Abstract
Ataxia-telangiectasia mutated (ATM) plays a central role in DNA damage responses, and its loss leads to development of T-cell malignancies. Here, we show that ATM loss also leads to intrinsic mitochondrial abnormalities in thymocytes, including elevated reactive oxygen species, increased aberrant mitochondria, high cellular respiratory capacity, and decreased mitophagy. A fraction of ATM protein is localized in mitochondria, and it is rapidly activated by mitochondrial dysfunction. Unexpectedly, allelic loss of the autophagy regulator Beclin-1 significantly delayed tumor development in ATM-null mice. This effect was not associated with rescue of DNA damage signaling but rather with a significant reversal of the mitochondrial abnormalities. These data support a model in which ATM plays direct roles in modulating mitochondrial homeostasis and suggest that mitochondrial dysfunction and associated increases in mitochondrial reactive oxygen species contribute to the cancer-prone phenotype observed in organisms lacking ATM. Thus, ataxia-telangiectasia should be considered, at least in part, as a mitochondrial disease.
Introduction
Ataxia-telangiectasia (A-T) is an autosomal recessive disease in which several pathologies emerge in the first 2 decades of life, including telangiectasias, immunodeficiency, radiosensitivity, insulin resistance, cerebellar ataxia, and T-lymphoid tumors.1 Among these, cerebellar ataxia and T-cell malignancies are the most debilitating phenotypes of this disorder. Interestingly, mouse models of A-T exhibit most of the phenotypes manifest in the human disease, with the exception of neurodegeneration.2,3 A-T is caused by loss of function mutations in the Ataxia Telangiectasia Mutated (ATM) gene, which encodes a serine/threonine protein kinase that belongs to the phosphoinositide 3-kinase (PI3K)–related protein kinase family.4
ATM plays a central role in initiating cellular responses to DNA double-strand breaks.5 However, some of the phenotypic abnormalities seen in A-T patients are not easily explained by defects in DNA damage response (DDR) pathways, including insulin resistance and lung and telangiectasia pathologies.6,7 Indeed, even the neurodegeneration, cancer predisposition, and premature aging phenotypes could result from defects other than abnormal responses to DNA breakage. Reports suggesting that a fraction of ATM localizes to cytoplasmic compartments in various cell types8 and that cerebellar cells lacking ATM exhibit an increase in cytoplasmic vesicles suggest the potential of non-nuclear functions of ATM.9 Because treatment of ATM-null mice with antioxidants can ameliorate intrinsic defects in stem cell renewal10,11 and delay their tumor onset,12,13 it has been suggested that increased accumulation of intracellular reactive oxygen species (ROS) associated with ATM dysfunction contributes to these pathologies.14-16 To date, the increased oxidative damage associated with ATM deficiency has largely been attributed to the defects in the DDR pathway.
Mitochondria are essential organelles in all eukaryotic cells that direct cellular energy (ATP) production via oxidative phosphorylation.17 During this process, electrons are transferred through a series of 5 macromolecular complexes known as the electron transport chain (ETC) in which a proton gradient is produced across the inner mitochondrial membrane to form an electrochemical membrane potential.17 This membrane potential is then used by the last complex of the chain (F0F1 ATP synthase) to generate ATP. Importantly, mitochondria are also major sites of ROS production and, accordingly mitochondrial dysfunction is detrimental to the organism. For example, oxidative damage can be caused by the ROS produced via accidental escape of electrons from complexes I and III of the ETC17 that can then damage biologic macromolecules such as lipids, proteins, and DNA. Indeed, mitochondrial dysfunction is implicated in the pathology of numerous diseases.18
Previous studies, largely using immortalized cell lines established from patients with A-T, have reported that cells lacking ATM function exhibit alterations in mitochondrial homeostasis.19,20 However, there are some inconsistencies between these studies, such as discrepancies in the nature of mitochondrial DNA content abnormalities. Here, we report that in vivo loss of ATM results in striking mitochondrial dysfunction in thymocytes, leading to elevated mitochondrial number and increased mitochondrial ROS production. The increase in mitochondrial content is associated with defects in the intracellular destruction of abnormal mitochondria (mitophagy). Unexpectedly, allelic loss of the autophagic regulatory gene Beclin-1 causes marked delays in lymphomagenesis in ATM-null mice. Although no rescue of DDR responses was manifest, many of the mitochondrial abnormalities displayed in ATM-null thymocytes were modulated by Beclin-1 heterozygosity. These findings establish links between ATM and Beclin-1 in mitochondrial homeostasis, control of ROS production, and tumor predisposition.
Methods
Mice
All animal protocols were approved by Institutional Animal Care and Use Committees at St Jude Children's Research Hospital and Scripps–Florida. ATM+/− and Beclin-1+/− mice were provided by Peter McKinnon2 and Nathaniel Heintz,21 respectively. ATM+/− and Beclin-1+/− mice were maintained more than 80% C57BL/6 (5-8 weeks old). ATM+/− mice were crossed to Beclin-1+/− mice to obtain ATM+/−Beclin-1+/− offspring. These mice were then intercrossed to obtain wild-type, ATM−/−Beclin-1+/+, and ATM−/−Beclin-1+/− mice. Eμ-Myc transgenic mice (C57Bl/6)22 were crossed to Beclin-1+/− mice (C57Bl/6) to generate the 2 desired cohorts Eμ-Myc Beclin-1+/− and Eμ-Myc Beclin-1+/+ that were followed for their survival. For radiosensitivity experiments, mice were exposed to whole body irradiation (6 or 8 Gy) using a Shepherd Mark I irradiator (cesium 137 source, model 68A).
Cell culture, transfection, and reagents
Mouse embryonic fibroblasts (MEFs) were generated from embryonic day 13.5 embryos by mincing embryonic carcass with sterile scalpels followed by digestion with 0.05% trypsin (10 minutes). Cells were then passed through a 181/2-gauge needle several times, and 15 mL of DMEM (15% FBS, 1% antibiotics, 4mM l-glutamine, and 0.05mM β-mercaptoethanol) was added. One milliliter of these cells was collected to isolate total cellular DNA and protein (passage 0). Remaining cells were divided into two 25-cm2 flasks (BD Biosciences) and incubated at 37°C in a 5% CO2 chamber (passage 1). Cells were passed every 2 days. Immortalized cells were maintained in DMEM or RPMI-1640 (15% FBS, 1% antibiotics, and 4mM l-glutamine). For cell transfection, cells were seeded in 4-well chamber slides (Lab-Tek; Nalge Nunc International) at 50% to 70% confluence followed by transfection with 0.5 μg YFP-Parkin (Addgene plasmid 23955) per well for 24 hours using Lipofectamine 2000 (Invitrogen) as indicated by the manufacturer. Carbonyl cyanide m-chlorophenyldrazone (CCCP) was purchased from Santa Cruz Biotechnology (sc-202984) and dissolved in DMSO to obtain a concentration of 100mM. For cell culture, CCCP was diluted in DMEM to a final concentration of 50μM, and cells were incubated for 3 hours at 37°C in a 5% CO2 chamber. For treatment with the ATM inhibitor, CP466722 (CP),23 thymocytes were spun down and resuspended in DMEM containing 10μM CP and incubated at 37°C in a 5% CO2 chamber (3 or 5 hours). Addition of 50μM CCCP was performed 3 hours before cell collection. Cells were stained with MitoTracker Green FM (Invitrogen) as described in the next section before flow cytometry analysis.
Antibodies, immunoblotting, and immunofluorescence
The following antibodies were used for immunoblotting or immunofluorescence: p62 (BD Biosciences); Beclin-1, Parkin, and Tom20 (Santa Cruz Biotechnology); LC3, total-p53, γ-H2AX, phospho-p53, and DJ-1 (Cell Signaling Technology); β-actin (Sigma-Aldrich), phospho-SMC1 (anti-sera raised in mouse against phosphorylated S957 of mouse SMC124 ); phospho-KAP1 (Bethyl Laboratories); Topoisomerase I (Topogen), lactate dehydrogenase and cytochrome c oxidase subunit-IV (COX-IV; Abcam); and anti–phospho-mouse ATM-S1987 (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Western blotting was performed as described previously.25 Immunofluorescence was performed using an E800 microscope (Nikon).
Cell fractionation
Normal human fibroblasts were fractionated into nuclear, cytoplasmic, and mitochondrial fractions using the MS861 cell fractionation kit (Mitoscience). Purity of the fractions was assessed by blotting against Topoisomerase I (nuclear), lactate dehydrogenase (cytoplasmic), and COX-IV (mitochondrial) proteins. Equal volumes of each fraction were loaded to guarantee analysis of the same number of cells per fraction. We obtained 10 μg of total cellular protein by lysing cells with Nonidet P-40 buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 0.5% Nonidet P-40, 50mM NaF, and 1× proteinase inhibitor cocktail; Roche Diagnostics), which was analyzed as control.
Mitochondrial mass and membrane potential measurements
MitoTracker Green FM (Invitrogen) was diluted in DMEM (200nM final concentration). Thymocytes and B220+ B lymphocytes were incubated at 37°C (20 minutes), cells were then spun down, and medium was replaced. Cells were analyzed by flow cytometry. Nonyl acridine orange (NAO; Invitrogen) was diluted to 5nM in 1× PBS-5% FBS. Cells were incubated with NAO (10 minutes). Cells were washed with PBS and resuspended in PBS-5% FBS and analyzed by flow cytometry. For mitochondrial membrane potential, cells were stained with 100nM tetramethylrhodamine, ethyl ester (Molecular Probes) at 37°C (10 minutes) and quickly analyzed by flow cytometry (propidium iodide was added to exclude dead cells).
Measurement of cellular ATP, and complex I enzyme activity assays
Cellular ATP levels were quantified in viable thymic cells using the ATP bioluminescent somatic cell assay kit (Sigma-Aldrich) as indicated by the manufacturer. The Complex-I Enzyme Activity Dipstick Assay kit developed by Mitoscience (MS130) was used to determine the activity of this complex as indicated by the company. The same amount of protein extracts from mice of different genotypes was analyzed in the assays. Standard curves were generated to verify the accuracy of the assay. The signal intensity was determined using ImageJ 1.44 (National Institutes of Health).
Oxygen consumption analyses
Respiration was measured in intact cells using a Seahorse XF24 analyzer.26 Cells were seeded in plates coated with poly-l-lysine. After 5 hours, the cells were loaded into the machine to determine the oxygen consumption rate (OCR). Respiration was measured sequentially after addition of oligomycin (0.5mM), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (1mM), and rotenone (0.5mM). After each injection, OCR was measured for 3 minutes, the medium was mixed, and then respiration was measured again for 3 minutes.
Please refer to supplemental Methods for additional descriptions of methods.
Image acquisition
Electron microscopy was imaged using a JEOL 1200 electron microscope with an AMT XR 111 camera. Scale bars placed in each EM picture. Immunofluorescence was observed using a Nikon Eclipse Ti microscope and pictures were taken using a Nikon Digital Sight DS-U3 camera. Images were analyzed and prepared for publication using the NIS-Elements BR 3.2 program. Magnification of objective lenses was either 40×0.55 or 10×/0.25. The numeric aperture used was 0.55 and the camera settings were 1280 × 1024 for all the immunofluorescence images.
Results
Mitochondrial dysfunction in ATM-null thymocytes
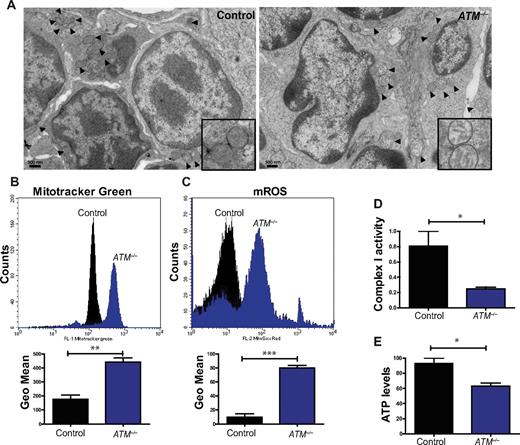
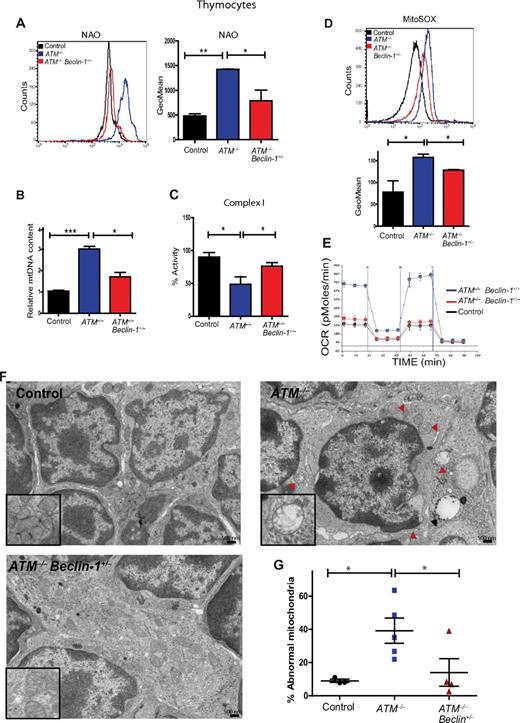
Humans and mice lacking the ATM protein kinase are prone to developing T-lymphoid tumors,2,3 a phenotype generally attributed to defects in the DDR pathway. Analysis of thymocytes isolated from 5- to 8-week-old mice revealed striking increases in the number of altered mitochondria in the absence of ATM, including those that were swollen, had poorly organized cristae, or both (Figure 1A; supplemental Figure 1A). Characterization of the thymic population demonstrated that ATM−/− thymocytes exhibit increases in mitochondrial mass as assessed by staining with MitoTracker Green, a dye that binds to mitochondria regardless of their membrane potential (Figure 1B), as well as marked increases in mitochondrial reactive oxygen species (mROS; Figure 1C). Previous studies reported that ATM−/− cells have increases in cellular ROS,14-16 but the source of these molecules had not been clarified. In agreement with the mitochondrial mass assay, measurement of mitochondrial membrane potential revealed that ATM-null display an increased number of cells with high membrane potential relative to controls (supplemental Figure 1B). Deficiency of complex I of the electron transport chain leads to abnormal generation of mROS,17 and complex I activity in ATM−/− thymocytes was significantly decreased (Figure 1D). ATM−/− thymocytes also had significantly reduced levels of cellular ATP (Figure 1E). Thus, loss of ATM results in mitochondrial dysfunction and concomitant elevation of mROS levels in vivo. Furthermore, the data suggest that the increased oxidative stress in ATM−/− mice probably occurs as a result of the elevated mROS, possibly because of diminished complex I activity.
Mitochondrial dysfunction in ATM-null thymocytes. (A) Ultrastructural abnormalities in ATM-deficient thymic cells. Representative pictures taken by transmission electron microscopy in young mice from wild-type or ATM−/− mice. ATM−/− thymic tissue showed a number of mitochondria with disorganized structure and swollen appearance. Arrowheads denote mitochondria. Magnification, × 6000. Boxes show higher magnifications of the mitochondria. (B-C) Increased mitochondrial mass and mROS in ATM−/− thymocytes. Freshly isolated thymic cells were stained with 200nM MitoTracker Green (MTG) probe (B) or 5μM superoxide indicator MitoSOX (C) and analyzed by flow cytometry. Representative histograms of MTG and MitoSOX fluorescence intensity per genotype are illustrated. Graph shows the averaged mean intensity for each genotype (n ≥ 3/group; Student t test: **P ≤ .001, ***P ≤ .0001). (D-E) ATM-deficient thymocytes display reduced activity of complex I of the ETC and diminished levels of cellular ATP. (D) Total protein was extracted from freshly isolated viable thymocytes of 5- to 8-week-old mice of the indicated genotypes. The activity of complex I of the ETC was analyzed using equal amount of protein per sample (Student t test: *P ≤ .02; n ≥ 3/genotype). (E) ATM−/− thymocytes display reductions in cellular ATP levels. Total cellular ATP levels were measured in equal number of freshly isolated viable thymic cells isolated from 5- to 8-week-old mice of the indicated genotypes. Shown are the relative ATP levels for each cohort (Student t test: *P ≤ .02; n ≥ 3/genotype).
Mitochondrial dysfunction in ATM-null thymocytes. (A) Ultrastructural abnormalities in ATM-deficient thymic cells. Representative pictures taken by transmission electron microscopy in young mice from wild-type or ATM−/− mice. ATM−/− thymic tissue showed a number of mitochondria with disorganized structure and swollen appearance. Arrowheads denote mitochondria. Magnification, × 6000. Boxes show higher magnifications of the mitochondria. (B-C) Increased mitochondrial mass and mROS in ATM−/− thymocytes. Freshly isolated thymic cells were stained with 200nM MitoTracker Green (MTG) probe (B) or 5μM superoxide indicator MitoSOX (C) and analyzed by flow cytometry. Representative histograms of MTG and MitoSOX fluorescence intensity per genotype are illustrated. Graph shows the averaged mean intensity for each genotype (n ≥ 3/group; Student t test: **P ≤ .001, ***P ≤ .0001). (D-E) ATM-deficient thymocytes display reduced activity of complex I of the ETC and diminished levels of cellular ATP. (D) Total protein was extracted from freshly isolated viable thymocytes of 5- to 8-week-old mice of the indicated genotypes. The activity of complex I of the ETC was analyzed using equal amount of protein per sample (Student t test: *P ≤ .02; n ≥ 3/genotype). (E) ATM−/− thymocytes display reductions in cellular ATP levels. Total cellular ATP levels were measured in equal number of freshly isolated viable thymic cells isolated from 5- to 8-week-old mice of the indicated genotypes. Shown are the relative ATP levels for each cohort (Student t test: *P ≤ .02; n ≥ 3/genotype).
Previous observations using cultured, immortalized human cell lines suggested that ATM deficiency is associated with reductions in mitochondrial content.19,20 Because ATM−/− thymocytes paradoxically showed increased mitochondrial mass, we assessed mitochondrial DNA content in commonly used immortalized A-T cell lines. In accord with published data, cultured, immortalized human or mouse cells lacking ATM displayed either equal or reduced levels of mDNA content relative to paired control cell lines (supplemental Figure 2A-C). To explore the potential impact of culture stress on mitochondrial content in the presence or absence of ATM, MEFs were newly established and relative mitochondrial DNA content was assessed as they were passaged in culture. Consistent with the increase in mitochondrial mass in ATM−/− thymocytes, newly plated (P0) ATM−/− MEFs exhibited significant increases in mitochondrial DNA content relative to P0 wild-type MEFs (supplemental Figure 2D), and this difference in mitochondrial DNA content was initially sustained as cells aged in culture (supplemental Figure 2D). However, with increasing passage mitochondrial DNA content increased in both wild-type and ATM−/− MEFs. Thus, cell culture conditions and cellular passage number seem to influence mitochondrial DNA homeostasis, and it is best to use in vivo cells or very early passage cells for these studies whenever possible.
Abnormal mitophagy but efficient conventional autophagy in the absence of ATM
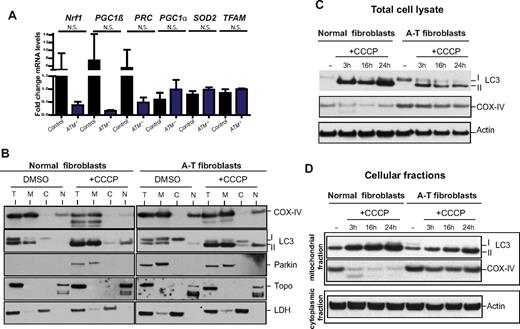
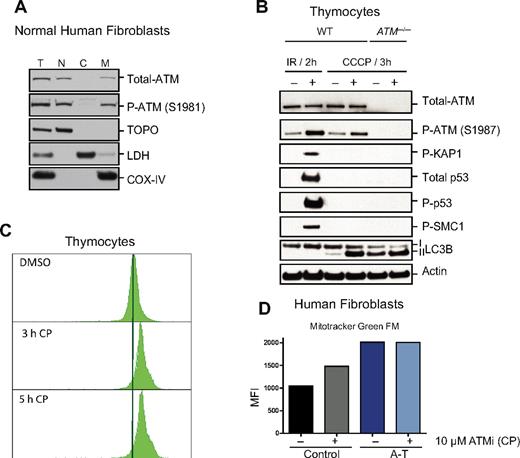
The abnormal increase in mitochondrial mass observed in ATM-deficient thymocytes (Figure 1B) could be because of increased mitochondrial biogenesis or decreased clearance of abnormal mitochondria by autophagy (mitophagy), or both. Several genes involved in mitochondrial biogenesis27 were evaluated, but none were not significantly increased in ATM−/− versus wild-type thymocytes (Figure 2A), suggesting that the increased mitochondrial content in ATM-null thymocytes is not because of increased mitochondrial biogenesis. Mitochondrial damage provoked by treatment with the mitochondrial uncoupler CCCP typically results in autophagic digestion of the damaged mitochondria and to reductions in mitochondria and mitochondrial proteins, a process known as mitophagy.28 The induction of mitophagy after mitochondrial damage is dependent on accumulation of Parkin protein in mitochondria.28 CCCP treatment of normal human fibroblasts led to increased levels of mitochondrial Parkin (Figure 2B). Interestingly, endogenous Parkin protein was already located in the mitochondria of untreated ATM-null fibroblasts, suggesting basal mitochondrial damage, and its levels did not increase further with CCCP treatment (Figure 2B). Moreover, expression of exogenous Parkin (YFP-Parkin) revealed that although normal human fibroblasts show a diffuse expression of this protein under normal conditions, ATM-null fibroblasts display a more punctate distribution that overlaps with the mitochondrial marker TOM-20 (supplemental Figure 3). Furthermore, although mitochondrial damage induced by CCCP treatment of wild-type human fibroblasts led to expected decreases in the levels of the mitochondrial protein COX-IV, human ATM-null fibroblasts did not exhibit decreases in COX-IV levels after CCCP treatment (Figure 2C [total cell lysate] and D [mitochondrial fractions]), even though the autophagy process was induced in these cells (determined by the presence of phosphatidylethanolamine-modified LC3 (LC3-II) at the mitochondria; Figure 2B-D). These observations suggest that the process of clearing damaged mitochondria by mitophagy is defective in ATM-null cells. Thus, defective mitophagy, but not increased mitochondrial biogenesis, seems to contribute to the increased mitochondrial content of ATM−/− mouse thymocytes.
ATM deficiency is associated with abnormal mitophagy, not increased mitochondrial biogenesis. (A) Expression of genes involved in mitochondrial biogenesis is intact in ATM-deficient and wild-type thymocytes. Nrf1, PGC1β, PRC, PGC1α, SOD2, and TFAM mRNAs were measured by reverse transcription real-time PCR analyses. mRNA levels were normalized using Actin mRNA as an internal control. N.S. indicates not statistically significant. (B-D) Accumulation of Parkin and impaired mitophagy in A-T human fibroblasts. (B) Parkin levels are elevated in mitochondria of A-T fibroblasts without induction of mitochondrial damage by CCCP. Normal or A-T human fibroblasts were treated with DMSO or 50μM CCCP for 4 hours, and equal number of cells per sample was submitted to fractionation into mitochondrial (M), cytoplasmic (C), and nuclear (N) subregions. Equal volume of the fractions was analyzed by Western blot for the expression of Parkin, COX-IV (mitochondrial marker), Topoisomerase I (Topo; nuclear marker), LC3 (autophagic marker), and lactate dehydrogenase (LDH; cytoplasmic marker). We also analyzed 20 μg of total (T) protein. The small amount of LC3 present at the nuclear fraction after CCCP treatment is probably because of a residual mitochondrial material that fractionated with the nuclear compartment because CCCP could potentially induce damage to the mitochondrial membrane and thus make these organelles more leaky. (C) Normal or A-T human fibroblasts were treated with DMSO or 50μM CCCP for 3, 16, or 24 hours, and proteins were isolated followed by immunoblotting against COX-IV, LC3 (autophagic marker), and Actin (loading control) in total cell lysate. (D) Normal or A-T human fibroblasts were treated with CCCP as in panel C followed by cellular fractionation as in panel B, and mitochondrial protein fractions were analyzed by immunoblotting against COX-IV and LC3. Actin levels in the cytoplasmic fractions of these samples also were analyzed to verify equal loading based on cell number.
ATM deficiency is associated with abnormal mitophagy, not increased mitochondrial biogenesis. (A) Expression of genes involved in mitochondrial biogenesis is intact in ATM-deficient and wild-type thymocytes. Nrf1, PGC1β, PRC, PGC1α, SOD2, and TFAM mRNAs were measured by reverse transcription real-time PCR analyses. mRNA levels were normalized using Actin mRNA as an internal control. N.S. indicates not statistically significant. (B-D) Accumulation of Parkin and impaired mitophagy in A-T human fibroblasts. (B) Parkin levels are elevated in mitochondria of A-T fibroblasts without induction of mitochondrial damage by CCCP. Normal or A-T human fibroblasts were treated with DMSO or 50μM CCCP for 4 hours, and equal number of cells per sample was submitted to fractionation into mitochondrial (M), cytoplasmic (C), and nuclear (N) subregions. Equal volume of the fractions was analyzed by Western blot for the expression of Parkin, COX-IV (mitochondrial marker), Topoisomerase I (Topo; nuclear marker), LC3 (autophagic marker), and lactate dehydrogenase (LDH; cytoplasmic marker). We also analyzed 20 μg of total (T) protein. The small amount of LC3 present at the nuclear fraction after CCCP treatment is probably because of a residual mitochondrial material that fractionated with the nuclear compartment because CCCP could potentially induce damage to the mitochondrial membrane and thus make these organelles more leaky. (C) Normal or A-T human fibroblasts were treated with DMSO or 50μM CCCP for 3, 16, or 24 hours, and proteins were isolated followed by immunoblotting against COX-IV, LC3 (autophagic marker), and Actin (loading control) in total cell lysate. (D) Normal or A-T human fibroblasts were treated with CCCP as in panel C followed by cellular fractionation as in panel B, and mitochondrial protein fractions were analyzed by immunoblotting against COX-IV and LC3. Actin levels in the cytoplasmic fractions of these samples also were analyzed to verify equal loading based on cell number.
Oxidative stress typically triggers increases in conventional autophagy in cells as a protective mechanism.29 Because ATM loss results in both mitophagy deficiency and increased oxidative damage, we evaluated the expression of markers involved in autophagy30,31 in ATM−/− MEFs. At early passage (P0), ATM-deficient MEFs expressed reduced levels of p62 and increased levels of LC3-II, both signs of increased basal autophagy (supplemental Figure 4). Thus, although autophagy can be efficiently induced in ATM-deficient cells (Figure 2B-D), apparently even at higher levels than ATM-proficient cells (supplemental Figure 4), the clearance of abnormal mitochondria via mitophagy is impaired (Figure 2B-D).
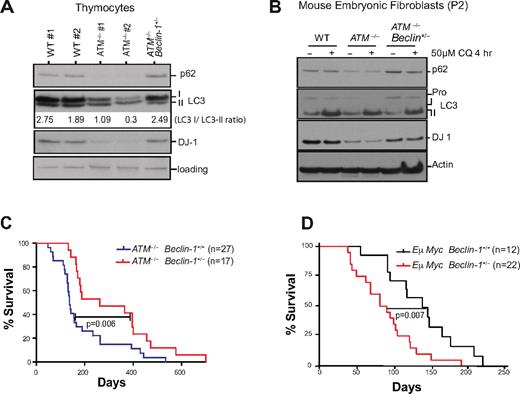
Effects of ATM deficiency on autophagy and mitophagy raised questions regarding the potential roles for alterations in autophagic processes in the various pathologies observed ATM-deficient mice. To explore this possibility, we bred ATM-null animals to a Beclin-1 heterozygous background21 (supplemental Figure 5). Consistent with the phenotypes manifest in MEFs, analyses of ATM-null thymocytes showed that autophagy is elevated relative to wild-type thymocytes, in that there were marked reductions in the levels of p62/Sequestosome and there was an increased ratio of LC3-II versus unmodified LC3 (LC3-I; Figure 3A). Allelic loss of Beclin-1 alleviated the autophagic induction in both ATM-null thymocytes (Figure 3A) and early passage MEFs (Figure 3B).
Beclin-1 heterozygosity reverts the induction of macroautophagy and reduced levels of the oxidative sensor DJ-1 in ATM-null cells but delays tumorigenesis in ATM-null mice. (A) Allelic loss of Beclin-1 reverses the increases in autophagic signaling and the reduced levels of DJ-1 manifest in ATM−/− thymocytes. Proteins from freshly isolated thymocytes were analyzed by SDS-PAGE immunoblotting for detection of the autophagic markers p62 and LC3 and the oxidative stress sensor DJ-1. Band intensities of the cytoplasmic (-I) and autophagosome-associated (-II) forms of LC3 were determined using the ImageJ processing program, and the ratio of the LC3-I/LC3-II forms was calculated for each sample. (B) Allelic loss of Beclin-1 reverses the increased autophagic response and the reductions in DJ-1 protein levels observed in early passage ATM−/− MEFs. P2 MEFs were treated with either DMSO or 50μM chloroquine (CQ; Sigma-Aldrich) for 4 hours, and total proteins were analyzed for the autophagic markers p62 and LC3, or for DJ-1. Transient treatment with CQ did not significantly alter p62 expression but affected LC3-I/II conversion in MEFs. (C-D) Allelic loss of Beclin-1 delays death of ATM−/− mice (C), while accelerating onset of B-cell lymphoma in Eμ-Myc–transgenic mice (D). Kaplan-Meier survival plots of mice with desired genotypes are shown. P values were obtained by the log-rank (Mantel-Cox) test. The nonsurviving ATM−/− and ATM−/−Beclin-1+/− all died of T-cell lymphomas.
Beclin-1 heterozygosity reverts the induction of macroautophagy and reduced levels of the oxidative sensor DJ-1 in ATM-null cells but delays tumorigenesis in ATM-null mice. (A) Allelic loss of Beclin-1 reverses the increases in autophagic signaling and the reduced levels of DJ-1 manifest in ATM−/− thymocytes. Proteins from freshly isolated thymocytes were analyzed by SDS-PAGE immunoblotting for detection of the autophagic markers p62 and LC3 and the oxidative stress sensor DJ-1. Band intensities of the cytoplasmic (-I) and autophagosome-associated (-II) forms of LC3 were determined using the ImageJ processing program, and the ratio of the LC3-I/LC3-II forms was calculated for each sample. (B) Allelic loss of Beclin-1 reverses the increased autophagic response and the reductions in DJ-1 protein levels observed in early passage ATM−/− MEFs. P2 MEFs were treated with either DMSO or 50μM chloroquine (CQ; Sigma-Aldrich) for 4 hours, and total proteins were analyzed for the autophagic markers p62 and LC3, or for DJ-1. Transient treatment with CQ did not significantly alter p62 expression but affected LC3-I/II conversion in MEFs. (C-D) Allelic loss of Beclin-1 delays death of ATM−/− mice (C), while accelerating onset of B-cell lymphoma in Eμ-Myc–transgenic mice (D). Kaplan-Meier survival plots of mice with desired genotypes are shown. P values were obtained by the log-rank (Mantel-Cox) test. The nonsurviving ATM−/− and ATM−/−Beclin-1+/− all died of T-cell lymphomas.
To corroborate the apparent autophagic induction in ATM−/− MEFs and rescue by allelic loss of Beclin-1, we also assessed LC3 expression by immunofluorescence. LC3 was undetectable in early passage (P2) wild-type MEFs, although treatment with the lysosomotropic drug chloroquine induced LC3 accumulation (supplemental Figure 6). In contrast, ATM−/− P2 MEFs expressed detectable levels of LC3 even in the absence of chloroquine treatment, and Beclin-1 heterozygosity reduced accumulation of LC3 protein (supplemental Figure 6). Interestingly, in all genotypes the accumulation of LC3 protein increased with passage, although ATM−/− cells still had higher levels of LC3 than either wild-type or ATM−/−Beclin-1+/− MEFs (supplemental Figure 6, P4). These data support the findings that autophagy is induced in the oxidatively-stressed ATM-deficient cells and suggest that allelic loss of Beclin-1 dampens this response.
The DJ-1 protein is thought to help cells overcome damage caused by oxidative stress and loss of DJ-1 triggers mitochondrial depolarization and fragmentation, and the induction of autophagy.32,33 Given that ATM loss leads to an increase in mROS, we also analyzed the expression of DJ-1 in these cells. DJ-1 protein levels were reduced in ATM−/− thymocytes and P2 MEFs and were rescued by Beclin-1 heterozygosity (Figure 3A-B). Reduced DJ-1 levels in ATM-deficient cells would be predicted to further augment oxidative stress in mitochondria, and both increased ROS and damaged mitochondria would contribute to induction of autophagic responses.34
Allelic loss of Beclin-1 delays tumorigenesis in ATM-null mice
ATM-null mice develop T-cell lymphomas with high penetrance, and Beclin-1 heterozygosity has been associated with increased tumor development.21,35 Unexpectedly, allelic loss of Beclin-1 significantly delayed lymphoid tumor onset and increased survival in ATM−/− mice (mean survival of 262 days in ATM−/−Beclin-1+/− mice vs 137 days in ATM−/− mice; Figure 3C). This tumor-protective effect of Beclin-1 heterozygosity was not a reflection of something unusual about these particular Beclin-1+/− mice because crossing these same Beclin-1+/− mice with Eμ-Myc transgenic mice, a model of human Burkitt lymphoma,22 accelerated tumor onset (mean survival of 80 days for Eμ-Myc Beclin-1+/− mice vs 143 days for Eμ-Myc Beclin-1+/+ mice; Figure 3D). The tumor-promoting effect of Beclin-1+/− in the Eμ-Myc background is consistent with the tumor-suppressive effects of this protein in a variety of settings21,35 ; however, its impact on tumor development in the context of ATM deficiency is clearly different.
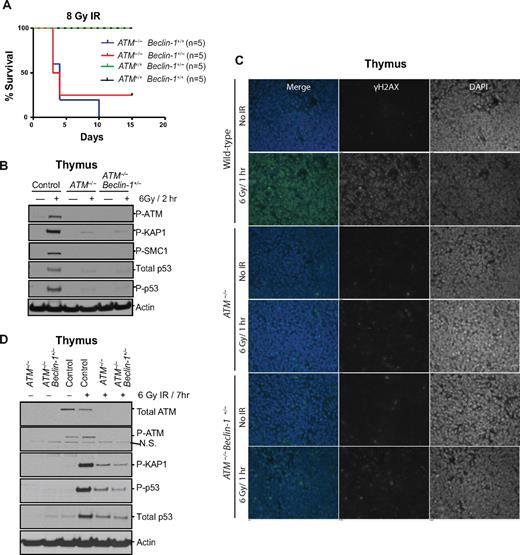
Given that tumor predisposition in ATM-null mice is typically attributed to defects in the DDR, we explored the impact of Beclin-1 heterozygosity on DNA damage signaling and outcome. Exposure of 6- to 8-week-old mice to 8-Gy ionizing radiation (IR) demonstrated no impact of Beclin-1 heterozygosity on survival. Although all wild-type and Beclin-1+/− mice survived the total body irradiation without significant morbidity, most ATM−/− and ATM−/−Beclin-1+/− mice rapidly died on IR exposure, with a mean survival of 4 days (Figure 4A). Furthermore, analyses of DDR signaling components in thymic tissue harvested 2 hours after exposure of the mice to 6-Gy IR demonstrated that thymocytes from ATM−/− and ATM−/−Beclin-1+/− mice had essentially identical profound deficits in this signaling pathway relative to wild-type thymocytes (Figure 4B). In addition, no differences in γH2AX immunostaining of paraffin embedded thymic tissues from irradiated ATM−/− versus ATM−/−Beclin-1+/− mice were observed (Figure 4C). The lack of rescue of the DDR abnormalities by Beclin-1 heterozygosity was not simply because of a delay of DDR signaling as the defects persisted for at least 7 hours after irradiation (Figure 4D).
Allelic loss of Beclin-1 does not affect DNA damage responses. (A) Both ATM−/− and ATM−/−Beclin-1+/− mice display radiosensitivity. Survival of control, ATM−/−, and ATM−/−Beclin-1+/− mice exposed to 8-Gy total body irradiation is shown (P = .85 between ATM−/− and ATM−/−Beclin-1+/− mice, log-rank (Mantel-Cox) test. (B-D) The DDR pathway is equally defective in ATM−/− and ATM−/−Beclin-1+/− mice. (B) Immunoblotting of total protein, phosphorylation status, or both of various ATM substrates, including P-KAP1, P-SMC1, total p53, P-p53, and P-ATM itself in thymocytes from mice sacrificed 2 hours after being exposed to 6-Gy irradiation. Actin was used as loading control. (C) Thymic tissues were isolated from nonirradiated or whole body–irradiated animals (6 Gy/1 hour) and embedded in paraffin followed by immunofluorescence staining against the genomic instability marker γH2AX (green). 4,6-diamidino-2-phenylindole (blue) was used to visualize nuclei (magnification, 40×0.055). (D) The lack of rescue of the DDR abnormalities by Beclin-1 heterozygosity is not because of a delay of DDR signaling. Animals were irradiated with 6 Gy, and tissues were harvested 7 hours later followed by isolation of total proteins from thymocytes. The expression or phosphorylation status of proteins involved in DDR (P-KAP, total-p53, P-p53, and P-ATM) was analyzed. Both ATM−/− and ATM−/−Beclin-1+/− thymocytes showed a mild increase in the levels of proteins involved in DDR (P-KAP, total-p53, and P-p53) compared with cells exposed for 2 hours (B), but no difference was observed between these 2 genotypes at this time point (D). Actin served as loading control.
Allelic loss of Beclin-1 does not affect DNA damage responses. (A) Both ATM−/− and ATM−/−Beclin-1+/− mice display radiosensitivity. Survival of control, ATM−/−, and ATM−/−Beclin-1+/− mice exposed to 8-Gy total body irradiation is shown (P = .85 between ATM−/− and ATM−/−Beclin-1+/− mice, log-rank (Mantel-Cox) test. (B-D) The DDR pathway is equally defective in ATM−/− and ATM−/−Beclin-1+/− mice. (B) Immunoblotting of total protein, phosphorylation status, or both of various ATM substrates, including P-KAP1, P-SMC1, total p53, P-p53, and P-ATM itself in thymocytes from mice sacrificed 2 hours after being exposed to 6-Gy irradiation. Actin was used as loading control. (C) Thymic tissues were isolated from nonirradiated or whole body–irradiated animals (6 Gy/1 hour) and embedded in paraffin followed by immunofluorescence staining against the genomic instability marker γH2AX (green). 4,6-diamidino-2-phenylindole (blue) was used to visualize nuclei (magnification, 40×0.055). (D) The lack of rescue of the DDR abnormalities by Beclin-1 heterozygosity is not because of a delay of DDR signaling. Animals were irradiated with 6 Gy, and tissues were harvested 7 hours later followed by isolation of total proteins from thymocytes. The expression or phosphorylation status of proteins involved in DDR (P-KAP, total-p53, P-p53, and P-ATM) was analyzed. Both ATM−/− and ATM−/−Beclin-1+/− thymocytes showed a mild increase in the levels of proteins involved in DDR (P-KAP, total-p53, and P-p53) compared with cells exposed for 2 hours (B), but no difference was observed between these 2 genotypes at this time point (D). Actin served as loading control.
Allelic loss of Beclin-1 rescues mitochondrial dysfunction in ATM-null thymocytes
Because ATM deficiency results in mitochondrial dysfunction, we asked whether Beclin-1 heterozygosity ameliorated these abnormalities. Notably, ATM−/− thymocytes had marked increases in their apoptotic index compared with controls, and this phenotype was significantly reduced by Beclin-1 heterozygosity (supplemental Figure 7). Allelic loss of Beclin-1 significantly reversed the abnormal increase in mitochondrial mass observed in ATM−/− thymocytes using either NAO or mitochondrial (mt)DNA content to quantitate mitochondrial mass (Figure 5A-B). In addition, immunofluorescence staining for the mitochondrial marker Tom20 in paraffin-embedded thymic tissues showed that the levels of this protein are elevated in ATM−/− thymocytes relative to control thymocytes, whereas Beclin-1 heterozygosity rescued this phenotype (supplemental Figure 8). Importantly, Beclin-1 heterozygosity also led to significant reductions in the mROS and complex I activity abnormalities seen in ATM-deficient thymocytes (Figure 5C-D), and even to significant decreases in mitochondrial mass in wild-type thymocytes (supplemental Figure 9). This suggests a potential role of Beclin-1 in modulating mitochondrial homeostasis in T cells, possibly independent of its conventional role in autophagy.
Allelic loss of Beclin-1 rescues mitochondrial abnormalities in ATM-null thymocytes. (A) Allelic loss of Beclin-1 partially reverses the increase in mitochondrial mass observed in ATM−/− mice. Freshly isolated thymic cells were stained with 5nM NAO dye. A representative histogram of NAO fluorescence intensity is illustrated. Graphs illustrate the average mean intensity of NAO for each genotype (n ≥ 3/genotype). (B) Beclin-1 heterozygosity reverses the abnormal increase in mtDNA observed in ATM−/− thymocytes. The ratio of mitochondrial/nuclear DNA was quantified in viable thymic cells of mice with desired genotypes by real-time PCR using primers for mitochondrial ND2 and nuclear 18S rRNA genes. Mean values are shown (n ≥ 3/genotype). (C-D) Beclin-1 heterozygosity reverses defects in complex I activity and mROS levels in ATM−/− thymocytes. Complex I activity (C) and mROS levels (D) were measured as described in Figure 1. For complex I activity, the relative activities of desired genotypes were graphed. For mROS, a representative histogram of MitoSOX fluorescence intensity per genotype is illustrated. Graph shows the averaged mean intensity for each genotype (n ≥ 3/group). P values were obtained by implementing the Student t test analysis in experiments shown in panels A-E (*P ≤ .04, **P ≤ .01, ***P ≤ .001). (E) Allelic loss of Beclin-1 rescues the increase in cellular respiration observed in the ATM−/− mice. The rate of oxygen consumption was measured in viable thymic cells of desired genotypes (n = 3/group). Shown is the OCR of thymic cells as function of time in minutes. Equal numbers of viable cells were used per sample for these experiments. (F-G) Allelic loss of Beclin-1 reduces mitochondrial ultra-structure abnormalities in ATM-null thymocytes. (F) Whole thymii of 5- to 8-week-old mice of the indicated genotypes were analyzed by transmission electron microscopy, and representative photomicrographs are shown. Mitochondria with disrupted cristae structure and with swollen appearance were considered abnormal. At least 15 images (magnification, ×10 000) were scored per sample. Red arrowheads illustrate abnormal mitochondria having disorganized cristae structure seen in ATM−/− thymic cells. The black arrow denotes an autophagic vacuole. (G) Percentage of abnormal mitochondria quantified in the whole thymus of mice shown in panel F. Each dot, triangle, or square represents a single mouse. Statistical analyses were performed using the Student t test (*P ≤ .03).
Allelic loss of Beclin-1 rescues mitochondrial abnormalities in ATM-null thymocytes. (A) Allelic loss of Beclin-1 partially reverses the increase in mitochondrial mass observed in ATM−/− mice. Freshly isolated thymic cells were stained with 5nM NAO dye. A representative histogram of NAO fluorescence intensity is illustrated. Graphs illustrate the average mean intensity of NAO for each genotype (n ≥ 3/genotype). (B) Beclin-1 heterozygosity reverses the abnormal increase in mtDNA observed in ATM−/− thymocytes. The ratio of mitochondrial/nuclear DNA was quantified in viable thymic cells of mice with desired genotypes by real-time PCR using primers for mitochondrial ND2 and nuclear 18S rRNA genes. Mean values are shown (n ≥ 3/genotype). (C-D) Beclin-1 heterozygosity reverses defects in complex I activity and mROS levels in ATM−/− thymocytes. Complex I activity (C) and mROS levels (D) were measured as described in Figure 1. For complex I activity, the relative activities of desired genotypes were graphed. For mROS, a representative histogram of MitoSOX fluorescence intensity per genotype is illustrated. Graph shows the averaged mean intensity for each genotype (n ≥ 3/group). P values were obtained by implementing the Student t test analysis in experiments shown in panels A-E (*P ≤ .04, **P ≤ .01, ***P ≤ .001). (E) Allelic loss of Beclin-1 rescues the increase in cellular respiration observed in the ATM−/− mice. The rate of oxygen consumption was measured in viable thymic cells of desired genotypes (n = 3/group). Shown is the OCR of thymic cells as function of time in minutes. Equal numbers of viable cells were used per sample for these experiments. (F-G) Allelic loss of Beclin-1 reduces mitochondrial ultra-structure abnormalities in ATM-null thymocytes. (F) Whole thymii of 5- to 8-week-old mice of the indicated genotypes were analyzed by transmission electron microscopy, and representative photomicrographs are shown. Mitochondria with disrupted cristae structure and with swollen appearance were considered abnormal. At least 15 images (magnification, ×10 000) were scored per sample. Red arrowheads illustrate abnormal mitochondria having disorganized cristae structure seen in ATM−/− thymic cells. The black arrow denotes an autophagic vacuole. (G) Percentage of abnormal mitochondria quantified in the whole thymus of mice shown in panel F. Each dot, triangle, or square represents a single mouse. Statistical analyses were performed using the Student t test (*P ≤ .03).
Given the striking effects of ATM deficiency on thymocyte cell death, mitochondrial number, and mROS, we also assessed the effects of ATM loss on cellular respiratory capacity. Consistent with the mitochondrial mass and mtDNA data, ATM−/− thymocytes displayed marked increases in OCRs compared with wild-type thymocytes, and Beclin-1 heterozygosity significantly rescued this abnormality (Figure 5E). Immortalized A-T fibroblasts also displayed significant increases in OCRs similar to those seen in ATM-deficient thymocytes (supplemental Figure 10), despite their decreased mitochondrial DNA content (supplemental Figure 2A), a discrepancy that could result from alterations in mitochondrial fusion or fission in the absence of ATM. Finally, Beclin-1 heterozygosity also led to significantly fewer abnormal mitochondria in ATM-null thymocytes (Figure 5F-G). Together, these data demonstrate that Beclin-1 heterozygosity reduces the mitochondrial abnormalities seen in ATM-null thymocytes, suggesting a direct role for Beclin-1 in mitochondrial function. Furthermore, the delayed tumor development in ATM-null mice associated with Beclin-1 heterozygosity correlates with rescue of mitochondrial defects, not rescue of DDR abnormalities.
Nrf2 and its transcriptional targets (eg, Nqo1) facilitate cellular responses to oxidative stress, and all are typically induced in settings of increased oxidative stress.36 Consistent with the increased mROS, ATM-deficient thymocytes had significantly elevated levels of Nrf2, and Nqo1 transcripts (supplemental Figure 11A-B). Furthermore, Beclin-1 heterozygosity reverted the increases in their expression. Similarly, Nrf2 levels were increased in ATM-null human fibroblasts and were rescued by down-regulation of Beclin-1 with small interfering RNAs (supplemental Figure 11C). These observations further demonstrate the increased oxidative stress associated with ATM deficiency and the reversion of this phenotype by Beclin-1 heterozygosity.
The c-Myc oncogene plays an important role in mitochondrial biogenesis, and its overexpression is sufficient to trigger increases in mitochondrial content.37 Because Beclin-1 heterozygosity accelerates lymphoma onset in Eμ-Myc mice (Figure 3D), we also analyzed the mitochondrial mass in premalignant (5-week-old) Eμ-Myc Beclin-1+/− versus Eμ-Myc Beclin-1+/+ and wild-type B cells. As expected, Eμ-Myc B cells showed significant increases in mitochondrial mass relative to B cells from wild-type littermates (supplemental Figure 12). However, in contrast to its impact on the ATM-null thymocytes, Beclin-1 heterozygosity did not affect the mitochondrial content of Eμ-Myc B cells (supplemental Figure 12). In contrast to its impact on wild-type thymocytes (supplemental Figure 9), Beclin-1 heterozygosity did not decrease mitochondrial content in wild-type B cells (WT vs Beclin-1+/−; supplemental Figure 12), suggesting that many of these phenotypic effects are cell-type dependent.
ATM localizes to mitochondria and is activated by mitochondrial dysfunction
The observation that ATM loss is associated with mitochondrial defects suggested that ATM might have a direct function in this organelle. Indeed, although predominantly a nuclear protein, ATM was easily detectable in mitochondrial fractions of normal human fibroblasts (Figure 6A). Furthermore, treatment of mouse thymocytes with the mitochondrial uncoupler CCCP led to rapid activation of the ATM kinase (Figure 6B). However, CCCP treatment did not induce phosphorylation and/or stabilization of the damage-inducible ATM substrates KAP-1, SMC1, or p53 (Figure 6B). Thus, mitochondrial damage induced by CCCP leads to ATM activation and occurs in the absence of detectable DNA damage signaling. The increased conversion of LC3-I to LC3-II after CCCP treatment confirmed that induction of mitochondrial damage increased autophagy in both wild-type and ATM-deficient cells (Figure 6B). Importantly, acute treatment of wild-type thymocytes with an ATM kinase inhibitor CP,23 resulted in rapid increases in mitochondrial mass (Figure 6C), although this increase in mitochondrial mass is probably not solely because of mitophagy deficiency because this phenomenon occurred very rapidly. Again, changes in mitochondrial fusion or fission dynamics because of ATM loss could be contributing to these phenomena. Consistent with the thymocyte data, treatment of wild-type human fibroblasts with the ATM inhibitor also triggered rapid increases in mitochondrial mass, whereas mitochondrial mass in ATM-deficient fibroblasts was not further elevated by this treatment (Figure 6D). Together, these data demonstrate that a fraction of the total ATM protein in cells is localized in mitochondria and that mitochondrial damage can directly activate the ATM kinase in the absence of DNA damage signaling. Furthermore, the data show that loss of ATM activity results in rapid changes in mitochondrial homeostasis, suggesting a mitochondrial role for ATM independent of nuclear or mitochondrial DNA damage.
ATM localizes to mitochondria and becomes activated by mitochondrial dysfunction without evidence of DNA damage. (A) ATM protein localizes to mitochondria. Normal human fibroblasts were fractionated into nuclear (N), cytoplasmic (C), and mitochondrial (M) fractions and the basal levels of total and phospho-ATM (S1981) were analyzed by immunoblotting. Topoisomerase I (Topo), lactate dehydrogenase (LDH), and COX-IV antibodies were used as N, C, and M markers, respectively. We also analyzed 10 μg of total (T) protein. (B) ATM is activated in response to dysfunctional mitochondria without evidence of DNA damage induction. Viable thymic cells isolated from 6- to 8-week-old wild-type (WT) or ATM-null mice were either treated with DMSO or 50μM of the mitochondrial uncoupler CCCP for 3 hours followed by total protein isolation and analysis by immunoblotting for various ATM substrates, including P-KAP1, P-SMC1, total p53, P-p53, and P-ATM itself. As a positive control for ATM activity in vivo, total protein lysates from irradiated (6 Gy) or nonirradiated control mice were included in the assay. LC3-II also was analyzed to confirm increased basal autophagy in ATM-null cells and induction of this process by CCCP treatment. (C-D) Transient inhibition of ATM results in mitochondrial mass abnormalities. (C) Wild-type thymocytes were treated with DMSO alone or with 10μM CP (ATM kinase inhibitor) for 3 or 5 hours before staining with MitoTracker Green FM (MTG) for 15 minutes. Flow cytometry histograms of 10 000 viable cells per sample are shown. (D) Control or A-T human fibroblasts were treated with either DMSO or 10μM CP for 3 hours before staining with MTG dye and analysis by flow cytometry. Shown is the mean of fluorescence intensity (MFI) in each sample.
ATM localizes to mitochondria and becomes activated by mitochondrial dysfunction without evidence of DNA damage. (A) ATM protein localizes to mitochondria. Normal human fibroblasts were fractionated into nuclear (N), cytoplasmic (C), and mitochondrial (M) fractions and the basal levels of total and phospho-ATM (S1981) were analyzed by immunoblotting. Topoisomerase I (Topo), lactate dehydrogenase (LDH), and COX-IV antibodies were used as N, C, and M markers, respectively. We also analyzed 10 μg of total (T) protein. (B) ATM is activated in response to dysfunctional mitochondria without evidence of DNA damage induction. Viable thymic cells isolated from 6- to 8-week-old wild-type (WT) or ATM-null mice were either treated with DMSO or 50μM of the mitochondrial uncoupler CCCP for 3 hours followed by total protein isolation and analysis by immunoblotting for various ATM substrates, including P-KAP1, P-SMC1, total p53, P-p53, and P-ATM itself. As a positive control for ATM activity in vivo, total protein lysates from irradiated (6 Gy) or nonirradiated control mice were included in the assay. LC3-II also was analyzed to confirm increased basal autophagy in ATM-null cells and induction of this process by CCCP treatment. (C-D) Transient inhibition of ATM results in mitochondrial mass abnormalities. (C) Wild-type thymocytes were treated with DMSO alone or with 10μM CP (ATM kinase inhibitor) for 3 or 5 hours before staining with MitoTracker Green FM (MTG) for 15 minutes. Flow cytometry histograms of 10 000 viable cells per sample are shown. (D) Control or A-T human fibroblasts were treated with either DMSO or 10μM CP for 3 hours before staining with MTG dye and analysis by flow cytometry. Shown is the mean of fluorescence intensity (MFI) in each sample.
Discussion
The ATM protein kinase is a central mediator of signaling pathways that result from DNA double-strand breaks,5 and the various pathologies observed in patients with ATM-deficiency have generally been attributed to defects in these nuclear functions of ATM. Here, we demonstrate that ATM also has a major role in modulating mitochondrial function and ROS generation in vivo and in vitro. ATM-deficient thymocytes have significant reductions in mitochondrial electron transport chain activity, decreases in intracellular ATP levels, altered mitochondrial ultrastructural organization, and enhanced mROS. These observations are consistent with previous studies demonstrating that ATM deficiency is associated with the accumulation of intracellular ROS,14-16 but the data suggest that the mitochondrion is the major source for elevated ROS. Unexpectedly, increased mitochondrial numbers, membrane potential, and cellular respiration also were associated with the ATM-deficient state. Data from both mouse thymocytes and human fibroblasts suggest that decreased mitophagy, rather than increased mitochondrial biogenesis, is associated with the increased mitochondrial content.
ATM−/− thymocytes from young mice exhibit an unusual combination of increased mitochondrial content, elevated mROS, and increased oxygen consumption, but decreased complex I activity and ATP production. The ATM deficiency and the increased mROS may induce mitochondrial uncoupling that eventually results in reduced synthesis of ATP and an increased oxygen cost for ATP production. The increased respiratory capacity, even in the immortalized ATM-null fibroblasts, could reflect cellular attempts to compensate for the deficiencies in complex I and total ATP levels.
Treatment of ATM−/− mice with ROS scavengers delays tumor onset.12,13 This striking observation is consistent with the genetic and biochemical data presented here, and both sets of findings suggest that defects in repairing endogenous DNA breaks, such as those that occur during normal metabolic processes or that are associated with immunoglobulin gene rearrangements in lymphoid tissues, are not the only drivers of tumorigenesis in ATM−/− mice or people. In fact, the increased intracellular ROS associated with ATM deficiency may function synergistically with alterations in DDR signaling to facilitate tumorigenesis (supplemental Figure 13).
Mitochondrial defects have been reported in immortalized or transformed cell lines derived from A-T patients.19,20 However, the reported nature of the mitochondrial defects was inconsistent, because 1 study reported that transformed human A-T fibroblasts have decreased mtDNA content compared with control fibroblasts,19 but another study showed that immortalized A-T lymphoblasts had mtDNA contents similar to control lymphoblasts.20 We analyzed both lymphoblasts and fibroblasts lacking ATM of either mouse or human origin and also found context-specific effects, with immortalized human A-T fibroblasts or lymphoblasts exhibiting a decreased mtDNA content, whereas immortalized ATM-deficient MEFs had mtDNA content similar to that of immortalized wild-type MEFs. However, examining viable thymocytes, the major site of tumor development in ATM-deficient mice and humans, we found that loss of ATM in vivo results in consistent increases in mitochondrial mass and DNA, mROS, the number of cells with high membrane potential, and increased cellular respiration. Exploring these apparent discrepancies, we found that in vitro culture of cells influences mitochondrial content and that early passage (nonimmortalized) MEFs lacking ATM mimicked in vivo thymocytes in having significant increases in mtDNA compared with wild-type MEFs of the same passage. Because these cells were passaged in culture, their mtDNA content increased regardless of ATM status. Thus, cell type and culture conditions, and immortalization all potentially affect both mitochondrial homeostasis and autophagic responses.
Beclin-1+/− mice develop spontaneous tumors,21,35 and allelic loss of Beclin-1 is observed in human cancers.38 Unexpectedly, we found that Beclin-1 heterozygosity delayed, rather than promoted, T-cell lymphomagenesis in ATM-null mice. Thus, Beclin-1 cannot be simply considered a tumor suppressor gene, because its impact on cell fate is context-dependent. Interestingly, the delayed tumor onset in this genetic background was not associated with an improved DDR signaling but rather with a partial rescue of the mitochondrial abnormalities. Overall, the data suggest that ATM deficiency leads to dysmorphic and dysfunctional mitochondria, increased mROS, and decreased mitophagy. These abnormalities are all quantitatively reduced by allelic loss of Beclin-1, suggesting that Beclin-1 has roles in addition to its functions as a regulator of the autophagy pathway. The increased basal autophagy in ATM-null cells seems to be a secondary effect of the stress associated with mitochondrial dysfunction and increased ROS and is rescued by allelic loss of Beclin-1 because of the rescue of the underlying mitochondrial stress. Alternatively, because tumor cells exhibit signs of increased metabolic stress, and autophagy is 1 mechanism by which tumor cells survive such stress,39,40 we cannot exclude the possibility that Beclin-1 heterozygosity also might delay tumor development by compromising the survival of developing malignant cells. Assessing the impact of loss of other components of the autophagy pathway, such as ATG5 or ATG7, on mitochondrial dysfunction and tumor development in ATM-null mice may help sort out these possibilities. Notably, we reported previously that administration of the antimalarial drug chloroquine (a known inhibitor of autophagy) in ATM−/− mice also retarded their tumor incidence,25 consistent with the notion that autophagy functions to sustain tumorigenesis arising in the absence of ATM.
In addition to its well-characterized role in DDR signaling, ATM also seems to be important in signaling pathways not directly associated with DNA damage, including insulin signaling,6,7 the pentose phosphate pathway,41 and now mitochondrial function and ROS generation. In fact, these results suggest that A-T should be considered among the diseases that are disorders of mitochondria. ATM is a serine/threonine kinase that preferentially phosphorylates SQ sites in proteins,42,43 but whether ATM regulates mitochondrial homeostasis by phosphorylating mitochondrial proteins is not yet clear. We show here that a fraction of ATM localizes to the mitochondria and can be activated by mitochondrial dysfunction in the absence of DNA damage. These findings are consistent with recent observations that oxidation can directly activate the ATM kinase,44 and they suggest that ATM is capable of sensing damage in this organelle and that it may phosphorylate resident mitochondrial proteins to handle the stress. Alternatively, ATM could modulate mitochondrial homeostasis by regulating other proteins that impact mitochondrial function. DJ-1 functions in a signaling pathway along with Parkin and PINK-1 to regulate the destruction of defective mitochondria by mitophagy.45,46 We observed relatively low levels of DJ-1 protein in thymocytes from young ATM-null mice. The function of DJ-1 is not well understood, but some studies have suggested that this protein localizes to the mitochondria and is critical in the response to oxidative stress32,33,47 and mutations in the DJ-1 gene are observed in Parkinson disease along with mutations in its partner proteins: Parkin and PINK-1.48 Because mitophagy seems to be impaired in ATM-deficient cells, it would be interesting to explore whether the ATM kinase plays a role in the regulation of these proteins, which also could affect mitophagy and ROS generation. Linking ATM function to this complex could also shed light on the neurodegenerative phenotype in A-T patients.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Kastan laboratory for advice and technical support throughout the course of this study, especially Diane Woods and Joanna Remack. They also thank Chunying Yang and Stephanie Prater (Scripps-Florida); Dr Sharon Frase, and the Electron Microscopy Core; and Dr Richard Ashmun and the Flow Cytometry Core at St Jude Children's Research Hospital for assistance with various experiments. They thank Richard Youle for providing the YFP-Parkin plasmid.
This work was supported by National Institutes of Health grants CA71387, CA157216, CA076379, and P30CA21765; by monies from the State of Florida to Scripps-Florida; and by the American Lebanese Syrian Associated Charities of St Jude Children's Research Hospital.
National Institutes of Health
Authorship
Contribution: Y.A.V.-V. designed and performed research, analyzed data, and wrote the manuscript; K.H.M., J.T.-M., S.M., M.S., and F.C.D. performed research and analyzed data; J.L.C. and D.R.G. contributed with vital reagents, analyzed data, funded research, and revised the manuscript; and M.B.K. funded and designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.B.K. is Department of Pharmacology and Cancer Biology, Duke Cancer Institute, Durham, NC.
Correspondence: Michael B. Kastan, Department of Pharmacology and Cancer Biology, Duke Cancer Institute, Seeley Mudd Bldg, Duke University Medical Center, Mail Box 3917, 10 Bryan Searle Dr, Durham, NC 27710; e-mail: michael.kastan@duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal