Abstract
Criteria of response and definition of resistance and intolerance to hydroxyurea (HU) in polycythemia vera (PV) were proposed by the European LeukemiaNet (ELN). Such criteria were evaluated in 261 PV patients (median follow-up, 7.2 years) treated with HU for a median of 4.4 years. Complete response, partial response, and no response were observed in 24%, 66%, and 10% of patients, respectively. Achieving ELN response (complete or partial) or hematocrit response did not result in better survival or less thrombosis and bleeding. On the contrary, having no response in leukocyte count was associated with higher risk of death (HR, 2.7; 95% confidence interval [CI], 1.3%-5.4%; P = .007), whereas lack of response in platelet count involved a higher risk of thrombosis and bleeding. Resistance and intolerance to HU was registered in 11% and 13% of patients, respectively. Resistance to HU was associated with higher risk of death (HR, 5.6; 95% CI, 2.7%-11.9%; P < .001) and transformation (HR, 6.8; 95% CI, 3.0%-15.4%; P < .001). In summary, fulfilling the ELN definition for response to HU was not associated with a benefit in the clinical outcome in PV, whereas response in platelet and white blood cell counts were predictive of less thrombohemorrhagic complications and better prognosis, respectively. Resistance to HU was an adverse prognostic factor.
Introduction
Hydroxyurea (HU) is the cytoreductive agent of choice for patients with polycythemia vera (PV) at high risk of developing thrombotic complications,1-4 and it is also useful in controlling PV-related symptoms, splenomegaly, leukocytosis, marked thrombocytosis, and the hematocrit in patients with high need or poor tolerance to phlebotomy.1-5 The bulk of the available evidence shows that HU bears a low leukemogenic potential. Besides, side effects of HU are infrequent and usually occur with high doses of the drug.3,5-7
Although there is general consensus that current therapy of PV should be aimed at preventing vascular complications without increasing the risk of hematologic transformation, there has been less agreement on how to monitor the response to therapy. Recently, a group of experts convened by the European LeukemiaNet (ELN) agreed on a set of standardized criteria of clinicohematologic response in PV,8 to be used mainly in clinical trials. In addition, a unified definition of resistance and intolerance to HU in PV has been proposed previously9 to help clinicians decide on discontinuation of the drug. For PV patients treated with HU, the above-mentioned group of experts recommends using the ELN criteria to monitor the clinicohematologic response and to apply the resistance and intolerance criteria to decide moving the patients to second-line therapies.10 It should be noted, however, that the above-mentioned definitions were the result of an experts' consensus and are not supported by hard evidence-based data. The present study was aimed at assessing the value of the ELN criteria for response, resistance, and intolerance to HU in a large series of PV patients and at analyzing the impact in the patients' outcome of fulfilling or not such criteria.
Patients and methods
Study design
The medical charts of all patients diagnosed with PV in 5 institutions in Spain were reviewed. In every case, the diagnosis of PV was reassessed using the updated criteria of the World Health Organization.11 Patients were eligible for inclusion in the study if they met the updated diagnostic criteria and had received cytoreductive therapy with HU. The indication of HU was decided according to the criterion of the attending hematologist on the basis of the clinical guidelines and prevailing recommendations at that time. Informed consent for the scientific use of the patients' clinicohematologic data and biologic samples was obtained following the Declaration of Helsinki in accordance with the requirements of the local ethics committees of all participating institutions.
In all patients, the main clinicohematologic data at presentation of PV were collected, including age, sex, cardiovascular risk factors (smoking habit, arterial hypertension, hypercholesterolemia, and diabetes), PV-related symptoms, spleen size measured by palpation and ultrasound imaging, hemoglobin (Hb) level, and white blood cell (WBC) and platelet counts. Reason for initiation, date of start, and treatment duration were obtained for each cytoreductive, antiplatelet and anticoagulant medication administered during the patients' follow-up. Occurrence of thrombosis and bleeding, hematologic progression to myelofibrosis or acute leukemia, second neoplasms, and causes of death also were registered.
Response to HU was categorized using the recently published ELN criteria.8 A complete response (CR) was defined as hematocrit less than 0.45 L/L without phlebotomy, normalization of the platelet count (< 400 × 109/L), WBC count less than 10 × 109/L, normal spleen size on imaging, and absence of disease-related symptoms. Partial response (PR) was defined as the absence of CR but achievement of a hematocrit less than 0.45 L/L without phlebotomy or a response in 3 or more of the other criteria. Any response that did not satisfy the PR criteria was classified as nonresponse. Loss of response was recognized when a responder no longer met the criteria for response in 2 consecutive measurements separated by at least 1 month. The date of acquisition and loss of CR or PR as well as of the individual items of response (hematocrit, WBC count, platelet count, spleen size, and disease-related symptoms) were registered for each patient during HU therapy. Consequently, in each patient, the time at risk of developing thrombotic or hemorrhagic complications after starting on HU was categorized as “in response (CR/PR),” “no response,” or “intermittent response,” with the latter being used for patients who alternated periods of CR or PR with periods of no response.
The occurrence of resistance and intolerance to HU was recorded in those patients fulfilling 1 of the following ELN criteria9 : need for phlebotomy to keep hematocrit less than 0.45 L/L after 3 months of at least 2 g/day of HU; uncontrolled myeloproliferation (platelet count > 400 × 109/L and WBC count > 10 × 109/L after 3 months of at least 2 g/day of HU; failure to reduce massive splenomegaly by more than 50% as measured by palpation after 3 months of at least 2 g/day of HU; absolute neutrophil count less than 1 × 109/L or Hb level less than 100 g/L or platelet count less than 100 × 109/L at the lowest dose of HU required to achieve a CR or a PR; or presence of leg ulcers or other unacceptable HU-related nonhematologic toxicities such as mucocutaneous manifestations, gastrointestinal symptoms, pneumonitis, or fever at any HU dose.
Outcomes
The primary outcome of the study was survival from diagnosis of PV. Secondary end points included transformation of PV into myelofibrosis or acute leukemia and the incidence of thrombosis (either arterial or venous) or major bleeding while on treatment with HU.
Thrombosis was defined according to the International Classification of Diseases, 9th revision. Minor occlusive events, such as erythromelalgia and superficial thrombophlebitis of the extremities, were not considered. Severe hemorrhage was defined as a symptomatic bleeding in a critical organ or an overt hemorrhage requiring transfusion or associated with a Hb decrease more than 20 g/L without transfusion.
Statistical methods
Overall survival curves were drawn by the method of Kaplan and Meier. Because the selection of patients was based on having received HU, survival analyses from diagnosis were left-truncated at the date of HU start to account for the fact that patients had to survive until that date to be observable in the present series. Relative survival was calculated by the cohort method described by Dickman et al.12 Relative survival is defined as the ratio of the actuarial survival observed in the group of patients to their expected survival derived from a subset of the general population matched to the patients by age, sex, and calendar year of diagnosis. A relative survival of 1 means that patients with PV are dying at the same rate as their counterparts in the general population so that there is no excess mortality attributable to PV. Estimates of expected survival were calculated by the Ederer II method12 from Spanish life tables stratified by age, sex, and calendar year that were obtained from the Human Mortality Database (www.mortality.org).
The investigation of the prognostic factors for survival was performed by Cox proportional hazards regression. Variables evaluated for their potential prognostic significance were age, sex, cardiovascular risk factors, hematologic values at diagnosis, response and resistance to HU according to the ELN criteria, and thrombosis and bleeding (either previously or during HU therapy). Response and resistance to HU were analyzed as time-dependent covariates.
The cumulative incidence of thrombosis while on HU therapy, as well as the cumulative incidence of transformation to myelofibrosis or acute leukemia, was calculated by taking death as a competing risk. Multivariate analyses of factors predicting the hematologic transformation of PV were done within the framework of competing risks by the method of Fine and Gray.13
The incidence rate of thrombosis or bleeding while the patient was on HU was calculated as the number of events per 1000 person-years of follow-up. The incidence rate method allows to separately accounting for the events occurring while the patient was in complete response to HU, intermittent response, or no response. Multivariate analyses of factors influencing on the incidence rate of thrombosis or bleeding were done by Poisson regression with correction for overdispersion when needed. In Poisson regression, the exponentiated coefficients of covariates can be regarded as incident rate ratios (IRRs) and are comparable with the hazard ratios (HRs) in Cox models. Variables analyzed for their independent association with the incidence rate of thrombosis or bleeding were age, sex, previous history of thrombosis or bleeding, cardiovascular risk factors, hematologic values at diagnosis, periods of treatment with antiplatelet or oral anticoagulants, and response to HU according to the ELN criteria.
All the statistical analyses were performed with Stata Version 11 (www.stata.com). The time-splitting method14 was used to calculate incidence rates and fitting the Poisson models. Curves of the cumulative incidence of thrombosis and the competing risk of dying without thrombosis were drawn by the “stcompet” Stata module developed by Coviello.15 Relative survival was calculated by using the Stata routines developed by Paul Dickman (Karolinska Institutet, Stockholm, Sweden; available at www.pauldickman.com).
Results
Patient characteristics and response to HU
In total, 261 patients were included in the study. Their main clinical and hematologic data at diagnosis are shown in Table 1. Median interval between diagnosis and HU start was 0.3 years, with 80% of patients starting on HU at diagnosis or within the next 3 years. Reasons for initiating HU were as follows: age more than 60 years (n = 121), history of thrombohemorrhagic complications (n = 94), extreme thrombocytosis (n = 15), microvascular symptoms refractory to antiplatelet therapy (n = 14), and other (n = 17). In the majority of patients, an initial HU dose of 1000 mg/day was scheduled, and it was subsequently adjusted to keep the hematocrit below 0.45 L/L, without significant cytopenias. In total, 219 patients received antiplatelet therapy at some time while on treatment with HU, and 46 patients received anticoagulation therapy. Out of the study period, 57 patients received other cytoreductive therapies, including radioactive phosphorus (32P; n = 29), anagrelide (n = 17), busulfan (n = 12), and interferon (n = 8).
Main clinicohematologic characteristics at diagnosis in 261 patients with PV treated with HU
| Clinicohematologic characteristic . | Value . |
|---|---|
| Age, y* | 64 (16-88) |
| Sex, male/female | 118/143 |
| Cardiovascular risk factors, no. (%)† | 183 (71) |
| PV-related symptoms, no. (%)‡ | 111 (43) |
| Thrombosis prior to HU start, no. (%) | 90 (34.5) |
| Palpable splenomegaly, no. (%) | 64/257 (24.5) |
| Radiologic splenomegaly, no. (%) | 96/187 (51) |
| Hemoglobin, g/L* | 178 (120-238) |
| Leukocytes, × 109/L* | 10.7 (4.2-29.0) |
| > 10 × 109/L, no. (%) | 147 (56) |
| Platelets, × 109/L* | 520 (155-1481) |
| > 500 × 109/L, no. (%) | 136 (52) |
| JAK2 mutation, no. (%) | 214/225 (95) |
| V617F, no. (%) | 209/214 (98) |
| Exon 12, no. (%) | 5/214 (2) |
| Clinicohematologic characteristic . | Value . |
|---|---|
| Age, y* | 64 (16-88) |
| Sex, male/female | 118/143 |
| Cardiovascular risk factors, no. (%)† | 183 (71) |
| PV-related symptoms, no. (%)‡ | 111 (43) |
| Thrombosis prior to HU start, no. (%) | 90 (34.5) |
| Palpable splenomegaly, no. (%) | 64/257 (24.5) |
| Radiologic splenomegaly, no. (%) | 96/187 (51) |
| Hemoglobin, g/L* | 178 (120-238) |
| Leukocytes, × 109/L* | 10.7 (4.2-29.0) |
| > 10 × 109/L, no. (%) | 147 (56) |
| Platelets, × 109/L* | 520 (155-1481) |
| > 500 × 109/L, no. (%) | 136 (52) |
| JAK2 mutation, no. (%) | 214/225 (95) |
| V617F, no. (%) | 209/214 (98) |
| Exon 12, no. (%) | 5/214 (2) |
Data are median (range).
Presence of either diabetes, arterial hypertension, smoking, or hypercholesterolemia.
Presence of either microvascular disturbances or pruritus.
Patients had been on treatment with HU for a median of 4.4 years (range, 0.1-21). Overall, 62 (24%) patients achieved a CR and 173 (66%) a PR, after a median of 4.6 months on therapy, whereas 25 (10%) patients did not attain any type of response according to the ELN criteria. It must be noted that the spleen size was not routinely assessed by imaging, thereby precluding the categorization of response as complete in many cases. Median daily dose of HU to achieve the best response was 1000 mg (range, 250-2500), and the median daily maintenance dose for responders was 850 mg (range, 250-2500). During the follow-up, 99 patients lost the response, 25 of them permanently and 74 were held intermittently in response. HU treatment had to be withdrawn in 74 patients because of toxicity (n = 30), lack of efficacy (n = 18), hematologic transformation or occurrence of a second malignancy (n = 13), concern of potential leukemogenic effect (n = 3), and other reasons (n = 10). The aggregated follow-up period according to the type of response was 777 person-years for sustained responders, 476 person-years for intermittent responders, and 473 person-years for no responders.
In total, 30 patients (11.5% of the total series) met at least 1 of the ELN criteria defining resistance to HU, after a median of 5.8 years (range, 0.2-18) from HU start. One patient needed regular phlebotomy to keep the hematocrit below 0.45 L/L after 3 months of at least 2 g/day of HU. Six patients developed uncontrolled myeloproliferation despite having received 2 g/day of HU for more than 3 months. Twenty-four patients developed neutropenia (absolute neutrophil count < 1 × 109/L), anemia (Hb < 100 g/L), or thrombocytopenia (platelet count < 100 × 109/L) at the lowest dose of HU required to achieve a CR or a PR. One patient met 2 resistance criteria, and no patient met the criteria defined as failure to reduce splenomegaly by more than 50% as measured by palpation after 3 months of at least 2 g/day of HU.
Thirty-three patients (12.6% of the series) fulfilled the ELN criteria of intolerance to HU. Seventeen patients developed leg ulcers at various HU doses, ranging from 500 mg to 2500 mg/day (median, 1000 mg/day). Other unacceptable mucocutaneous manifestations occurred in 13 cases, 1 patient developed fever probably related to the HU and 5 had gastrointestinal toxicity. Two patients suffered from 2 types of intolerant side effects, and 6 patients presented both resistance and intolerance criteria.
Survival
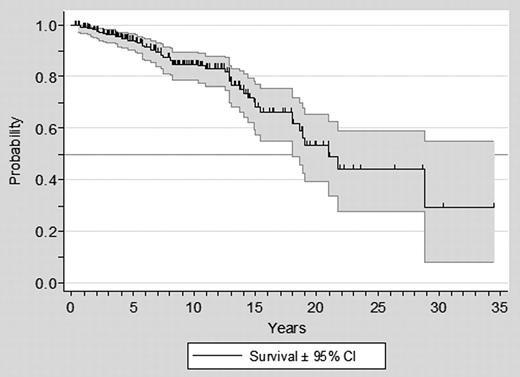
At the study closing date, median follow-up from PV diagnosis was 7.2 years (range, 0.13-34), 48 (18%) patients had died, and 24 (9%) were lost to follow-up. Median survival from diagnosis was 19 years, with a 10-year projected survival of 81% (Figure 1). When measured from the start on HU, the total time under observation amounted to 1726 person-years, and the median survival was 18 years. Eight cases of progression to acute leukemia, 20 of transformation to myelofibrosis, and 35 second neoplasms were diagnosed during the follow-up. Nineteen patients died after transformation to myelofibrosis or acute leukemia. In the remaining cases, the causes of death were a second neoplasia (n = 9), cardiac disease (n = 6), thrombosis (n = 5), infection (n = 4), bleeding (n = 2), and other (n = 3).
Overall survival from diagnosis in 261 patients with PV treated with HU.
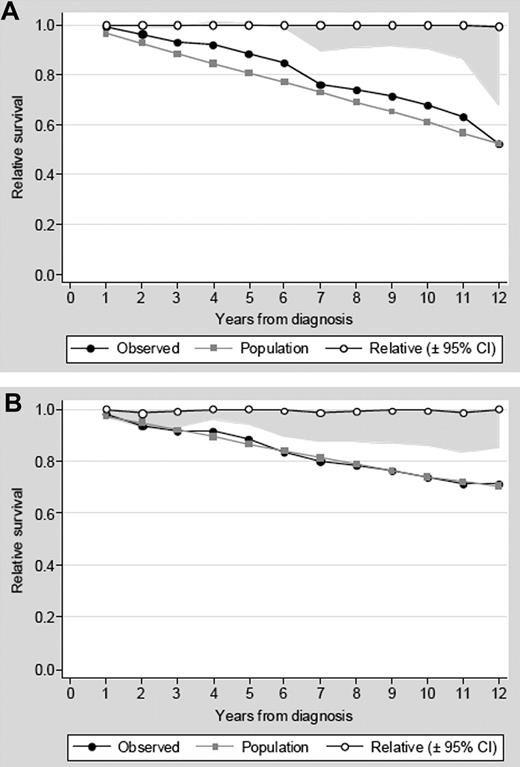
Baseline clinical factors predicting for survival at the multivariate analysis are shown in Table 2. Age > 65 years, male sex, and leukocytosis more than 10 × 109/L at diagnosis were associated with a significantly shorter survival. As can be seen in Figure 2, when relative survival curves were computed to evaluate the intrinsic prognostic value of age and sex, patients older than 65 years and males had a mortality rate similar to that seen in the age- and sex-matched general population. These findings indicate that the poorer prognosis associated with older age and male sex was driven exclusively by demographic factors and could not be attributed to PV.
Multivariate analysis of initial factors predicting survival in 261 patients with PV treated with HU
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age > 65 y | 3.0 | 1.6-5.9 | .001 |
| Male sex | 2.3 | 1.2-4.3 | .008 |
| Cardiovascular risk factors* | 1.6 | 0.8-3.2 | .2 |
| History of thrombosis | 1.4 | 0.8-2.6 | .2 |
| Hematologic values at diagnosis | |||
| Hemoglobin > 180 g/L | 0.7 | 0.4-1.3 | .2 |
| Leukocyte count > 10 × 109/L | 2.9 | 1.5-5.9 | .002 |
| Platelet count > 500 × 109/L | 0.9 | 0.5-1.8 | .9 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age > 65 y | 3.0 | 1.6-5.9 | .001 |
| Male sex | 2.3 | 1.2-4.3 | .008 |
| Cardiovascular risk factors* | 1.6 | 0.8-3.2 | .2 |
| History of thrombosis | 1.4 | 0.8-2.6 | .2 |
| Hematologic values at diagnosis | |||
| Hemoglobin > 180 g/L | 0.7 | 0.4-1.3 | .2 |
| Leukocyte count > 10 × 109/L | 2.9 | 1.5-5.9 | .002 |
| Platelet count > 500 × 109/L | 0.9 | 0.5-1.8 | .9 |
Presence of either diabetes, arterial hypertension, smoking, or hypercholesterolemia.
Relative survival of patients with PV. Relative survival of patients with PV and age more than 65 years (A) or male sex (B). Relative survival is the quotient of the observed survival to the expected survival in the general population matched to the patients by age, sex, and calendar year. A relative survival of 1 implies that patients are not dying at a higher rate than their counterparts in the general population.
Relative survival of patients with PV. Relative survival of patients with PV and age more than 65 years (A) or male sex (B). Relative survival is the quotient of the observed survival to the expected survival in the general population matched to the patients by age, sex, and calendar year. A relative survival of 1 implies that patients are not dying at a higher rate than their counterparts in the general population.
Fulfilling the ELN definition of response, either PR or CR, had no benefit for prolonging survival. When the different items included in the composite ELN response definition were individually assessed by multivariate analysis, lack of response in hematocrit, platelet count, PV-related symptoms, or spleen size did not have any prognostic relevance (data not shown). By contrast, failure to achieve a response in the WBC count was associated with a significantly shorter survival (HR, 2.7; 95% confidence interval [CI], 1.3%-5%.4; P = .007), after adjustment for age, sex, initial hematologic values, cardiovascular risk factors, and development of thrombosis or bleeding during follow-up. Furthermore, lack of sustained response in the WBC count was a predictor of hematologic transformation (HR, 3.2; 95% CI, 1.5%-7.1%; P = .004), after adjustment for age, sex, initial hematologic values, and exposure to busulfan or 32P).
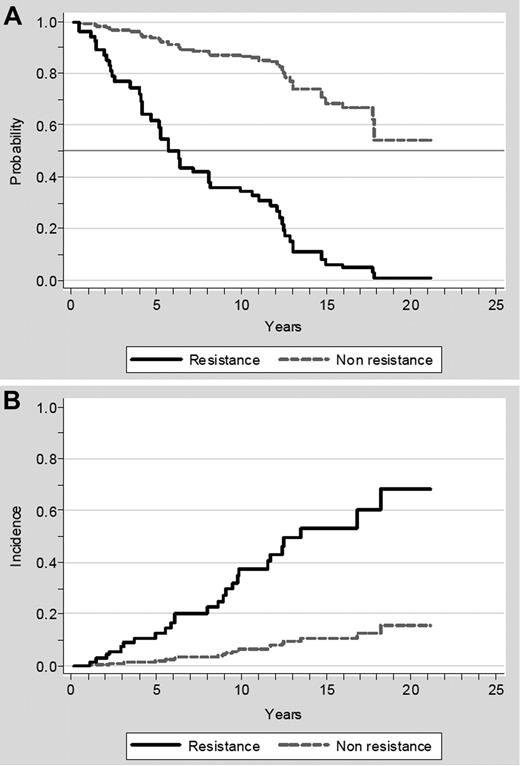
Resistance to HU was associated with a 5.6-fold increase in the risk of death after adjustment for other relevant prognostic factors, including age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, and WBC count response (Table 3). As can be seen in Table 3, when resistance to HU and no response in the WBC count were assessed simultaneously, both variables retained an independent prognostic significance, whereas leukocytosis more than 10 × 109/L at diagnosis lost its prognostic value. Median survival after developing resistance to HU was 1.2 years (range, 0.1-4.3). Figure 3A shows the adjusted survival curves according to whether the patients developed resistance to HU or not. Of note, 5 of the 8 patients progressing to acute leukemia and 11 of the 20 patients evolving to myelofibrosis had fulfilled the ELN criteria for resistance. In the multivariate analysis, resistance to HU was associated with an increased risk of transformation into acute leukemia or myelofibrosis (HR, 6.8; 95% CI, 3.0%-15.4%; P < .001) adjusted for age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, WBC count response, and exposure to busulfan or radioactive phosphorous. The adjusted cumulative incidence of hematologic transformation according to resistance to HU is shown in Figure 3B. Intolerance to HU, as defined by the ELN, did not show any association with the subsequent survival or the risk of transformation (data not shown).
Effect of response in WBC count and resistance to HU on survival in 261 patients with PV
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age > 65 y | 4.1 | 1.9-9.0 | < .001 |
| Male sex | 2.0 | 1.1-3.6 | .03 |
| Cardiovascular risk factors* | 1.8 | 0.9-3.7 | .1 |
| Hematologic values at diagnosis | |||
| Hemoglobin > 180 g/L | 0.8 | 0.4-1.6 | .6 |
| WBC > 10 × 109/L | 1.6 | 0.8-3.4 | .2 |
| Platelet count > 500 × 109/L | 1.0 | 0.5-1.9 | .9 |
| Thrombosis during follow-up | 1.6 | 0.8-3.2 | .1 |
| Bleeding during follow-up | 0.8 | 0.3-1.9 | .6 |
| No response in WBC† | 2.7 | 1.3-5.4 | .007 |
| Resistance to HU | 5.6 | 2.7-11.9 | < .001 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age > 65 y | 4.1 | 1.9-9.0 | < .001 |
| Male sex | 2.0 | 1.1-3.6 | .03 |
| Cardiovascular risk factors* | 1.8 | 0.9-3.7 | .1 |
| Hematologic values at diagnosis | |||
| Hemoglobin > 180 g/L | 0.8 | 0.4-1.6 | .6 |
| WBC > 10 × 109/L | 1.6 | 0.8-3.4 | .2 |
| Platelet count > 500 × 109/L | 1.0 | 0.5-1.9 | .9 |
| Thrombosis during follow-up | 1.6 | 0.8-3.2 | .1 |
| Bleeding during follow-up | 0.8 | 0.3-1.9 | .6 |
| No response in WBC† | 2.7 | 1.3-5.4 | .007 |
| Resistance to HU | 5.6 | 2.7-11.9 | < .001 |
Presence of either diabetes, arterial hypertension, smoking, or hypercholesterolemia.
Persistence of WBC count > 10 × 109/L despite treatment with HU.
Effect of resistance to HU on survival and on risk of transformation to acute leukemia or myelofibrosis. (A) Effect of resistance to HU on survival. The survival curves were adjusted for age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, and WBC count response. (B) Effect of resistance on the risk of transformation to acute leukemia or myelofibrosis. The cumulative incidence of transformation was computed taking death as a competing risk and adjusted for age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, WBC count response, and exposure to busulfan or radioactive phosphorus.
Effect of resistance to HU on survival and on risk of transformation to acute leukemia or myelofibrosis. (A) Effect of resistance to HU on survival. The survival curves were adjusted for age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, and WBC count response. (B) Effect of resistance on the risk of transformation to acute leukemia or myelofibrosis. The cumulative incidence of transformation was computed taking death as a competing risk and adjusted for age, sex, hematologic values at diagnosis, thrombosis, hemorrhage, WBC count response, and exposure to busulfan or radioactive phosphorus.
Thrombosis and bleeding
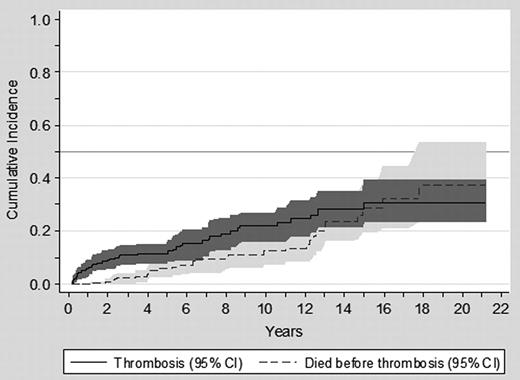
Over the 1726 person-years of follow-up on HU therapy, 55 thrombotic episodes (35 arterial and 20 venous) were observed in 45 patients (10 patients suffered 2 thrombotic events each), and 26 major hemorrhagic events in 23 patients (3 patients experiencing 2 bleeding episodes each). The cumulative incidence of thrombosis after starting HU and the competing risk of dying without thrombosis are shown in Figure 4. As can be seen, the cumulative incidence of thrombosis at 10 years was 22%.
Cumulative incidence of thrombosis and the competing risk of dying without thrombosis in patients with PV on treatment with HU.
Cumulative incidence of thrombosis and the competing risk of dying without thrombosis in patients with PV on treatment with HU.
Table 4 shows the incidence rates of major thrombosis or bleeding over the periods in which patients remained in sustained response versus those in intermittent or no response. Achieving a sustained response, either a PR or a CR, did not reduce the incidence rate of thrombosis or bleeding. When the different items included in the composite ELN response definition were individually considered, being in sustained response as related to PV-related symptoms, the spleen size by palpation, the hematocrit, or the WBC count was not associated with any significant reduction in the incidence rate of vascular events. On the contrary, the incidence rates of thrombosis and bleeding were significantly reduced while patients were in sustained response in the platelet count (Table 4). Of note, the protective effect of a sustained platelet response persisted after adjustment for age, sex, cardiovascular risk factors, initial hematologic values, history of thrombosis or bleeding before starting on HU, and periods of time on antiplatelet or anticoagulant therapy (IRR, 0.35; 95% CI, 0.2%-0.7%; P = .002 and IRR, 0.35; 95% CI, 0.1%-0.8%; P = .01 for thrombosis and bleeding, respectively). In most of the multivariate models, age more than 65 years and a previous history of thrombosis emerged as significant predictors of a higher incidence of thrombotic events while on HU (data not shown). No factor other than the response in the platelet count was independently associated with the incidence rate of bleeding, including the use of antiplatelet drugs or oral anticoagulants.
Incidence rate of thrombosis and bleeding according to response criteria in 261 PV patients treated with HU
| Type of sustained response . | Follow-up person-years . | Thrombosis . | Bleeding . | ||||
|---|---|---|---|---|---|---|---|
| No. of events . | Incidence rate (× 1000 person-years) . | P . | No. events . | Incidence rate (× 1000 person-years) . | P . | ||
| ELN response | |||||||
| Yes | 777.3 | 22 | 28.3 | .5 | 10 | 12.8 | .5 |
| No | 949.0 | 33 | 34.7 | 16 | 16.8 | ||
| Hematocrit < 0.45 L/L | |||||||
| Yes | 1101 | 36 | 32.7 | .8 | 16 | 14.5 | .8 |
| No | 625 | 19 | 30.4 | 10 | 16.0 | ||
| WBC count < 10 × 109/L | |||||||
| Yes | 1484.6 | 45 | 30.3 | .4 | 21 | 14.1 | .4 |
| No | 241.7 | 10 | 41.4 | 5 | 20.7 | ||
| Platelet count < 400 × 109/L | |||||||
| Yes | 1223.5 | 32 | 26.1 | .04 | 12 | 9.8 | .009 |
| No | 502.8 | 23 | 45.7 | 14 | 27.8 | ||
| Type of sustained response . | Follow-up person-years . | Thrombosis . | Bleeding . | ||||
|---|---|---|---|---|---|---|---|
| No. of events . | Incidence rate (× 1000 person-years) . | P . | No. events . | Incidence rate (× 1000 person-years) . | P . | ||
| ELN response | |||||||
| Yes | 777.3 | 22 | 28.3 | .5 | 10 | 12.8 | .5 |
| No | 949.0 | 33 | 34.7 | 16 | 16.8 | ||
| Hematocrit < 0.45 L/L | |||||||
| Yes | 1101 | 36 | 32.7 | .8 | 16 | 14.5 | .8 |
| No | 625 | 19 | 30.4 | 10 | 16.0 | ||
| WBC count < 10 × 109/L | |||||||
| Yes | 1484.6 | 45 | 30.3 | .4 | 21 | 14.1 | .4 |
| No | 241.7 | 10 | 41.4 | 5 | 20.7 | ||
| Platelet count < 400 × 109/L | |||||||
| Yes | 1223.5 | 32 | 26.1 | .04 | 12 | 9.8 | .009 |
| No | 502.8 | 23 | 45.7 | 14 | 27.8 | ||
Discussion
In the present study, the ELN criteria of response to HU were evaluated in a large cohort of PV patients with a long follow-up under this therapy. Most patients achieved a CR or a PR with HU, as shown by the fact that only 10% of cases failed to reach any degree of response. However, a substantial proportion of responders subsequently lost the response. Consequently, when analyzing the entire monitored period on HU, patients spent more time in intermittent or no response (949 person-years) than in sustained response (777 person-years).
With regard to clinical outcomes, achieving a sustained response, as defined by the ELN composite criterion, had no influence on the subsequent survival or the incidence of vascular events. Similar results have been recently reported by us and others in patients with essential thrombocythemia managed with HU, where the ELN definition of response showed no prognostic value with regard to survival or the risk of developing thrombohemorrhagic complications.16,17 Given the retrospective design of the present study, evaluation of spleen size by imaging was not available in many patients, what precluded the categorization of response as complete in many cases. Nevertheless, the ELN definition of response kept lacking any association with clinical outcomes even after assuming that patients without palpable splenomegaly would have met the ELN requirement of normal spleen size.
It must be noted that the ELN definition of response reflects the proliferative status of PV and was specifically designed to help evaluate new therapies aimed at modifying the biology of PV. As the ELN expert panel explicitly admitted, vascular complications and hematologic transformation were deliberately not considered.8 Moreover, the ELN definition of response is a composite of several items whose influence on clinical outcomes may vary from one to another. In this sense, when the different items of response were individually considered, only sustained response in the platelet and WBC count were associated with meaningful clinical outcomes, whereas the remaining variables included in the ELN definition lacked any prognostic significance. We are aware of the limitations of the present analysis, inherent to its retrospective design, which might have resulted in biases from patient selection and uncontrolled drug prescription, and also that our findings require confirmation in prospective studies. Meanwhile, our results emphasize the importance that PV patients on HU therapy achieve a response in the platelet and WBC count criteria of the ELN definition.
Sustained response in the hematocrit was not associated with better survival or a lower frequency of thrombosis, a finding in agreement with that observed in the European Collaboration on Low-dose Aspirin in Polycythemia Vera study.18 Other studies, however, have reported an increased risk of thrombosis in patients with poorly controlled hematocrit.5,19,20 Results on the lack of association between high hematocrit values and thrombotic risk must be interpreted with caution. First, we evaluated only the 0.45 L/L threshold, because this is the cut-off value defined by the ELN Expert Panel. Second, our patients were on cytoreductive treatment and under regular clinical monitoring, so that very high, uncontrolled hematocrit values, which bear the highest risk of thrombosis, were not allowed. Taken into consideration our results and those previously reported, it would be advisable targeting the hematocrit at 0.45 L/L but it does not seem necessary to maintain patients at all times below such threshold.
The association found in the present study between lack of response in platelet count and increased risk of thrombosis was unexpected, because it is in disagreement with previous studies reporting no association between thrombocytosis, either at presentation of PV or during follow-up, and thrombotic complications.5,18 In this sense, the lack of response to HU in the platelet count evaluated here has not the same meaning as the sustained thrombocytosis evaluated in previous publications because of differences between the above-mentioned studies and ours in the length of time in response, treatment modalities used, and statistical methodology.
Previous studies have shown that leukocytosis at PV diagnosis is a risk factor for the development of arterial thrombosis and acute leukemia, with both of these complications resulting in a shorter survival.21-23 In fact, these data were the rationale for including the WBC count in the ELN response criteria. Our study is the first addressing this issue in patients with PV treated with HU. We found that persistence of leukocytosis despite treatment with HU was associated with a higher risk of hematologic transformation and shorter survival. In addition, when leukocytosis at diagnosis and response in the WBC count were explored together in multivariate models, the latter cancelled the prognostic impact of the former, suggesting that treatment response in the leukocyte count could identify a subset of cases with less aggressive disease than those with persistent leukocytosis. In contrast, we could not demonstrate that response in the WBC count resulted in a reduced incidence of thrombosis.
Resistance to HU was infrequent, occurring in only 11% of our patients. It must be noted that primary resistance was extremely rare, with the majority of cases developing resistance late in the course of the disease, as illustrated by the 6-year median time elapsed from the start on HU to the diagnosis of resistance. Moreover, only one patient continued requiring regular phlebotomy to keep the hematocrit less than 0.45 L/L after 3 months of at least 2 g/day of HU, whereas 6 patients presented uncontrolled myeloproliferation. When the individual items included in the definition of resistance were assessed, cytopenia at the lowest dose of HU required to achieve a response emerged as the most common cause of resistance to HU. This latter observation suggests that resistance to HU is more a reflection of reduced hematopoietic reserve and impending hematologic transformation than a dose-dependent process. Indeed, patients fulfilling the ELN criteria for resistance had a 6.8-fold higher risk of hematologic transformation, and a significantly shorter survival than for patients not developing resistance. Resistance to HU seems, therefore, to be a meaningful dynamic prognostic factor in PV patients that may help clinicians in the therapeutic decision-making. Finally, intolerance to HU also was infrequent, being observed in only 13% of patients, a figure similar to that reported in previous studies.5-7 Intolerance to HU, as defined by the ELN criteria, can be a reason for moving patients to a second-line therapy but does not entail any prognostic significance.
In summary, no significant association was observed in the present study between the composite ELN definition of response and the clinical outcomes of patients with PV on HU treatment. Nevertheless, achieving a response in the platelet and WBC counts were predictive of less thrombohemorrhagic complications and lower risk of hematologic transformation and better prognosis, respectively. Furthermore, resistance to HU emerged as an adverse prognostic factor in PV.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants Retics RD06/0020/004, EC10-136, and FIS PI10/01 807, PI10/00 236, and PS09/02 324 from the Instituto de Salud Carlos III, Spanish Ministry of Health and grant Asociación Española Contra el Cáncer Catalunya 2011.
Authorship
A.A.-L. designed the study, collected the data, performed the statistical analysis, analyzed and interpreted the results, and wrote the paper; A.P. designed the study, performed the statistical analysis, analyzed and interpreted the results, and wrote the paper; F.C. designed the study, interpreted the results, and wrote the paper; E.A.-R. collected the data, interpreted the results, and wrote the paper; J.C.H.-B. collected the data, interpreted the results, and wrote the paper; F.F.-M. collected the data and approved the final version; A.A. collected the data and approved the final version; M.G. collected the data and approved the final version; B.M. collected the data and approved the final version; H.G. collected the data and approved the final version; A.T. collected the data and approved the final version; B.B. performed molecular studies, collected the data, and approved the final version; C. Burgaleta collected the data, interpreted the results, and approved the final version; V.V. interpreted the results and approved the final version; and C. Besses designed the study, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Alvarez-Larrán, Department of Hematology, Hospital del Mar, Passeig Marítim 25-29, 08003 Barcelona, Spain; e-mail: 95967@parcdesalutmar.cat.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal