot analysis of CDC25A protein level in JAK2WT- and JAK2V617F- expressing BA/F3-EPOR cells. S indicates steady-state culture conditions; −, 12 hours of EPO deprivation; +, 12 hours of EPO restimulation (10 IU/mL). β-actin was used as a loading control. Western blots are representative of 3 independent experiments.
We next investigated the influence of EPO in CDC25A regulation. In JAK2V617F cells, the addition of EPO changed neither CDC25A level nor the protein and phosphorylation levels of JAK2, Akt, STAT5, or Erk (Figure 1A). Similarly, serum starvation did not decrease CDC25A protein expression in these cells (data not shown). By contrast, EPO deprivation resulted in a dramatic reduction of CDC25A protein level in JAK2WT cells. The phosphorylation of Akt, STAT5, and Erk was also reduced in these conditions (Figure 1A). These results suggest that not only CDC25A is a target for EPO signaling in nonmutated JAK2 expressing cells but also that JAK2V617F could substitute for EPO-mediated CDC25A regulation. This EPO- and JAK2V617F-dependent regulation of CDC25A was confirmed in another cellular model, BA/F3 cells coexpressing the EPOR and the WT and V617F forms of JAK2 (Figure 1C).
Myeloproliferative leukemia virus oncogene (MPL; or thrombopoietin receptor) W515L mutations have been described in 5%-10% of PMF and 1%-3% ET cases. In vitro effects of MPLW515L are similar to those of JAK2V617F, triggering spontaneous MPL and JAK/STAT pathway activation and accelerating G1/S transition.24 In consequence, we tested the status of CDC25A protein expression downstream of this mutated receptor. BA/F3-MPLWT cells were grown in the presence of TPO, whereas BA/F3-MPLW515L ones were independent of TPO. As shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), the status of CDC25A protein expression observed in BA/F3-MPLW515L was similar to that described in BA/F3 JAK2V617F cells. These results show constitutive up-regulation of CDC25A downstream of JAK2V617F and MPLW515L oncogenes.
CDC25A is overexpressed in human and murine JAK2V617F-expressing primary cells
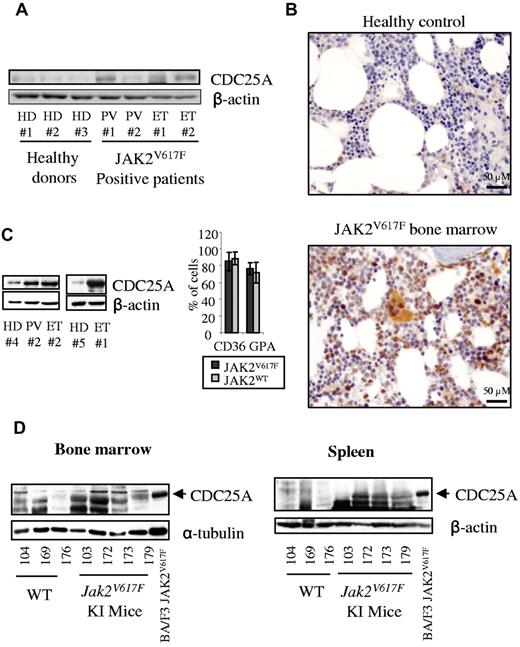
We next investigated whether JAK2V617F could influence CDC25A levels in primary murine and human hematopoietic cells. First, we examined CDC25A expression in human CD36+-purified marrow cells issued from 4 JAK2V617F-positive patients and 3 healthy donors (Figure 2A). Among these, all patients but one displayed CDC25A overexpression compared with healthy donors. These results were confirmed by immunohistochemistry on BM biopsies of JAK2V617F patients (3 PV, 1 ET), where CDC25A was found overexpressed in megakaryocytes and erythroblasts (Figure 2B).
CDC25A is overexpressed in JAK2V617F-positive primary cells. (A) CDC25A expression was analyzed by Western blot in CD36+ cells purified from the BM of 4 JAK2V617F-positive patients (PV, ET) and 3 healthy donors (HD). (B) Paraffin-embedded BM biopsies from 3 JAK2V617F-positive PV patients, 1 JAK2V617F-positive ET patient, and 2 HDs were subjected to an immunohistochemical staining for CDC25A. Pictures representative of JAK2V617F-positive and control BM biopsies are shown. (C) Purified CD34+ cells from a JAK2V617F-positive PV patient, 2 JAK2V617F-positive ET patients, and 2 representative healthy controls (HD) were grown in liquid erythroid differentiation medium. Cells were harvested at day 7, corresponding to a proerythroblast stage, and subjected to a Western blot analysis for CDC25A (left). The presence of CD36 and GPA markers was visualized by flow cytometry (right). (D) BM (left) and spleen lysates (right) from 3 WT and 4 Jak2V617F KI mice were subjected to Western blot analysis for CDC25A. CDC25A expression in BA/F3 JAK2V617F cell line was used as molecular weight control. β-actin or α-tubulin levels were used as control.
CDC25A is overexpressed in JAK2V617F-positive primary cells. (A) CDC25A expression was analyzed by Western blot in CD36+ cells purified from the BM of 4 JAK2V617F-positive patients (PV, ET) and 3 healthy donors (HD). (B) Paraffin-embedded BM biopsies from 3 JAK2V617F-positive PV patients, 1 JAK2V617F-positive ET patient, and 2 HDs were subjected to an immunohistochemical staining for CDC25A. Pictures representative of JAK2V617F-positive and control BM biopsies are shown. (C) Purified CD34+ cells from a JAK2V617F-positive PV patient, 2 JAK2V617F-positive ET patients, and 2 representative healthy controls (HD) were grown in liquid erythroid differentiation medium. Cells were harvested at day 7, corresponding to a proerythroblast stage, and subjected to a Western blot analysis for CDC25A (left). The presence of CD36 and GPA markers was visualized by flow cytometry (right). (D) BM (left) and spleen lysates (right) from 3 WT and 4 Jak2V617F KI mice were subjected to Western blot analysis for CDC25A. CDC25A expression in BA/F3 JAK2V617F cell line was used as molecular weight control. β-actin or α-tubulin levels were used as control.
We next estimated the expression of CDC25A protein during erythroid differentiation in liquid culture in both normal and JAK2V617F marrow cells. Purified CD34+ cells from healthy donors and JAK2V617F-positive patients were cultured in liquid medium in conditions inducing their differentiation as described in the Methods. CDC25A expression was analyzed at the proerythroblast stage. At day 7, morphology (data not shown) and cytometry analysis (Figure 2C) revealed that CD36+GPA+ proerythroblasts represented approximately 75% of cultured cells in both normal and JAK2V617F cells. At this stage, CDC25A was clearly overexpressed in JAK2V617F cells compared with healthy donor cells.
Finally, we took advantage of our new model of KI mice expressing JAK2V617F to confirm these results. As we recently reported, these mice displayed splenomegaly and spleen myeloid metaplasia, with the percentage of erythroblasts in this tissues being as high as 60%.6 Thus, BM and spleen from 4 Jak2V617F and 3 control littermates were analyzed by Western blot for CDC25A expression. Mice were from 2 to 6 months of age in each group. As shown in Figure 2D, CDC25A protein level was greater in both spleen and BM cells expressing JAK2V617F compared with controls expressing JAK2WT. Altogether, these data show up-regulation of CDC25A in JAK2V617F cells, suggesting a potential link between the JAK2V617F oncogene and CDC25A protein expression level.
JAK2 kinase activity is required for CDC25A up-regulation
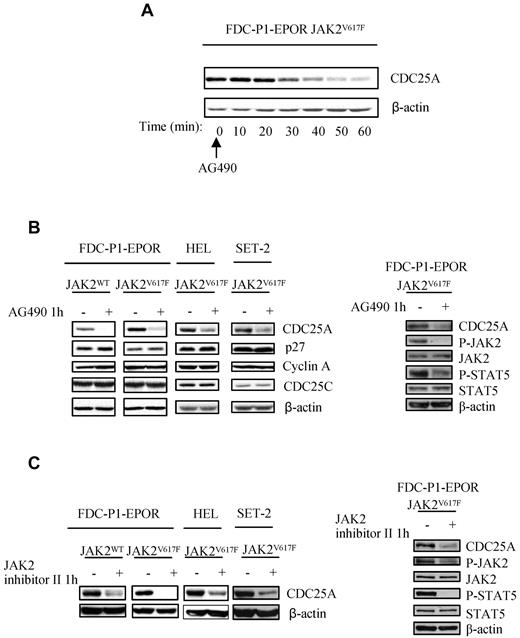
The JAK2V617F kinase has been shown to promote G1/S transition in the HEL cell line.7 To understand the functional link between JAK2V617F and cell cycle progression, we assessed the expression of CDC25A and other cell cycle regulators known to be molecular targets of oncogenic signaling pathways. We conducted Western blot analysis of p27Kip1, cyclin A, and CDC25A in response to JAK2 inhibition by AG490 in FDC-P1 cell lines expressing either JAK2WT or JAK2V617F and in the human JAK2V617F-expressing SET-2 and HEL cell lines. Treatment with AG490, leading to JAK2 and STAT5 phosphorylation inhibition, resulted in a rapid decrease in CDC25A protein expression in JAK2V617F cells (Figure 3A-B right), without modifications of p27Kip1 and cyclin A levels (Figure 3B left). Similar effects were observed with another more specific JAK2 kinase inhibitor (JAK2 inhibitor II; Figure 3C). Because we did not observe major reproducible modifications of cell proliferation or survival on 1 hour of treatment with these inhibitors (data not shown), we conclude that JAK2V617F-dependent CDC25A regulation is cell cycle and cell death independent. Interestingly, PI3K/Akt and Erk activities known to be up-regulated downstream of JAK2V617F and involved in CDC25A regulation in other models remained unchanged after AG490 treatment (supplemental Figure 2A). Moreover, their respective inhibitions had no influence on CDC25A expression in FDC-P1-EPOR-JAK2V617F (supplemental Figure 2B-C). These results indicate that the JAK2V617F oncogene influences CDC25A protein expression through its kinase activity.
JAK2 activity is required for CDC25A up-regulation. (A) JAK2V617F-expressing FDC-P1 EPOR cells were treated with AG490 (50μM), an inhibitor of JAK2, harvested at indicated time, and subjected to Western blot analysis for CDC25A. (B) Left, JAK2WT and JAK2V617F-expressing FDC-P1 EPOR, HEL, and SET-2 cell lines were treated with AG490 (50μM, 1 hour) and subjected to Western blot analysis for the cell cycle regulators CDC25A, p27Kip1, cyclin A, and CDC25C. Right, Western blot analysis of P-STAT5, STAT5, P-JAK2, and JAK2 was performed in JAK2V617F-expressing FDC-P1 EPOR cell lines in response to AG490 treatment. (C) Left: JAK2V617F-expressing FDC-P1–EPOR and HEL cell lines were treated with JAK2 inhibitor II (50μM, 1 hour), an inhibitor of JAK2, and subjected to Western blot analysis for CDC25A. Right: Western blot analysis of P-STAT5, STAT5, P-JAK2, and JAK2 was performed in JAK2V617F-expressing FDC-P1 EPOR cell lines in response to JAK2 inhibitor II treatment. β-actin levels were used as control. Western blots are representative of at least 3 independent experiments.
JAK2 activity is required for CDC25A up-regulation. (A) JAK2V617F-expressing FDC-P1 EPOR cells were treated with AG490 (50μM), an inhibitor of JAK2, harvested at indicated time, and subjected to Western blot analysis for CDC25A. (B) Left, JAK2WT and JAK2V617F-expressing FDC-P1 EPOR, HEL, and SET-2 cell lines were treated with AG490 (50μM, 1 hour) and subjected to Western blot analysis for the cell cycle regulators CDC25A, p27Kip1, cyclin A, and CDC25C. Right, Western blot analysis of P-STAT5, STAT5, P-JAK2, and JAK2 was performed in JAK2V617F-expressing FDC-P1 EPOR cell lines in response to AG490 treatment. (C) Left: JAK2V617F-expressing FDC-P1–EPOR and HEL cell lines were treated with JAK2 inhibitor II (50μM, 1 hour), an inhibitor of JAK2, and subjected to Western blot analysis for CDC25A. Right: Western blot analysis of P-STAT5, STAT5, P-JAK2, and JAK2 was performed in JAK2V617F-expressing FDC-P1 EPOR cell lines in response to JAK2 inhibitor II treatment. β-actin levels were used as control. Western blots are representative of at least 3 independent experiments.
CDC25A is not regulated at the mRNA level downstream of JAK2V617F
We then asked whether CDC25A transcript levels were modified on JAK2V617F inhibition. By using quantitative PCR (supplemental Figure 3A), we found that FDC-P1–EPOR-JAK2V617F cells did not display greater CDC25A mRNA levels compared with FDC-P1–EPOR-JAK2WT. Furthermore, whereas treatment with AG490 resulted in a dramatic decrease in CDC25A protein expression in JAK2V617F-expressing cells (Figure 3B), no decrease in CDC25A mRNA transcripts was found in FDC-P1–EPOR-JAK2V617F, HEL, and SET2 cells (supplemental Figure 3B). These data show a decoupling between CDC25A protein and mRNA levels in JAK2V617F expressing cells, suggesting that in these cells, CDC25A is regulated through a translational or a posttranslational mechanism.
Translational regulation of CDC25A by JAK2V617F
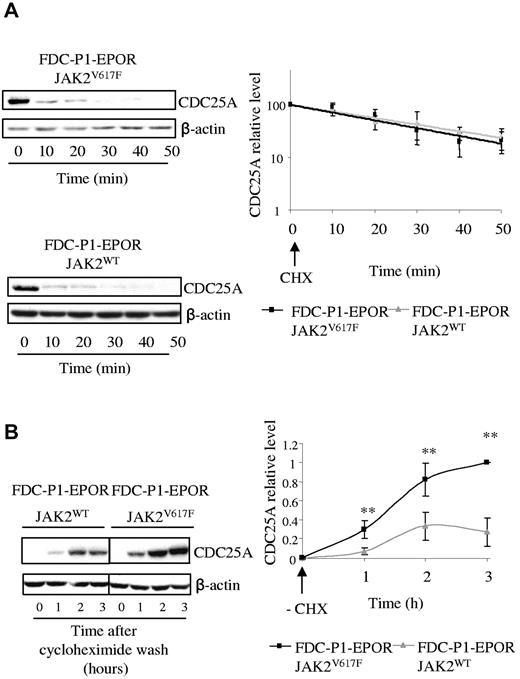
First, we investigated whether expression of JAK2V617F could affect the stability of the CDC25A protein. For this purpose, FDC-P1–EPOR-JAK2V617F and FDC-P1–EPOR-JAK2WT cells were incubated with the protein synthesis inhibitor cycloheximide, and CDC25A protein expression was measured by Western blot. As shown in Figure 4A, CDC25A degradation was similar in the 2 cell lines, with an estimated half-life of approximately 20 minutes, a value expected from previous studies.25 By contrast, when cycloheximide was removed, CDC25A was re-expressed faster and at a greater magnitude in JAK2V617F cells, compared with JAK2WT cells (Figure 4B), indicating that CDC25A protein synthesis was greater downstream of JAK2V617F. These data suggest that JAK2V617F facilitates CDC25A synthesis without influencing its stability.
The presence of the JAK2V617F mutation induces an increase in CDC25A protein translation. (A) JAK2V617F and JAK2WT-expressing FDC-P1 EPOR cells were incubated with 10 μg/mL of cycloheximide (CHX), an inhibitor of protein translation, harvested at indicated times, and subjected to Western blot analysis of CDC25A (left). Quantification of CDC25A protein levels was performed by densitometric analysis normalized to β-actin level (right). The results are expressed as mean ± SD for 3 independent experiments. (B) JAK2V617F and JAK2WT-expressing FDC-P1–EPOR cells were incubated for 1 hour with 10 μg/mL of CHX, then washed (−CHX), and harvested at the indicated times. Cell lysates were prepared and subjected to Western blot to analyze the reappearance of CDC25A (left). CDC25A levels were determined by densitometric analysis normalized to β-actin level (right). The results are the means ± SD for 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane.
The presence of the JAK2V617F mutation induces an increase in CDC25A protein translation. (A) JAK2V617F and JAK2WT-expressing FDC-P1 EPOR cells were incubated with 10 μg/mL of cycloheximide (CHX), an inhibitor of protein translation, harvested at indicated times, and subjected to Western blot analysis of CDC25A (left). Quantification of CDC25A protein levels was performed by densitometric analysis normalized to β-actin level (right). The results are expressed as mean ± SD for 3 independent experiments. (B) JAK2V617F and JAK2WT-expressing FDC-P1–EPOR cells were incubated for 1 hour with 10 μg/mL of CHX, then washed (−CHX), and harvested at the indicated times. Cell lysates were prepared and subjected to Western blot to analyze the reappearance of CDC25A (left). CDC25A levels were determined by densitometric analysis normalized to β-actin level (right). The results are the means ± SD for 3 independent experiments. Vertical lines have been inserted to indicate a repositioned gel lane.
Translational regulation of CDC25A by the eIF2α pathway
Protein synthesis is controlled by different translation regulators, among which eIF2α plays an important role in cap-dependent translation initiation.26 Furthermore, a role for eIF2α in CDC25A synthesis regulation has been recently described.27 The function of eIF2α is regulated by phosphorylation on ser 51, which results in eIF2α sequestration and subsequent loss of function.28 Thus, we investigated the status of eIF2α phosphorylation downstream of JAK2V617F.
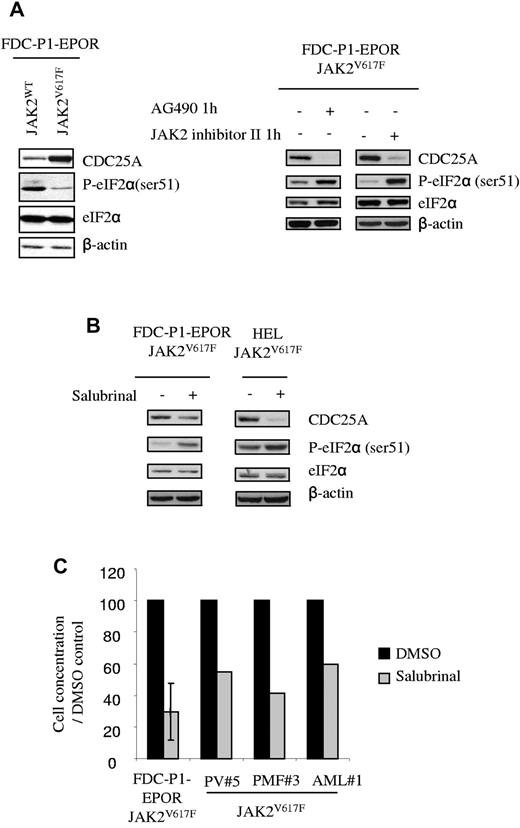
We first compared eIF2α phosphorylation level in JAK2WT and JAK2V617F cells, in the presence or the absence of JAK2 inhibitors (AG490 or JAK2 inhibitor II) for 1 hour. As shown in Figure 5A, eIF2α ser51 phosphorylation was lower in JAK2V617F compared with JAK2WT cells and was restored by the JAK2 inhibitor AG490 and JAK2 inhibitor II, demonstrating for the first time that eIF2α is indirectly regulated by JAK2V617F. To investigate the link between eIF2α and CDC25A in JAK2V617F cells, we used salubrinal, a compound that inhibits the function of eIF2α by interfering with ser 51 dephosphorylation. As shown in Figure 5B, treatment with salubrinal resulted in a concomitant increase of eIF2α ser51 phosphorylation and a decrease in CDC25A protein level, thus establishing a correlation between CDC25A expression and eIF2α phosphorylation profile. Importantly, the proliferation of FDC-P1–EPOR-JAK2V617F cells was dramatically reduced in the presence of salubrinal (Figure 5C), as well as the growth of primary cells from 3 independent JAK2V617F-positive samples. Altogether, these data suggest that eIF2α is an important regulator of CDC25A protein translation in JAK2V617F cells and of JAK2V617F positive cells proliferation.
eIF2α regulates CDC25A downstream of JAK2V617F. (A) CDC25A and eIF-2α protein levels, as well as eIF-2α phosphorylation on ser 51, were analyzed by Western blot in JAK2WT and JAK2V617F-expressing FDC-P1–EPOR cells under normal conditions of culture or after 1 hour of treatment with AG490 or JAK2 inhibitor II. (B) CDC25A expression was analyzed by Western blot after 24 hours of treatment with Salubrinal, an inhibitor of eIF2α dephosphorylation, in JAK2V617F-expressing FDC-P1–EPOR cells or HEL cell lines. β-actin levels were used as control. Western blots are representative of 3 independent experiments. (C) FDC-P1–EPOR JAK2V617F and JAK2V617F-positive cells from 3 different patients (PV patient 5, PMF 3, and AML 1) were cultured in the presence or the absence of salubrinal at 75μM. Cells were stained with trypan blue and counted after 48 hours.
eIF2α regulates CDC25A downstream of JAK2V617F. (A) CDC25A and eIF-2α protein levels, as well as eIF-2α phosphorylation on ser 51, were analyzed by Western blot in JAK2WT and JAK2V617F-expressing FDC-P1–EPOR cells under normal conditions of culture or after 1 hour of treatment with AG490 or JAK2 inhibitor II. (B) CDC25A expression was analyzed by Western blot after 24 hours of treatment with Salubrinal, an inhibitor of eIF2α dephosphorylation, in JAK2V617F-expressing FDC-P1–EPOR cells or HEL cell lines. β-actin levels were used as control. Western blots are representative of 3 independent experiments. (C) FDC-P1–EPOR JAK2V617F and JAK2V617F-positive cells from 3 different patients (PV patient 5, PMF 3, and AML 1) were cultured in the presence or the absence of salubrinal at 75μM. Cells were stained with trypan blue and counted after 48 hours.
eIF2α and CDC25A regulation by the STAT5 pathway
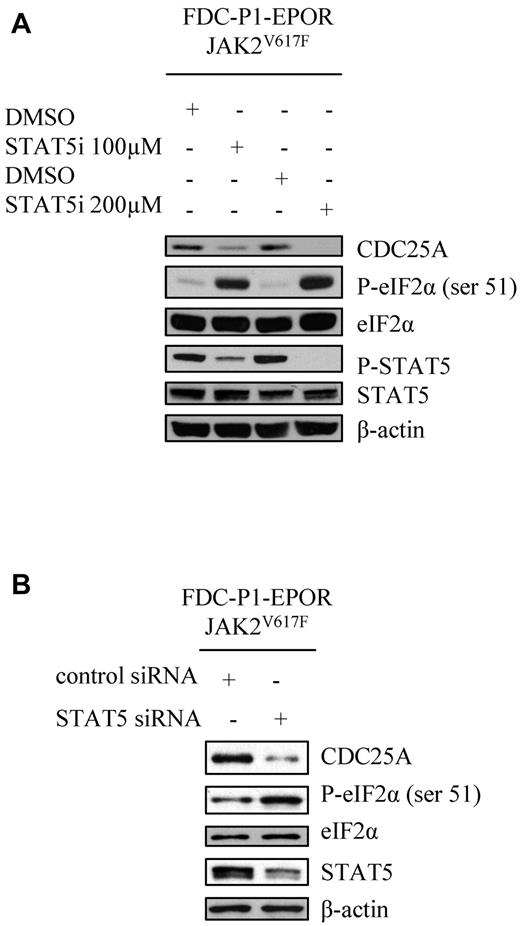
To identify the link between JAK2V617F and eIF2α, we tested the involvement of STAT5, the main direct JAK2 target. For this purpose, FDC-P1–EPOR-JAK2V617F cells were incubated with a STAT5 inhibitor for 1 hour at 2 different concentrations. As shown in Figure 6A, this treatment resulted in a dose-dependent reduction of STAT5 phosphorylation, an increase of eIF2α phosphorylation, and a concomitant decrease in CDC25A levels. The same results were obtained with the human HEL cell line (data not shown). Finally, these data were nicely confirmed by RNA interference-induced STAT5 down-regulation (Figure 6B).
STAT5 regulates eIF2α downstream of JAK2V617F. (A) JAK2V617F-expressing FDC-P1–EPOR cells were treated with DMSO or STAT5 inhibitor (100 or 200μM for 1 hour) and CDC25A, P-eIF2α, eIF2α, P-STAT5, and STAT5 protein levels were analyzed by Western blot. (B) JAK2V617F-expressing FDC-P1–EPOR cells were transduced with control or STAT5 siRNA and CDC25A, P-eIF2α, eIF2α and STAT5 protein levels were analyzed by Western blot 8 hours after transfection. β-actin levels were used as control. Western blots are representative of 3 independent experiments.
STAT5 regulates eIF2α downstream of JAK2V617F. (A) JAK2V617F-expressing FDC-P1–EPOR cells were treated with DMSO or STAT5 inhibitor (100 or 200μM for 1 hour) and CDC25A, P-eIF2α, eIF2α, P-STAT5, and STAT5 protein levels were analyzed by Western blot. (B) JAK2V617F-expressing FDC-P1–EPOR cells were transduced with control or STAT5 siRNA and CDC25A, P-eIF2α, eIF2α and STAT5 protein levels were analyzed by Western blot 8 hours after transfection. β-actin levels were used as control. Western blots are representative of 3 independent experiments.
Because STAT5 is a transcription factor, we then tested whether the protein level of the 4 kinases (PKR, PERK, HRI, and GCN2) and the phosphatases (GADD34/PP1 and PP2A), known to regulate eIF2α phosphorylation, were affected on AG490 treatment. The basal levels of these proteins were not modified in these conditions, suggesting that STAT5 does not regulate their expression in our model (data not shown). Thus, our study suggests that STAT5, the main direct target of JAK2V617F, is a key intermediate for the regulation of eIF2α by JAK2.
Effects of CDC25A inhibition on JAK2V617F-expressing BFU-E
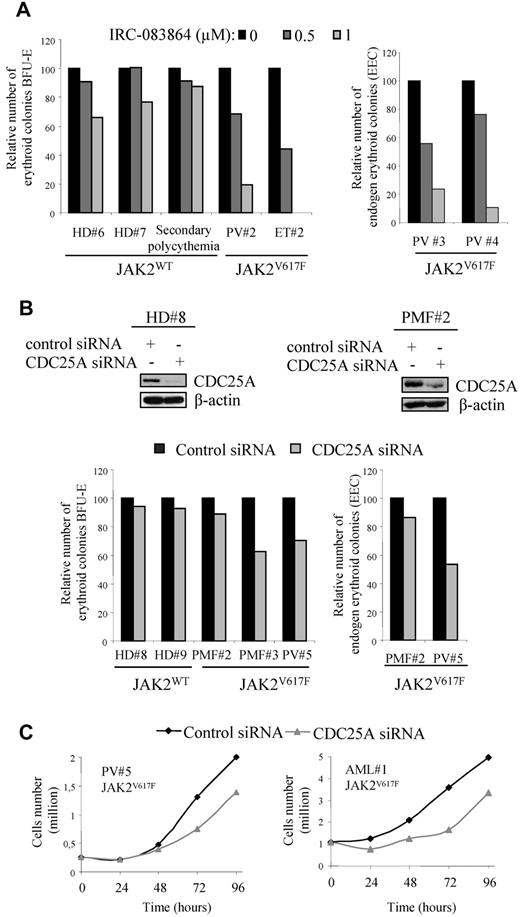
We firstly investigated the effect of the pan CDC25 inhibitor IRC-08386421 on BFU-E colony formation from JAK2V617F human primary cells. Purified marrow CD34+ cells were plated in methylcellulose for 14 days in the presence of adequate stimulating factors, as described in the Methods. As illustrated in Figure 7A (left), IRC-083864 inhibited in a dose-dependent manner the growth of BFU-E derived from 2 independent JAK2V617F-positive patients. In the same conditions, the growth of BFU-E from healthy donors (n = 2), or from a patient with secondary polycythemia (JAK2WT), was poorly affected. Importantly, the effect of CDC25 inhibition was also observed on the clonogenic potential of JAK2V617F-positive cells from 2 additional patients, grown in the absence of EPO (Figure 7A right). To confirm the specific role of CDC25A, we transfected purified normal or JAK2V617F-positive CD34+ cells with CDC25A siRNA and performed the same experiments. As shown in Figure 7B (top), CDC25A siRNA decreased CDC25A protein expression and reduced the growth of BFU-E derived from 2 JAK2V617F-positive patients, with no significant effect on BFU-E from healthy donors. For a third JAK2V617F-positive sample, the clonogenic potential was less affected by CDC25A down-regulation. As with the pharmacologic inhibitor, the inhibitory effect of CDC25A down-regulation on clonogenicity was reproduced in the absence of EPO (Figure 7B right).
Pharmacologic or genetic inhibition of CDC25A inhibits clonogenic capacities of CD34+ cells from JAK2V617F-positive patients. (A) CD34+ cells from BM of HD, JAK2V617F-positive PV, or ET patients, and a patient with secondary polycythemia (JAK2WT), were purified and plated at a concentration of 1.5-3.103 cells/mL in duplicate, in the presence or the absence of the CDC25 inhibitor IRC-083864 and in the presence (left) or the absence (right) of EPO. The erythroid colonies (BFU-E) were scored at day 14. Results are expressed as percentage of control (untreated). (B) CD34+ cells from HD, or from JAK2V617F-positive PV or PMF patients, were purified, cultured overnight, and transfected with control or CDC25A siRNA. Top, siRNA efficiency was evaluated by Western blot 24 hours after transfection. Western blots are representative of results obtained on control and JAK2V617F samples. Bottom, cells (1.5-3.103) were plated in duplicate, in the presence (left) or the absence of EPO (right). The erythroid colonies (BFU-E) were scored at day 14. Results are expressed as percentage of control (control siRNA). (C) The growth of cells in erythroid differentiation medium was followed by cell counting after trypan blue staining.
Pharmacologic or genetic inhibition of CDC25A inhibits clonogenic capacities of CD34+ cells from JAK2V617F-positive patients. (A) CD34+ cells from BM of HD, JAK2V617F-positive PV, or ET patients, and a patient with secondary polycythemia (JAK2WT), were purified and plated at a concentration of 1.5-3.103 cells/mL in duplicate, in the presence or the absence of the CDC25 inhibitor IRC-083864 and in the presence (left) or the absence (right) of EPO. The erythroid colonies (BFU-E) were scored at day 14. Results are expressed as percentage of control (untreated). (B) CD34+ cells from HD, or from JAK2V617F-positive PV or PMF patients, were purified, cultured overnight, and transfected with control or CDC25A siRNA. Top, siRNA efficiency was evaluated by Western blot 24 hours after transfection. Western blots are representative of results obtained on control and JAK2V617F samples. Bottom, cells (1.5-3.103) were plated in duplicate, in the presence (left) or the absence of EPO (right). The erythroid colonies (BFU-E) were scored at day 14. Results are expressed as percentage of control (control siRNA). (C) The growth of cells in erythroid differentiation medium was followed by cell counting after trypan blue staining.
In good correlation with the effects observed on the clonogenicity of these cells, CDC25A siRNA also reduced the proliferation potential in erythroid differentiation medium of 2 JAK2V617F samples, as shown in Figure 7C. These data demonstrate that CDC25A inhibition reduces the growth of JAK2V617F-positive erythroid progenitors while sparing normal BFU-E.
Discussion
This study shows that CDC25A is constitutively overexpressed downstream of the JAK2V617F oncogene. JAK2V617F is not the unique oncogenic tyrosine kinase capable of regulating this enzyme in hematopoietic cells. Indeed, our group has recently described that cells expressing the BCR-ABL, FLT3-ITD, and NPM-ALK oncogenes display an increase in levels of CDC25A protein compared with their WT counterparts.20
The mechanism by which JAK2V617F regulates CDC25A was examined. Our study supports the fact that JAK2V617F influences CDC25A expression by acting at the translational level. This mechanism of regulation is rather uncommon. For example, as NPM-ALK cells are concerned, our group has shown that this oncogene acts at the transcriptional level, whereas it may also affect CDC25A stability.20 As a matter of fact, stability appears as one of the major regulatory mechanism of CDC25A expression.13 This mechanism was further investigated in cancer cells models. Indeed, recent studies have emphasized the role of GSK3β in CDC25A stability. In this model, GSK3β interacts with and phosphorylates CDC25A, with this phosphorylation facilitating its ubiquitination and subsequent proteasome-dependent degradation. Conversely, GSK3β deregulation, a common event in cancer cells, results in overexpression of CDC25A. This mechanism has been described in a large variety of carcinomatous cells.15 Other studies have also very recently documented that CDC25A degradation could be counteracted by the specific ubiquitin hydrolase Dub3, with the deregulation of this enzyme resulting in high levels of CDC25A in a subset of human breast cancers.16
The mechanism by which JAK2V617F regulates CDC25A synthesis also was investigated. Our study suggests a role for eIF2α, a major regulator of cap-dependent translation initiation. The role of eIF2α is supported by 2 lines of evidences. First, JAK2V617F expression and function modulate eIF2α phosphorylation (see Figure 5), and thus presumably eIF2α function. Second, eIF2α inhibition by salubrinal resulted in the down-regulation of CDC25A in JAK2V617F cells. We identified STAT5, the main target of JAK2V617F, is involved in eIF2α regulation. Because basal eIF2α expression remained unchanged after STAT5 down-regulation, it is unlikely that eIF2α is transcriptionally regulated by STAT5. In addition, we did not detect any significant variations of the level of the 4 kinases (PKR, PERK, GCN2, and HRI) and the phosphatase complex (PP1/GADD34) known as key eIF2α regulators. In consequence, the molecular link(s) between STAT5 and eIF2α still remain(s) to be established.
More generally, the fact that JAK2V617F could affect eIF2α function may have important consequences for the biology of JAK2V617F cells. Indeed, eIF2α is considered as a master regulator of the stress response by regulating antiapoptotic proteins like Bcl-xL for instance.29 Interestingly, Bcl-xL accumulates in PV30 and BCR-ABL31 cells, presumably through a STAT5-dependent transcriptional mechanism.32 Thus, it is possible that, in MPN, transcriptional and posttranscriptional mechanisms cooperate to facilitate the accumulation of apoptosis inhibitors.
With regard to cell-cycle regulation, JAK2V617F has been shown to promote G1/S phase transition.7,33 Indeed, JAK2 inhibition with JAK inhibitor I induces p27kip1 up-regulation and a decrease in cyclin D2 after an 18-hour incubation followed with G1 arrest, suggesting that these proteins are implicated in JAK2V617F-induced proliferation.7 In our model, the rapid kinetics of CDC25A down-regulation in response to pharmacologic JAK2 inhibitors (30 minutes), together with the absence of modifications of other cell-cycle proteins expression, such as p27kip1 or cyclin A, and similar cell-cycle distribution between JAK2WT and mutated cell lines, strongly argue for CDC25A being an early target of JAK2V617F rather than just a cell proliferation marker.
CDC25A up-regulation was observed not only in cell lines but also in human CD36+ cells issued from JAK2V617F-positive PV patients and BM biopsies compared with healthy donors cells. Moreover, CDC25A overexpression was found in BM and spleen cells in Jak2V617F KI mice, an animal model that develops a PV-like disease.6 These results further support the notion that CDC25A overexpression is a common feature of JAK2V617F-related diseases and perhaps of other MPN. However, in some cases JAK2V617F and CDC25A levels were not correlated, suggesting additional ways of regulation of the protein.
Our study also shows that CDC25A is overexpressed at the proerythroblast stage of erythroid differentiation in JAK2V617F-positive patients. These results suggest that CDC25A could be involved in terminal erythroid differentiation. We previously observed an erythroid amplification and a selective advantage for JAK2V617F-mutated cells in late stages of erythropoiesis.34 Our hypothesis is that CDC25A could mediate this effect by stimulating proliferation. This hypothesis is supported by the fact that CDC25A depletion by siRNA results in decreased proliferation of FDC-P1-EPOR-JAK2V617F cells (data not shown).
The CDC25 inhibitor IRC-083864 and more specific CDC25A siRNA inhibit BFU-E in JAK2V617F-positive samples being less active in normal BFU-E, suggesting a therapeutic window between normal and JAK2V617F hematopoietic cells. However, clonogenic potential is not affected for one patient (PMF patient 2), suggesting either that CDC25A is not overexpressed in CD34+ cells of this patient (Figure 2), or that additional events can compensate CDC25A inhibition in some patients. Whatever, CDC25 inhibitors should represent a new class of drugs in most JAK2V617F-MPN therapy, beside the JAK2 inhibitors that are currently tested.35
To conclude, we propose a model in which JAK2V617F drives through STAT5 an eIF2α-mediated pathway that results in the stimulation of synthesis and accumulation of CDC25A, with this enzyme responsible for increased proliferation and expansion of hematopoietic cells in PV or ET, and, perhaps, in other MPN.
An Inside Blood analysis appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Anne Fernandez-Vidal for quantitative PCR; Michel March for immunohistochemistry technical assistance; Grégoire Prevost (Ipsen) for providing the CDC25 inhibitor; Fanny Fava (Tumorothèque Cancer-Est) for preparing the samples; and Fanny Grimal, Anne Quillet-Mary, and Christine Didier for helpful technical assistance.
This work was supported by grants from the Inserm, Institut National du Cancer (PL2008, INCa_Gov_1345), Fondation de France, Novartis, MPN research foundation. E.-F.G. was supported by a fellowship from the Ligue Nationale contre le Cancer.
Authorship
Contribution: E.-F.G. performed the experiments and collected and analyzed data; M.P. performed experiments; C.L. performed immunohistochemistry; C.M. and J.-L.V. developed and prepared the samples from KI mice models; F.D. established JAK2-expressing cell lines and provided MPN samples; E.H. provided MPN samples; S.G. established MPL-expressing cell lines; C.D., C.R., and G.L. provided MPN samples; N.B. provided the discarded fragments from hip surgery; B.D. and C.R. were involved in the design of the study; E.-F.G., G.L., S.M., and V.M.-D.M. designed the research and wrote the paper; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Véronique Mansat-De Mas, Laboratoire d'Hématologie, Pavillon Lefèbvre, CHU Purpan, Place du Dr Baylac, 31059 Toulouse Cedex, France; e-mail: demas.v@chu-toulouse.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal