Abstract
Phospholipids are of critical importance in mammalian cell biology, both through providing a permeability barrier and acting as substrates for synthesis of lipid mediators. Recently, several new families of bioactive lipids were identified that form through the enzymatic oxidation of membrane phospholipids in circulating innate immune cells and platelets. These comprise eicosanoids attached to phosphatidylethanolamine and phosphatidylcholine and form within 2-5 minutes of cell activation by pathophysiologic agonists, via the coordinated action of receptors and enzymes. In this review, we summarize what is currently known regarding their structures, mechanisms of formation, cell biology, and signaling actions. We show that phospholipid oxidation by acutely activated immune cells is a controlled event, and we propose a central role in regulating membrane biology and innate immune function during health and disease. We also review the mass spectrometry methods used for identification of the lipids and describe how these approaches can be used for discovery of new lipid mediators in complex biologic samples.
Introduction
During infection and injury, circulating innate immune cells and platelets respond acutely to a variety of pathophysiologic agonists that mediate profound changes in both their function and physical state. Significant alterations in the lipid compartment, including changes to the plasma membrane and generation of potent signaling mediators occur within 2-5 minutes of stimulation.1-4 Activation of neutrophils, monocytes, and platelets leads to several common events at the plasma membrane, including shape change, flattening, adhesion, phagocytosis, microvilli, and microparticle generation.5,6 Phospholipids are the building blocks of the cell membrane, forming a permeability barrier and providing substrates for generation of important signaling mediators, including platelet-activating factor, phosphoinositides, diacylglycerides, sphingosine-derived phospholipids, phosphatidic acids, and eicosanoids. All of these are formed acutely in immune cells through the actions of phospholipases and other enzymes, for example, phospholipase A2 (PLA2), which hydrolyzes phospholipids at sn2, generating fatty acid substrates for oxidation by cyclooxygenases (COX) and lipoxygenases (LOX).7,8 Thus, acute activation of innate immune cells and platelets results in significant remodeling of the lipid compartment. However, how this is organized on a molecular and cellular level, particularly in terms of membrane lipid composition and biophysics, is not well understood.
One group of phospholipid signaling mediators that has been extensively studied in recent years is oxidized phospholipids, generated through nonenzymatic redox cycling reactions that occur in chronic inflammation and atherosclerosis. Hundreds of species are known to form, with only a select few analyzed for structure and function to date.9 Early studies on these lipids focused on characterizing the biologic action of air-oxidized phosphatidylcholine (PC) in vitro.9-11 Later, investigators began to fractionate and focus on individual species, including 16:0-05:0(ALDO)–PC, and 16:0-05:0(COOH)–PC.12-14 Because these lipids are generated through nonenzymatic reactions, their formation is considered to be uncontrolled and associated with later-stage disease, as opposed to a regulated event triggered during innate immune cell activation.
The advent of new-generation benchtop mass spectrometers, coupled with high performance liquid chromatography (LC-MS/MS), has led to a significant resurgence in the study of lipids in health and disease.15-17 Over the last 10 years, structural identification of low abundance phospholipid molecular species in limited amounts of highly complex cellular lipid extracts has become possible. This has revolutionized the field, with recent highlights including the mass spectrometry analysis of phosphoinositides in neutrophils, studies on the role of phosphatidylcholine metabolites in cardiovascular disease and detailed profiling of the plasma lipidome in humans.18-20
In this review, we focus on how LC-MS/MS has been used to uncover new families of enzymatically oxidized phospholipids generated by activated human immune cells and platelets. These differ from the nonenzymatically oxidized phospholipids described earlier in this section because they compose a small number of specific molecular species and they are formed acutely on immune cell activation. We describe the identification and characterization of these new lipid species, followed by what is known regarding their in vivo generation and biologic functions. Lastly, we describe how recently developed high-resolution rapid-scanning instruments offer significant new possibilities in terms of powerful approaches that aim to discover new structures and have the potential to redefine how we study the biology and biochemistry of lipid mediators in the immune system.
Early studies on esterified eicosanoid generation by immune cells
During immune cell activation, PLA2 hydrolysis of phospholipids releases free arachidonate, which is oxidized by LOX, COX, or cytochrome P450 (CYP) to generate eicosanoids, including prostaglandins E2 and D2, thromboxane A2, leukotrienes, hydroxyeicosatetraenoic acids (HETEs), and others.21,22 Many are important signaling molecules in innate immunity, through regulating hemostasis, pain, fever, cell adhesion, proliferation, and tissue regeneration. Traditionally, eicosanoids have been considered free acid mediators, containing a carboxyl group at the site of PLA2 hydrolysis. In 1998, Brinckmann et al noted that alkaline hydrolysis of lipids from ionophore-activated human eosinophils released significant amounts of 15-HETE that had been attached to membrane lipids.23 However, neither the molecular structures of the HETE-containing lipids nor their mechanisms of generation were investigated. Around this time, several other groups conducted elegant studies on incorporation of exogenously added HETE standards into immune cells.24-26 However, it has since become clear that incorporation of exogenous HETEs into membranes is very different from the fate of endogenously generated eicosanoids formed via oxidation of cellular substrate, and so this will not be further discussed in this review.
In 2005, Marnett et al demonstrated the cellular generation of esterified prostaglandins for the first time, including both PGE2 and PGD2 attached to glycerol and ethanolamine.27-29 These lipids were initially identified in vitro, generated using purified COX-2, but then subsequently in murine macrophages with endogenous substrate. Shortly after, while investigating the cell biology of 15-LOX, we also noted that significant 15-HETE was acutely generated as esterified products by IL-4–treated human monocytes. To determine the molecular species to which this was attached, a targeted lipidomic approach was developed that used precursor scanning LC-MS/MS to “fish” for lipids that contained a HETE functional group. These studies led to the discovery of several additional families of phospholipid-esterified eicosanoids and are described in detail in the following sections. As part of this work, quantitative assays for all these lipids were established, and protocols are provided.30
Generation of esterified eicosanoids by monocyte/macrophages
The leukocyte 15-LOX is induced in human monocytes by Th2 cytokines, and its murine homolog 12/15-LOX is highly expressed by certain resident macrophage populations. In mice, genetic deletion of 12/15-LOX protects against atherosclerosis, diabetes, and hypertension.31-35 The 12/15-LOX−/− macrophages show defects in peroxisome proliferator-activated receptor (PPAR-γ) and Toll-like receptor 4 (TLR4) signaling, as well as altered IL-12 synthesis, and defective phagocytosis.34,36 The primary product of 15-LOX, 15-hydroperoxyeicosatetraenoic acid (15-HpETE), is rapidly reduced by glutathione peroxidases to 15-HETE, which can then be oxidized to the electrophilic lipid, 15-oxo-ETE, using prostaglandin dehydrogenase.37 How 12/15-LOX signals to control macrophage biology is not well understood because known free eiocosanoid metabolites (such as HETEs) do not effectively restore the phenotype of LOX-deficient cells.38
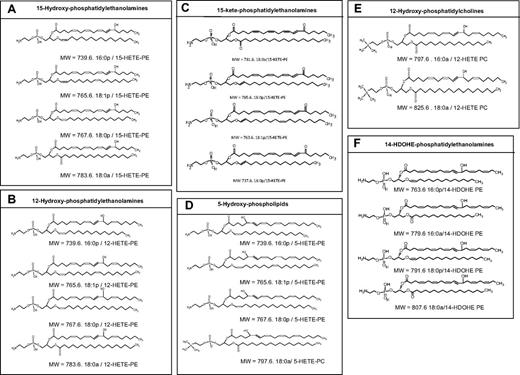
In 2007, precursor scanning of lipid extracts from ionophore-treated IL-4–induced human monocytes revealed 4 molecular species attached to 15-HETE, subsequently identified as 3 plasmalogen and 1 acyl phosphatidylethanolamine (16:0p, 18:1p, 18:0p, and 18:0a/15-HETE-phosphatidylethanolamine [PE]; Figure 1A). Chiral chromatography demonstrated that they contained primarily the 15S-HETE enantiomer, confirming their enzymatic generation, and that they composed ∼ 30% of the total 15-HETE generated (see also erratum for this study).39 Stable isotope labeling studies revealed that they are generated through direct oxidation of the intact phospholipid by 15-LOX. Analogous 12-HETE–containing PEs are also found in murine peritoneal macrophages, and these are absent in 12/15-LOX−/− (Figure 1B).40 Unlike free HETE, the lipids are not secreted, suggesting an autocrine or local mode of action. More recent studies on human monocytes and murine macrophages have uncovered several additional related families, including PEs containing either 15- or 12-HpETE, or 15- or 12-keto-eicosatetraenoic acid (KETE; V. Hammond and V.B.O., unpublished data, 2012; and Figure 1C). Up to 1.5% of the total cellular PE pool contains 15-HETE, raising the possibility that local concentrations of esterified eicosanoids could be high enough to regulate biophysical changes to the plasma membrane of activated monocytes/macrophages. Of relevance, 12/15-LOX–deficient macrophages are unable to mount a normal phagocytic response in vitro,41 and we recently observed that they contain larger numbers of cytoplasmic vesicles and abnormal mitochondria, which could represent defective autophagy or exosomal processing (V. Hammond and V.B.O., unpublished data, 2012).
Structures of esterified eicosanoids acutely generated by human and murine immune cells. (A) Lipids generated by human monocytes and bronchial epithelial cells. (B) Lipids generated by human platelets and murine peritoneal macrophages. (C) Lipids generated by human macrophages. (D) Lipids generated by human neutrophils. (E-F) Lipids generated by human platelets. Note that additional lipids not shown here include esterified HpODEs generated by monocytes and macrophages, and PGE2/D2-PEs generated by human platelets.
Structures of esterified eicosanoids acutely generated by human and murine immune cells. (A) Lipids generated by human monocytes and bronchial epithelial cells. (B) Lipids generated by human platelets and murine peritoneal macrophages. (C) Lipids generated by human macrophages. (D) Lipids generated by human neutrophils. (E-F) Lipids generated by human platelets. Note that additional lipids not shown here include esterified HpODEs generated by monocytes and macrophages, and PGE2/D2-PEs generated by human platelets.
In vitro, both 18:0a/15-KETE- and 18:0a/15-HETE-PE weakly activate PPAR-γ transcriptional activity, consistent with the known signaling defect of this transcription factor in murine macrophages deficient in 12/15-LOX (V. Hammond and V.B.O., unpublished data, 2012).36 The 18:0a/15-HETE-PE also inhibits induction of several cytokines (including TNF-α and G-CSF) by lipopolysaccharide (LPS).40 This probably results through HETE-PE binding to LPS-binding protein and/or CD14 and is similar to how nonenzymatically oxidized PC analogs were previously shown to act as Toll-like receptor 4 (TLR4) antagonists.42 In these experiments, amounts of HETE-PEs added (< 320 ng/4 × 106 cells) were somewhat higher than levels endogenously generated by monocytes (∼ 10 ng or 151 ng/4 × 106 for basal and activated cells, respectively).39 However, we note that HETE-PEs are poorly incorporated by leukocytes, with only 5%-10% becoming cell-associated over 24 hours (V. Hammond and V.B.O., unpublished data, 2012). Thus, added amounts will be much lower than that ultimately present in the cells and can be considered biologically relevant. In addition, exogenously added lipid will be present in the supernatant primarily, rather than cell associated, as for endogenous HETE-PE. Overall, these signaling actions suggest that HETE-PEs generated by monocyte/macrophage 12/15-LOX display anti-inflammatory bioactivities. This is consistent with the observation that this LOX isoform is constitutively expressed and basally active in noninflammatory activated resident macrophage populations and is cleared during acute peritoneal inflammation.38,40
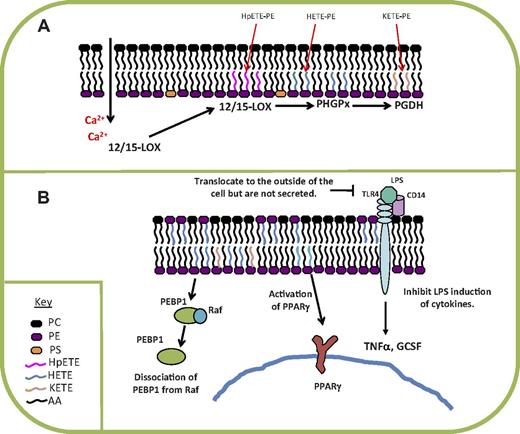
A potential role for esterified eicosanoids in Th2 inflammation is suggested by observations that 12-HETE-PEs are generated during murine lung allergy in vivo, corresponding with the time of greatest IL-4, IL-13, and eosinophil influx.40 Furthermore, two 15-HETE-PEs are generated in response to IL-13 and A23187 by human bronchial epithelial cells in culture.43 The 15-HETE-PE was recently shown to bind to PE-binding protein-1 in these cells and induce its dissociation from Raf-1.44 This has downstream effects in activating extracellular signal-regulated kinase and elevating IL-4Rα–related gene expression, suggesting that the pathway promotes Th2 pathways relevant to asthma pathogenesis. In these experiments, the concentration of HETE-PE added (782 ng/106 cells) is somewhat more than that routinely generated by these cells (typically 10-150 ng/106 cells). However, as for monocytes, HETE-PEs are poorly incorporated by bronchial epithelial cells in culture. Lastly, a recent study from Uderhardt et al showed that resident peritoneal macrophages express binding sites for soluble receptors for apoptotic cells, such as milk fat globule-EGF factor 8, through the presence of oxidized phospholipids on the surface of the cells.45 This occurs in a 12/15-LOX–dependent manner, regulates clearance of apoptotic cells, and maintains immunologic tolerance.45 The identity of the specific oxidized phospholipids involved is not fully clarified. A summary of mechanisms of formation and known signaling actions for 15-LOX–derived esterified eicosanoids in monocytes/macrophages is shown (Figure 2).
Summary of mechanism of formation and action of 12- and 15-HETE-PEs generated by human monocytes and murine peritoneal macrophages. (A) HETE-PEs and KETE-PEs are already present in the membranes of IL-4–cultured monocytes and peritoneal macrophages, but their levels are elevated ∼ 2-fold on ionophore activation. Generation involves direct oxidation of membrane phospholipids. (B) In vitro, HETE-PEs inhibit TLR4 signaling, activate PPAR-γ transcriptional activity, and stimulate dissociation of PEBP1 from Raf. PHGPx indicates, phospholipid hydroperoxide glutathione peroxidase; PGDH, prostaglandin dehydrogenase; PEBP1, PE-binding protein-1; and AA, arachidonic acid.
Summary of mechanism of formation and action of 12- and 15-HETE-PEs generated by human monocytes and murine peritoneal macrophages. (A) HETE-PEs and KETE-PEs are already present in the membranes of IL-4–cultured monocytes and peritoneal macrophages, but their levels are elevated ∼ 2-fold on ionophore activation. Generation involves direct oxidation of membrane phospholipids. (B) In vitro, HETE-PEs inhibit TLR4 signaling, activate PPAR-γ transcriptional activity, and stimulate dissociation of PEBP1 from Raf. PHGPx indicates, phospholipid hydroperoxide glutathione peroxidase; PGDH, prostaglandin dehydrogenase; PEBP1, PE-binding protein-1; and AA, arachidonic acid.
Generation of esterified eicosanoids by neutrophils
Neutrophils express a 5-LOX isoform that is activated by several agonists, including bacterial peptides, chemokines, and chemical stimuli, such as phorbol and calcium ionophore. The primary 5-LOX product, 5-HpETE, is the precursor for leukotriene synthesis, or can be converted by glutathione peroxidases to the more stable 5-HETE, which in turn can be oxidized to the potent chemoattractant, 5-oxo-ETE.46
We conducted precursor scanning of lipid extracts from ionophore-activated human neutrophils and detected 4 molecular species that were subsequently identified as one HETE-PC (16:0a/5-HETE-PC) and three 5-HETE-PEs (18:0p/, 18:1p/, and 16:0p/5-HETE-PE)47 (Figure 1D). No other positional isomers are present, suggesting that they are generated via 5-LOX activity. Similar to 15-HETE-PEs, they are retained by the cells and are localized equally in both nuclear and non-nuclear membrane fractions, consistent with the known translocation of 5-LOX that occurs on neutrophil activation.47 The 5-HETE-PC is highly unstable, being metabolized within 5 minutes of its acute generation to unknown products, whereas 5-HETE-PEs remain cell-associated for up to 3 hours after their synthesis.47 Generation of the lipids in response to N-formyl-methionine-leucine-phenylalanine can be significantly enhanced by priming with cytochalasin B, GM-CSF, or LPS, and requires calcium mobilization, phospholipase C, cytosolic PLA2, secretory PLA2 (sPLA2), 5-LOX activating protein, and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1. This indicates a highly coordinated mechanism of formation, involving receptors and intracellular signaling intermediates. Unlike macrophage HETE-PEs, the formation of these lipids requires hydrolysis of arachidonate, and 5-LOX–dependent formation of free 5-HETE, which is then re-esterified back into the phospholipid pool.47
The 5-HETE-PEs were detected in both human and murine peritonitis, coinciding with the time point of maximum neutrophil influx.47 Stimulation of their generation in vitro by live bacteria requires opsonization, indicating that complement or antibody is probably involved. Highest levels were found with Gram-positive infection, as expected because neutrophil influx was greatest in these patients.47
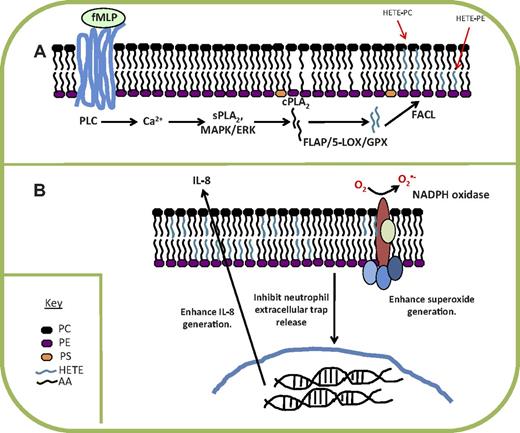
The 5-HETE-PEs regulate a number of neutrophil activities in vitro, including enhancing superoxide and IL-8 release, and inhibiting neutrophil extracellular trap (NET) release.47 These biologic actions were demonstrated at ∼ 7.8 μg added 5-HETE-PE per 106 cells, whereas in contrast neutrophils synthesize ∼ 400 ng/106 cells.47 However, as described earlier, exogenously added HETE-PEs are poorly incorporated by primary leukocytes, whereas almost all that is endogenously generated is retained.47 In support of the idea that 5-HETE-PEs mediate these actions when endogenously generated, we found that 2 pharmacologically distinct 5-LOX inhibitors (MK886 and zileuton) both enhanced NET generation in vitro.47 Thus, as predicted, inhibition of 5-LOX had the opposing effect to addition of exogenous 5-HETE-PE. NETs are proposed to trap bacteria in the bloodstream where they can be more effectively killed, and limit damage from neutrophil microbicidal proteins. The processes of NET formation are not clear but have been proposed to involve profound membrane alterations, including dissolving of nuclear and granular membrane, and then rupture of plasma membrane.48 Thus, agonist activation of nuclear and plasma membrane phospholipid oxidation by 5-LOX would be consistent with causing cell membrane disruption to facilitate NET release. Overall, whether this is a pro- or anti-inflammatory action in terms of killing bacteria or regulating host damage is not yet clear. A summary of mechanisms of formation and actions of 5-HETE–containing phospholipids is shown in Figure 3.
Summary of mechanism of formation and action of 5-HETE-PLs by human neutrophils. (A) Generation of the lipids is stimulated via receptor-dependent stimuli, including bacterial peptides, and intracellular signaling mediators. Hydrolysis of arachidonate by cPLA2 is required, then oxidation by 5-LOX, reduction by GPX, and re-esterification into the phospholipid membrane. (B) HETE-PEs enhance superoxide generation and IL-8 release while inhibiting NET formation. fMLP indicates N-formyl-methionine-leucine-phenylalanine; PLC, phospholipase C; cPLA2, cytosolic phospholipase A2; FACL, fatty acyl CoA ligase; and GPX, glutathione peroxidase.
Summary of mechanism of formation and action of 5-HETE-PLs by human neutrophils. (A) Generation of the lipids is stimulated via receptor-dependent stimuli, including bacterial peptides, and intracellular signaling mediators. Hydrolysis of arachidonate by cPLA2 is required, then oxidation by 5-LOX, reduction by GPX, and re-esterification into the phospholipid membrane. (B) HETE-PEs enhance superoxide generation and IL-8 release while inhibiting NET formation. fMLP indicates N-formyl-methionine-leucine-phenylalanine; PLC, phospholipase C; cPLA2, cytosolic phospholipase A2; FACL, fatty acyl CoA ligase; and GPX, glutathione peroxidase.
Generation of esterified eicosanoids by human platelets
Platelets express 2 eicosanoid generating enzymes, a platelet-specific 12-LOX and COX. The 12-LOX does not appear to play a major role in regulating direct platelet function because rather high levels of free 12-HETE are required for any effect on the cells.49 On the other hand, COX-1 generates eicosanoids that are of central importance in thrombosis and hemostasis.50 The primary COX-1 product, prostaglandin H2 is unstable and rapidly converted to thromboxane A2 by thromboxane synthase. Thromboxane A2 is a potent activator of platelet aggregation via the thromboxane receptor (TP) and also can stimulate smooth muscle contraction.51 Both these enzymes are activated acutely during platelet activation by thrombin or collagen in a calcium-dependent manner.
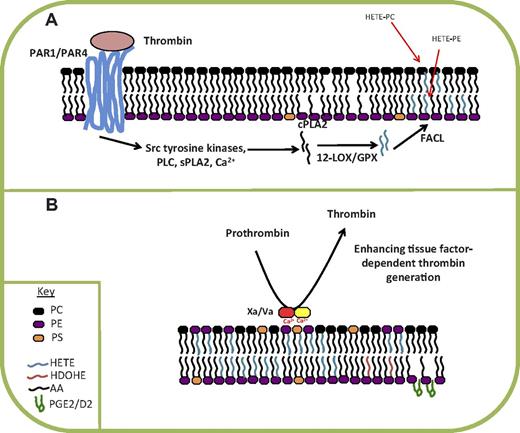
Precursor scanning of thrombin or ionophore-activated platelet lipid extracts for HETE-containing lipids identified 6 species, composing 2 PCs (16:0a/, 18:0a/12-HETE-PC) and 4 PEs (16:0p/, 18:1p/, 18:0p/, and 18:0a/12-HETE-PE; Figure 1B,E). The 12S-HETE was the prominent enantiomer, indicating the requirement for 12-LOX.52 Furthermore, they are absent in platelets from 12-LOX−/− mice (M. Aldrovandi and V.B.O., unpublished data, 2012). The lipids are generated within 5 minutes, and levels continue to increase slowly up to 30 minutes after activation. Esterified HETEs account for up to one-fourth of the total 12-HETE generated, and as for other cells, they remain cell associated. In resting cells, PE is predominantly facing the cytosol, but along with phosphatidylserine, is externalized on cell activation. This is in contrast to PC, which predominantly composes the outer leaflet. We found that a small amount of 12-HETE-PE becomes externalized after its synthesis.52 This suggests that oxidized phospholipids may regulate extracellular phospholipid-dependent signaling events. One important process that requires the presence of negatively charged PE and phosphatidylserine on the cell surface is coagulation. In this, several clotting factors become active through proteolytic cleavage in response to injury (release of tissue factor), associate with the plasma membrane, and generate thrombin. Coagulation factor activity can be tested in vitro by activating the cascade in human plasma using exogenous tissue factor, in the presence of liposomes of various composition. Using this assay, we found that HETE-PC dose-dependently stimulates tissue factor-dependent thrombin generation at concentrations found in human platelets.52 This is consistent with previous reports showing that nonenzymatically oxidized lipid can enhance coagulation, suggesting a role for the lipids in promoting hemostasis.53,54 As this is currently a preliminary finding, and its biologic relevance is not yet known, the mechanisms involved are currently being explored using recombinant coagulation factors, in vitro.
Thrombin stimulation of 12-HETE-PE/PC synthesis requires src tyrosine kinases, protein kinase C, and sPLA2 and calcium mobilization, and can be triggered by either protease activated receptors (PAR) 1 or 4.52 Similar to neutrophil 5-HETE-PEs, the platelet lipids are generated through PLA2 hydrolysis of phospholipid, oxidation by LOX, and then re-esterification into the plasma membrane. The time scale of generation is fast, similar to that for free 12-HETE generation, suggesting that the proteins involved may be coupled together in a tight complex. In support, we found that exogenously added 12-HETE-d8 does not become incorporated into PE or PC during the time scale of 12-HETE-PE/PC synthesis.47
Platelets also generate several additional phospholipid-esterified hydroxyl-fatty acids. These include 4 PE-esterified 14-hydroxydocosahexaenoic acids (HDOHEs), formed via 12-LOX oxidation of docosahexaneoic acid-containing phospholipids55 (Figure 1F). Their levels are lower than 12-HETE-PE/PCs, reflecting the lower amounts of unsaturated fatty acid substrate in the platelet membrane. Similar to 12-HETE-PE/PC, generation of esterified HDOHEs requires calcium and phospholipases. Intriguingly, platelets do not appear to generate esterified thromboxane, although we recently identified COX-1–derived PE esterified PGE2 and PGD2, formed on activation of platelets with thrombin (M. Aldrovandi and V.B.O., unpublished data, 2012). The mechanism of formation and proposed biologic actions of 12-HETE–containing phospholipids generated by human platelets are shown in Figure 4.
Summary of mechanism of formation and action of 12-HETE-PLs in human platelets. (A) The 12-HETE-PLs are generated in response to thrombin activation of PAR1 and PAR4, via several signaling intermediates. Hydrolysis of arachidonate by cPLA2 is required. (B) Some HETE-PEs translocate to the outside of the plasma membrane and can enhance tissue factor-dependent thrombin generation. sPLA2 indicates secretory phospholipase A2; FACL, fatty acyl CoA ligase; and PLC, phospholipase C.
Summary of mechanism of formation and action of 12-HETE-PLs in human platelets. (A) The 12-HETE-PLs are generated in response to thrombin activation of PAR1 and PAR4, via several signaling intermediates. Hydrolysis of arachidonate by cPLA2 is required. (B) Some HETE-PEs translocate to the outside of the plasma membrane and can enhance tissue factor-dependent thrombin generation. sPLA2 indicates secretory phospholipase A2; FACL, fatty acyl CoA ligase; and PLC, phospholipase C.
New generation mass spectrometry approaches for identification of immune cell lipids
The identification of esterified eicosanoids described in this review used a mass spectrometry method termed precursor scanning that takes advantage of the facile fragmentation of these species to generate a characteristic eicosanoid carboxylate anion on collision-induced dissociation. This mode is available on standard triple quadrupole instruments, and when combined with an ion trap (eg, Q-Trap), enables MS/MS to also be performed during elution, greatly aiding structural identification. Using this mode, lipid extracts were analyzed for families of lipids that contain a common functional group, specifically an eicosanoid.39,40,47,52,55 Because the mass spectrometry fragmentation patterns of both eicosanoids and phospholipids are already well known, the structural characterization of these lipids was straightforward. This approach could equally be used for lipids containing other functional groups of interest (eg, particular fatty acids or short chain modified lipids where the fragment generates a negative ion). Where a charged species is not formed on dissociation, neutral loss scanning can alternatively be used. Our methods coupled precursor scanning to high pressure liquid chromatography. Good separation is essential for analysis of complex lipid extracts because artifactual adducts of HETEs with other matrix constituents can form in the electrospray source that behave similar to esterified HETEs, when direct infusion of complex lipid extracts is performed. In our studies, an extraction method that extracts most lipid species was used, where cells or tissue (homogenized in the presence of antioxidants and metal chelators) is vortexed vigorously in the presence of hexane/isopropanol/acetic acid (2:20:30) at a ratio of 2.5 mL per milliliter sample. The method was described in full detail by Morgan et al.30
Another recently described approach involved derivatization of the PE headgroup using isotope-labeled 4-(dimethyamino)benzoic acid (DMABA).56 Generally, selective detection of PE by MS relies on neutral loss of m/z 141. However, this scan does not allow detection of plasmalogen species. Headgroup derivatization by DMABA enables all subclasses of PE to be detected by a common precursor ion scan in positive mode while also allowing detection in negative ion mode for collision-induced dissociation, with identification of radyl groups.57 Modification of this approach, where control lipids and 2,2′-azobis-(2-amidinopropane) hydrochloride lipid extracts from RAW264.7 cells were derivatized using d0-DMABA or d6-DMABA, respectively, allowed identification of several oxidized PEs, including truncated structures.56
Precursor scanning works only for lipid families where part of the structure is already known. More recently, we have begun to use high-resolution rapid scanning, coupled with peak alignment and integration analysis, which can be performed by hybrid ion trap-Orbitrap instruments. This method catalogs all molecular species present in a lipid mixture that form either positive or negative ions, within a defined mass window. Unbiased methods such as this have been used primarily in metabolomic studies, but the increasing scanning speed of newer instruments coupled to ultra-high pressure liquid chromatography, offers new exciting approaches that are especially suited to lipid discovery studies. Identification of lipids using databases, such as the Human Metabolome Database (www.hmdb.ca), can be carried out for known structures. A significant challenge lies in identification of unknowns, especially in complex mixtures of hundreds of lipids that are extracted from activated immune cells. Generation of spectral trees using MSn on high-resolution instruments will allow determination of elemental composition and will aid identification, whereas traditional derivatization approaches can determine functional groups. Although the MSn approach can be carried out in lipid mixtures, derivatization and analysis require purification, and this can be a significant challenge where lipids of interest may be present in very low amounts compared with other constituents.
Summary: common themes of oxidized phospholipid generation by immune cells
In this review, the identification and characterization of oxidized phospholipids that are generated acutely by human and murine immune cells are described. The lipids represent several families of related structures, containing an oxidized fatty acid moiety attached to a phospholipid. Generation of esterified eicosanoids is restricted to cells that express lipid oxidation enzymes. Thus, under basal or acute inflammatory conditions (eg, infection), esterified LOX products are generated by resident macrophages or neutrophils.40,47 Induction of 15-LOX by Th2 cytokines leads to generation of 15-HETE-PEs in monocytes and airway epithelium.43 Eosinophils are also a likely source of these lipids because they basally express 15-LOX and are present at elevated amounts in asthmatic airway along with IL-4 and IL-13. Platelets synthesize esterified HETEs, HDOHEs, and prostaglandins; thus, these lipids are probably generated during hemostasis or in conditions where platelet activation is elevated.52,55
COX isoforms are either constitutive (COX-1, platelet, renal, gastric) or inducible (COX-2 induced in most cell types by bacterial peptides or pro-inflammatory cytokines). We have observed acute generation of esterified COX-1 lipids in thrombin-activated platelets but not yet studied COX-2–expressing cells. Because macrophages expressing COX-2 have already been shown to synthesize glyceryl-PGE2/D2 in response to lipopolysaccharide, generation of phospholipid-esterified prostaglandins is also likely by isoform.58
Esterified eicosanoids are closely related to nonenzymatically oxidized phospholipids, which compose hundreds of molecules originally identified during air or chemical oxidation of unsaturated phospholipids in vitro.9 Indeed, the molecular structures described herein that originate from LOX or COX turnover will also be present at low concentrations in nonenzymatically generated mixtures. The key difference is that enzymes generate a restricted number of specific products, with oxygen insertion effectively dictated by the enzyme pathway involved. Nonenzymatically oxidized phospholipids display pleiotropic biologic actions, including some that are common to the lipids described herein, such as modulation of TLR and PPAR-γ signaling.9
Nonenzymatically oxidized phospholipids that originate from decomposition of phospholipid hydroperoxides have been measured in inflammation and atheroma.9 However, although the decomposition itself is likely to be uncontrolled, whether the primary hydroperoxides originate from enzymatic or nonenzymatic oxidation in vivo is not clear. An important difference in the processes that generate these 2 lipid classes is that enzymatically generated oxidized phospholipids are formed through controlled processes involving receptors and intracellular signaling pathways, using enzymes that are conserved among all mammalian species. This indicates that these lipids are of physiologic relevance and probably important in innate immune events, including hemostasis and bacterial killing. In contrast, nonenzymatic oxidized phospholipids will be generated during chronic inflammation and atherosclerosis, through redox cycling of metals or uncontrolled oxidant toxicity. In both cases, common bioactivities are displayed, although in chronic disease these processes will be occurring in an inappropriate and uncontrolled manner that contributes to the disease, rather than being required for maintenance of health and homeostasis. Thus, both are of importance in human health and disease, but the relative contribution of signaling will depend on the inflammatory context and the types and amounts of each species generated.
In conclusion, we describe several new families of enzymatically oxidized phospholipids formed by acutely activated cells. Their generation and retention in the cell occur in concert with profound changes in plasma membrane function. Thus, future work will determine how the lipids regulate membrane behavior at local level, in particular changes in shape and form, such as occurring during phagocytosis, aggregation, microparticle release, and chemotaxis.
Acknowledgments
This was supported in part by the National Institutes of Health (grant HL34303, R.C.M.) and the Wellcome Trust, European Union, and British Heart Foundation (V.B.O.).
National Institutes of Health
Authorship
Contribution: V.B.O. and R.C.M. wrote and edited the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valerie B. O'Donnell, Institute of Infection and Immunity, School of Medicine, Cardiff University, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: o-donnellvb@cardiff.ac.uk.