Jácomo and colleagues advance our understanding of the coagulopathy present in acute promyelocytic leukemia (APL). By means of elegant and intriguing experiments performed in an APL mouse model, they demonstrate that supplementation with L-methionine, an essential amino acid that assists in the metabolism of homocysteine (Hcy), is able to prevent the fibrinolytic abnormalities present in this leukemia.1
The unique features defining APL are the morphology of malignant promyelocytes, the presence of a coagulopathy responsible for the severe bleeding propensity, and a specific chromosomal balanced translocation t(15;17). However, despite the progress achieved in the treatment and biologic understanding of the molecular defect of this leukemia with the introduction of the vitamin A derivative all-trans-retinoic acid (ATRA), the controversy about the respective roles of primary fibrinolysis or proteolysis and diffuse intravascular coagulation (DIC) in the pathogenesis of the APL coagulopathy still persist, suggesting that it is due to a complex interaction of the leukemic promyelocytes with the coagulation system.2,3
The DIC theory is supported by the presence in APL of elevated levels of tissue factor and cancer procoagulant.4 In favor of the fibrinolytic mechanism is the presence of elevated levels of annexin II (ANXII) detected on APL leukemic cells5 and of normal antithrombin levels associated with acquired α2-antiplasmin deficiency.6 Antithrombin (formerly called ATIII) is a natural anticoagulant that is a major inhibitor of thrombin; whereas α2-antiplasmin or α2-plasmin inhibitor is the major, if not the sole, inhibitor of plasmin (Pl). Overall, the data favor a mechanism involving a primary activation of the fibrinolysis rather than DIC.3
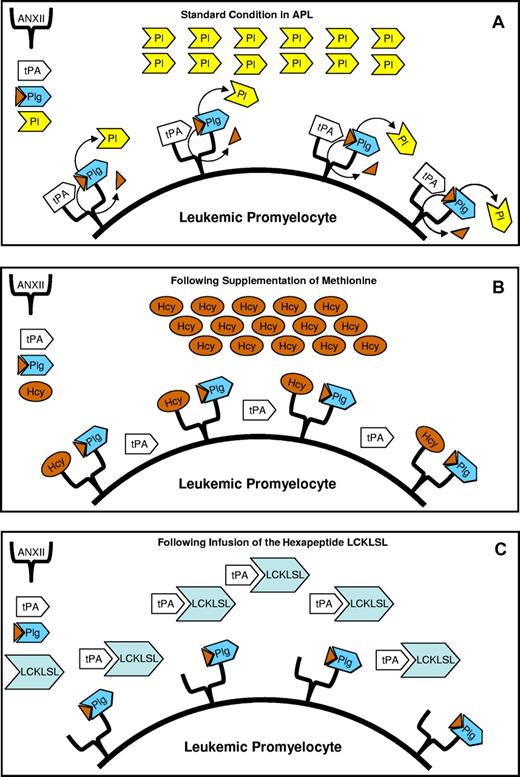
The fibrinolytic theory is clinically supported by the observation that the severe bleeding in APL is rarely associated with acute renal failure and schistocytes, which differ from other conditions associated with DIC. ANXII is a cell-surface receptor for both plasminogen and tissue plasminogen activator (tPA) present in endothelial cells, macrophages, and some tumor cells. In APL cells, ANXII levels are abnormally high and these high levels have been considered responsible for the increased production of plasmin favoring the bleeding diathesis (see figure panel A).5 The increased production of Pl observed in APL may be blocked by anti-ANXII antibody5 ; moreover, it has also been demonstrated that homocysteine (Hcy) competes with tPA for the same site of ANXII through its motif LCKLSL, a hexapeptide in the amino-terminal domain of ANXII.7
In APL cells, annexin II (ANXII) levels are abnormally high and have been considered responsible for the increased production of plasmin (Pl). In fact, ANXII is a receptor for both tissue plasminogen activator (tPA) and plasminogen (Plg). The close position of tPA to Plg favors the activation of Plg to Pl, the most important fibrinolytic enzyme responsible for the observed fibrinolysis in APL (A). Supplementation of methionine increases the levels of homocysteinemia (Hcy), producing hyperhomocysteinemia that inhibits the binding of tPA to ANXII, therefore reverting hyperfibrinolysis because tPA can no longer activate Plg to Pl (B). A lack of activation of Plg by tPA is also produced by the infusion, in leukemic mice, of the hexapeptide LCKLSL because tPA fails to link to the hexapeptide LCKLSL present in the molecule of ANXII (C).
In APL cells, annexin II (ANXII) levels are abnormally high and have been considered responsible for the increased production of plasmin (Pl). In fact, ANXII is a receptor for both tissue plasminogen activator (tPA) and plasminogen (Plg). The close position of tPA to Plg favors the activation of Plg to Pl, the most important fibrinolytic enzyme responsible for the observed fibrinolysis in APL (A). Supplementation of methionine increases the levels of homocysteinemia (Hcy), producing hyperhomocysteinemia that inhibits the binding of tPA to ANXII, therefore reverting hyperfibrinolysis because tPA can no longer activate Plg to Pl (B). A lack of activation of Plg by tPA is also produced by the infusion, in leukemic mice, of the hexapeptide LCKLSL because tPA fails to link to the hexapeptide LCKLSL present in the molecule of ANXII (C).
Jácomo and colleagues, using an APL mouse model, hypothesized that by inhibiting ANXII activity, either through the induction of hyperhomocysteinemia or by administering LCKLSL peptide, APL-associated hyperfibrinolysis could be reversed. The obtained results confirmed that the hyperhomocysteinemia induced by methionine inhibits the binding of tPA to ANXII, thereby preventing hyperfibrinolysis because tPA can no longer activate plasminogen (Plg) to Pl (panel B). The same result was obtained by infusing an excess of the hexapeptide LCKLSL in leukemic mice (panel C). The prevention of hyperfibrinolysis was demonstrated by the reduction of tPA at baseline concentrations in the APL mice. Hcy is an intermediary amino acid formed by the conversion of methionine to cysteine, and external supplementation of methionine may increase the levels of homocysteine.
The data presented by Jácomo et al in this study help shed light on the pathogenesis of the coagulopaty in APL. The results favor the theory that primary hyperfibrinolysis plays a major role in the pathogenesis of the APL coagulopathy. A drawback of this study is that it is solely based on an animal model and may not accurately reflect conditions in human APL. As a consequence, we don't know whether the results obtained from the animal model will be replicated in humans. From a clinical point of view the use of L-methionine to reverse hyperfibrinolysis and reduce the bleeding diathesis observed in APL during induction should be studied. However, it is very important to note that this approach may increase the thromboembolic tendency observed in some APL patients with the use of ATRA. In fact, hyperhomocysteinemia is an independent risk factor for atherosclerotic vascular disease and for recurrent venous thromboembolism. Therefore, given that the tissue factor and the cancer procoagulant are also elevated in APL cells,4 supplementation of methionine in newly diagnosed APL patients, even if limited to a few days, may increase the tendency for thromboembolism observed in some ATRA-treated patients. Alternatively, to try to avoid the increased risk of thrombosis, a surrogate would be to compare the bleeding and thromboembolic complications observed in APL patients with hyperhomocysteinemia to those observed in APL patients with normal Hcy levels.
In conclusion, although the introduction of ATRA in the treatment of APL has greatly reduced the bleeding diathesis observed in APL patients, early hemorrhagic deaths are still present and represent the main cause of induction failure in APL.8 Therefore, the bleeding diathesis of APL remains an active area of investigation. The results presented here can contribute to revitalize this area of research in APL.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal