Even in 2012, almost 45 years after the first successful allogeneic bone marrow transplants, infection remains the most common cause of nonrelapse transplant-related mortality.1
Patients undergoing allogeneic bone marrow or stem cell transplantation have a substantially increased risk of bacterial, fungal, and viral infection. The causes for these infections are quite diverse and relate to neutropenia after conditioning therapy, prior infectious complications during induction/salvage treatment, and immune deficits that occur after allogeneic or autologous stem cell transplantation. Multiple approaches to decrease the risk of infections after allogeneic stem cell transplantation have been explored including laminar air flow housing, the use of absorbable and nonabsorbable antibiotics, prophylactic antiviral and antifungal therapy, infusion of intravenous immunoglobulin, and use of cytokines to limit neutropenia.2
In addition to the well-described risk of infection associated with stem cell transplantation, recent data have indicated that there is a relationship between acute GVHD and infectious complications. Patients who develop infections with Clostridium difficile are at increased risk of developing gastrointestinal (GI) tract GVHD.3 Similarly, patients who develop GVHD are at increased risk of bacterial sepsis although the mechanisms underlying this finding, beyond those related to the immune suppression that occurs after the treatment of patients with acute GVHD, remain poorly understood.4
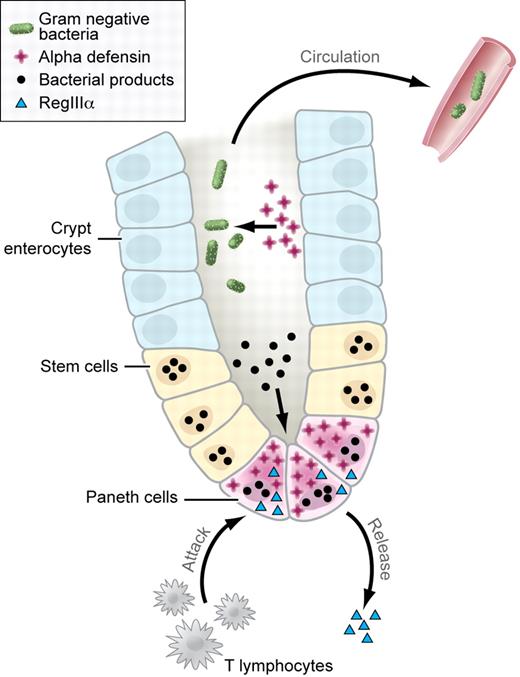
In this issue of Blood, Eriguchi et al provide a new mechanism for the relationship between infectious complications and acute GVHD involving the GI tract.5 They focused on the function of Paneth cells, located at the base of the crypts of Lieberkühn, in the small intestine of mammals (see figure).6 Paneth cell progenitors descend to the crypt base during their differentiation, which is mediated in part by Wnt signaling. These cells have a much longer half-life than other cells found in the small intestine, with labeling experiments indicating life-spans longer than 2 months. Paneth cells secrete a large number of antibiotic peptides and proteins in addition to proinflammatory mediators. These products include lysozyme, lipopolysaccharide binding protein, IL-17A, TNF, regenerating islet-derived protein IIIγ (RegIIIγ), IgA, and matrix metalloproteinases. In addition, 2 very important classes of antimicrobial peptides are generated by Paneth cells, defensins and cathelicidins. Murine Paneth cells secrete into the lumen of the small intestine very high levels of α-defensins, which have antimicrobial activity against a wide range of pathogens including gram negative and positive bacteria. Killing of these organisms is mediated by a variety of mechanisms, which predominantly focus on the formation of ion channels for potassium in lipid bilayers leading to efflux of potassium from within the bacterium. Thus, Paneth cells are critical to (1) the immune response to pathogens and (2) the maintenance of a noninflammatory commensal flora in the small bowel. Interestingly, using a proteomic screen, Ferrara et al demonstrated that RegIIIα, which is also generated by Paneth cells, is a biomarker for the presence of acute GVHD involving the lower GI tract.7
Biology of the crypts of Lieberkühn. Paneth cells generate defensins (red crosses) that are involved in controlling the growth of bacterial organisms in the lumen of the crypts of Lieberkühn (bacteria shown as green ovals). Production of defensins is partly mediated by the response of Paneth cells to bacterial products present in the lumen of the crypt (bacterial products shown as black circles). T-cell immune response (labeled) leads to loss of Paneth cells, inhibiting the generation of defensins, and bacterial translocation across the mucosa and into the systemic circulation. In addition, Paneth cell destruction leads to the release of RegIIIα (light blue triangle), which serves as a marker for the presence of GvHD. Professional illustration by Kenneth X. Probst.
Biology of the crypts of Lieberkühn. Paneth cells generate defensins (red crosses) that are involved in controlling the growth of bacterial organisms in the lumen of the crypts of Lieberkühn (bacteria shown as green ovals). Production of defensins is partly mediated by the response of Paneth cells to bacterial products present in the lumen of the crypt (bacterial products shown as black circles). T-cell immune response (labeled) leads to loss of Paneth cells, inhibiting the generation of defensins, and bacterial translocation across the mucosa and into the systemic circulation. In addition, Paneth cell destruction leads to the release of RegIIIα (light blue triangle), which serves as a marker for the presence of GvHD. Professional illustration by Kenneth X. Probst.
Using F1 into parent murine models of acute GVHD, Eriguchi et al found that GVHD involving the small bowel in recipient mice was associated with a substantial loss of Paneth cells. This was correlated with a statistically significant decrease in the generation of multiple different enteric defensins in recipients with acute GVHD of the small bowel. Of significant interest given the role that α-defensins are known to play in the development of the commensal flora of the GI tract, this change was accompanied by a marked loss of diversity of the microbiotica of the GI tract with a specific decrease in the common species, Firmicutes and Bacteroidetes. Using restriction fragment length polymorphism analysis of 16S rRNA genes from fecal pellets, they identified an organism in the small bowel of mice with GVHD. Sequencing demonstrated that this peak was almost certainly due to the presence of Escherichia coli in the intestine of recipients suffering from acute GVHD.
While these findings would be quite interesting alone, the authors further showed that the presence of E coli in the GI tract was associated with translocation of the organisms into the systemic circulation with increased titers of E coli found in the liver and mesenteric lymph nodes in mice that developed GVHD. The prophylactic administration of polymixin B orally, a cell membrane active antibiotic with a particular efficacy against gram negative bacteria, suppressed the growth of E coli. This correlated with a decrease in mortality in recipient mice treated with polymixin B and decreased GVHD pathology in the small intestine.
The report by Eriguchi and colleagues extends several recent findings regarding the mechanism of GVHD involving the GI tract. Previously, this same group had shown that the administration of the Wnt agonist, R-spondin1, markedly decreased GI tract GVHD, which was associated with preservation of Olfm4-expressing intestinal stem cells (ISCs).8 Paneth cells are generated from ISCs and serve a critical role in their maintenance. Thus, the loss of Paneth cells found in the current manuscript may be due to direct targeting of Paneth cells or indirect targeting of ISCs. Future work is needed to determine this. In addition, it is not clear from the current work if enhancing the presence of commensal bacteria in the GI tract could be used to treat GVHD because their approach was prophylactic. Quite recently, Jenq et al found that the reintroduction of a specific strain of Lactobacillus before transplantation led to an improvement in the survival of mice undergoing allogeneic bone marrow transplantation.9
The findings by Eriguchi et al and recent other work suggest novel approaches to the treatment of acute GVHD of the lower GI tract and to the possible prevention of bacterial sepsis. In addition to treatment with T cell–specific inhibitors, treatment of GI tract GVHD may require reconstitution of the nonpathogeneic commensal flora. Wouldn't it be ironic if the best approach to prevent enteric bacterial sepsis was to administer commensal anaerobic bacteria as part of the transplantation process?
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal