Abstract
Acute myeloid leukemia and acute lymphoblastic leukemia remain devastating diseases. Only approximately 40% of younger and 10% of older adults are long-term survivors. Although curing the leukemia is always the most formidable challenge, complications from the disease itself and its treatment are associated with significant morbidity and mortality. Such complications, discussed herein, include tumor lysis, hyperleukocytosis, cytarabine-induced cellebellar toxicity, acute promyelocytic leukemia differentiation syndrome, thrombohemorrhagic syndrome in acute promyelocytic leukemia, L-asparaginase-associated thrombosis, leukemic meningitis, neutropenic fever, neutropenic enterocolitis, and transfussion-associated GVHD. Whereas clinical trials form the backbone for the management of acute leukemia, emergent clinical situations, predictable or not, are common and do not readily lend themselves to clinical trial evaluation. Furthermore, practice guidelines are often lacking. Not only are prospective trials impractical because of the emergent nature of the issue at hand, but clinicians are often reluctant to randomize such patients. Extensive practical experience is crucial and, even if there is no consensus, management of such emergencies should be guided by an understanding of the underlying pathophysiologic mechanisms.
Introduction
Although inadequate for the cure of most patients, the initial therapeutic approach to acute myeloid leukemia (AML) is standardized. However, the management of hematologic emergencies is challenging and may be controversial. We present clinical vignettes describing 10 emergencies encountered in practice and the questions such emergencies generate. We then provide our view of how to treat adults supported by studies if available and, if not, by clinical experience and consensus. The approach to these emergencies is as important as the antileukemia therapy, and in no clinical setting is it fairer to say that “the devil is in the details.”
Tumor lysis syndrome
A 23-year-old man with a history of G6PD deficiency is diagnosed with pre-B acute lymphoblastic leukemia (B-ALL). At presentation, his white blood cell count (WBC) is 160 000/μL with 90% lymphoblasts and 450/μL neutrophils; the hemoglobin is 7.5 g/dL and platelet count is 10 000/μL. His electrolytes and renal function are normal, the uric acid is 11.0 mg/dL, and lactate dehydrogenase (LDH) is 1500 IU/L (normal, 100-250 IU/L). Remission induction chemotherapy is planned.
Questions
What is the risk of tumor lysis syndrome (TLS)? What is the optimal treatment for preventing TLS? What is the role of rasburicase and urine alkalinization?
TLS can appear before starting treatment (primary TLS) as a result of high turnover of the malignant cells or, more commonly, a short time after the beginning of treatment (secondary TLS).1 The risk of developing TLS and its severity is influenced by many factors, including tumor burden, potential for rapid cell lysis, and preexisting nephropathy.2 In acute leukemias, the risk is dependent on the WBC and the rate of response to therapy. In ALL with WBC > 100 000/μL, the risk for TLS is high. With lower WBC, the risk is moderate and low.3
A classification system has been developed,4 which is based on separate definitions for laboratory and clinical TLS. Laboratory TLS loosely is defined as the presence of 2 or more abnormal laboratory values of uric acid, potassium, phosphorus, or calcium at presentation or a 25% change in values from the pretreatment measurements. Clinical TLS is defined as the presence of laboratory TLS plus renal dysfunction, seizures, cardiac arrhythmia, or sudden death. An expert TLS panel has developed guidelines to assign patients to low, intermediate, and high risk of TLS.5
The current patient does not fit the diagnostic definitions of laboratory/clinical TLS, and we find such separation not as clinically useful as one might think. However, the high WBC, the anticipated high sensitivity to corticosteroids, with the elevated uric acid and LDH, place him at high risk.
The prevention and treatment of TLS are based on aggressive hydration, electrolyte correction, and reduction of uric acid levels. For a young adult without comorbidities, as much as 4000 to 5000 mL of intravenous fluids per 24 hours should be started 24 to 48 hours before induction chemotherapy, maintaining a urine output of at least 80 to 100 mL/m2 per hour.6 Loop diuretics are added for patients with low urine output.2 If hyperkalemia is present, conventional treatment is started promptly. For hyperphosphatemia, dietary phosphate restriction and oral phosphate binders are given. Administration of calcium is restricted to patients with symptomatic hypocalcemia because of its ability to increase the calcium-phosphate product and the risk of calcium-phosphate renal crystallization.
Allopurinol blocks the conversion of xanthine and hypoxanthine to uric acid by inhibiting xanthine oxidase and prevents the formation of uric acid but does not decrease the level of uric acid already present. Uric acid is reduced with allopurinol in 1 to 3 days, a time in which the kidneys are prone to damage. The intravenous preparation is as efficacious and safe as the oral route7 but is rarely used. Rasburicase, a recombinant urate oxidase, represents a new addition to the well-recognized therapeutic armamentarium. It reduces the level of uric acid by promoting the catabolism of uric acid to allantoin, a more soluble metabolite. Its efficacy and safety in TLS are established in several prospective studies,8,9 including multicenter randomized trials, which compared it with allopurinol, and provide a rationale for its prophylactic use.10,11 However, we use rasburicase in established TLS, particularly with elevated creatinine, uric acid greater than 9 mg/dL, or in patients not responding to allopurinol. Nevertheless, we recognize that fiduciary concerns and institutional practice may prevail. If rasburicase is to be used, a recent randomized trial suggested that a single dose (0.15 mg/kg) may be sufficient.12
The current patient is hyperuricemic and at very high risk for TLS. It is theoretically appropriate to treat him with rasburicase, but G6PD deficiency is a contraindication because hydrogen peroxide, a byproduct of rasburicase, cannot be broken down in G6PD-deficient patients and can lead to hemolysis or methemoglobinemia.13
Urinary alkalinization, considered in the past as standard of care, is no longer recommended. Despite increasing uric acid solubility, this benefit is outweighed by the potential increase precipitation of xanthine (which can be elevated in patients treated with allopurinol) and calcium phosphate in renal tubules.2,3
Despite optimal treatment, a minority of patients will require hemodialysis. Continuous venovenous hemofiltration as therapy or prophylaxis has been described.14
Although there are no data to support such a practice, if a patient with ALL presents with TLS, it is reasonable to start induction therapy emergently with only low doses of corticosteroids, such as prednisone 40 mg daily, until the WBC is less than 20 000/μL, assuming that less intensive therapy may slow the lysis of cells and enable the kidneys to maintain hemostasis.
Hyperleukocytosis
A 55-year-old woman is diagnosed with AML. She presents with a WBC of 88 000/μL with 78% blasts, a hematocrit of 31 mL/dL, and a platelet count of 3000/μL. She complains of profound fatigue, and after receiving one unit of packed red blood cells and a unit of single donor platelets, she develops dyspnea. Her oxygen saturation is 80%, and the temperature is 38.2°C. Chest radiograph reveals bilateral pulmonary infiltrates. The electrolytes are normal apart from potassium of 3.2mM/L.
Questions
Is the diagnosis leukostasis? What is the preferred treatment? How can blood transfusion be safely administered? What is the optimal treatment for an asymptomatic patient with newly diagnosed or relapsed AML and a WBC of 100 000/μL?
Hypeleukocytosis is important in AML because of the morbidity and mortality caused by leukostasis, TLS, and disseminated intravascular coagulation. A WBC count of 100 000/μL arbitrarily defines hyperleukocytosis in AML. Pathologically, leukostasis is characterized by intravascular accumulation of blasts occupying the vascular lumen, with or without the presence of fibrin,15 but this can usually only be diagnosed at autopsy.
Although a grading system has been developed to predict for leukostasis,16 this remains a clinical diagnosis often based on exclusion of other etiologies. A funduscopic examination, a practice sadly often neglected even by seasoned physicians, is most helpful in establishing the diagnosis when papilledema, blurred disc margins, dilated blood vessels, and retinal hemorrhages are present.
The CNS, including the eyes and lungs, are the most common sites for vascular obstruction, but other organs such as extremities, kidneys, heart, and penis, can also be affected. When an AML patient presents with dyspnea or stupor and the WBC is > 100 000/μL, in the absence of a clear etiology, a presumptive diagnosis of leukostasis is made. Leukostasis may occur where the clinical picture is less typical and/or the WBC may be less than 100 000/μL, particularly with rapidly increasing blast counts or in association with monocytic variants. In this patient, the differential diagnosis includes pulmonary infection, transfusion-related acute lung injury in a patient who receives single donor platelets several hours before the deterioration, as well as leukostasis.
It may be easy to inadvertently attribute pseudohypoxemia, known as the “leukocyte larceny” syndrome typical in hyperleukocytosis, to true pulmonary insufficiency.17 Similarly, plasma potassium should be measured to avoid a spuriously high level because of pseudohyperkalemia,18 another common phenomenon in hyperleukocytosis.
Perhaps surprisingly, there has been no prospective randomized trial of leukapheresis in patients with hyperleukocytosis and/or leukostasis.12 Historically, cranial irradiation was used for patients with neurologic symptoms. More recently, dexamethasone has been proposed because it inhibits up-regulation of adhesion molecules on leukemia and endothelial cells.19 Neither option has proven to be effective.
The transfusion of blood to a patient with hyperleukocytosis may be hazardous because of increasing the blood viscosity and potential for aggravating leukostasis. If needed, the transfusion should be administered cautiously during or immediately after leukapheresis. In the patient presented here, the risk of aggravation of leukostasis with a transfusion is minimal as the leukocrit is only approximately 4 mL/dL and, with a hematocrit of 31 mL/dL, this still leads to a total cytocrit of only 35 mL/dL, making increased blood viscosity unlikely.20
The management of asymptomatic patients with hyperleukocytosis is controversial. Table 1 summarizes the broad indications for leukapheresis in our centers.
WBC counts (× 109/L) as indication for leukapheresis in hyperleukocytosis
| . | Symptomatic . | Asymptomatic . |
|---|---|---|
| AML | > 50 000 | > 100 000 |
| ALL | > 150 000 | > 300 000 |
| CML | > 150 000 | No |
| CLL | > 500 000 | No |
| APL | No | No |
| . | Symptomatic . | Asymptomatic . |
|---|---|---|
| AML | > 50 000 | > 100 000 |
| ALL | > 150 000 | > 300 000 |
| CML | > 150 000 | No |
| CLL | > 500 000 | No |
| APL | No | No |
In the presence of unequivocal retinal findings, leukapheresis may be performed for even lower WBC counts.
CML indicates chronic myeloid leukemia; and CLL, chronic lymphocytic leukemia.
Leukapheresis usually requires 2 procedures, 12 to 24 hours apart, for successful leukoreduction, although in rare cases this may need to be further continued. In an emergent situation, in the absence of facilities to perform leukapheresis, judicious phlebotomies with concurrent blood and/or plasma replacement may be attempted.21
We recommend leukapheresis for the patient presenting for presumed leukostasis. We also treat with high doses of corticosteroids for the possibility of transfusion-related acute lung injury, as well as broad-spectrum antibiotics. Hydroxyurea would be instituted at diagnosis. Induction therapy would begin when the WBC is, arbitrarily, less than 50 000/μL. Blood potassium levels are measured in a heparinized tube to exclude life-threatening hypokalemia. We also favor treating with leukapheresis any asymptomatic AML patient with a WBC greater than 100 000/μL, as an initial therapeutic modality to prevent leukostasis. However, this decision is influenced by age, history of neurologic or vascular disease, and rate of rise in the WBC. In patients with asymptomatic ALL, with a very low risk of leukostasis, the WBC threshold is, again arbitrarily, more than 300 000/μL, performed primarily to prevent TLS. In contrast, leukapheresis should not be used for the hyperleukocytosis of acute promyelocytic leukemia (APL).22
Cytarabine-induced cerebellar toxicity
A 43-year-old man is diagnosed with AML. He achieves complete remission with induction therapy. Consolidation is started with high-dose cytarabine (HiDAC), 3 g/m2 over 1 hour, twice daily on days 1 to 6. Metabolic parameters at the beginning of treatment are normal, except for a creatinine of 1.45 mg/dL. On day 4, he complains of minimal gait disturbance. The next day, he develops gait ataxia, nystagmus, and dysarthria. The last dose of cytarabine is omitted.
Questions
Is it possible to predict and/or prevent cytarabine-induced neurotoxicity? What is the optimal management? Can HiDAC be safely administered to older patients or to patients with renal insufficiency? Is it possible to re-treat, with HiDAC, a patient who previously sustained cytarabine-induced cerebellar toxicity?
HiDAC is commonly used as postremission therapy for AML, and also in some ALL protocols, exploiting its ability to penetrate the blood-brain barrier. Neurologic toxicity related to HiDAC includes gait ataxia, nystagmus, dysmetria, and dysarthria.23,24 Seizures and cerebral dysfunction occur, and peripheral neuropathy is rarely described. Signs of cerebellar dysfunction usually emerge between the third and eighth day from start of treatment. In most patients, the symptoms resolve in 3 to 10 days,24 but in others symptoms persist and can be debilitating or even fatal.
The pathophysiology of HiDAC-induced neurotoxicity is poorly understood and the main goal is prevention because there is no effective treatment.25 Administration of pyridoxine hydrochloride as prophylaxis was common but has no protective effect. Anecdotal reports suggest removal of cerebrospinal fluid by lumbar puncture26 as well as methylprednisolone27 as treatment for symptomatic patients.
Retrospective studies report that increasing age and cumulative dose are the main risk factors,24 but others identify renal insufficiency and liver dysfunction.28,29 Other possible risk factors are rate of administration,25 CNS involvement, previous CNS pathology, concurrent medications (eg, antiemetic drugs),25 and previous cytarabine-related neurotoxicity.
Although CNS toxicity can occur at any age and even among patients with normal organ function, dose adjustments need to be made for older age and renal or hepatic insufficiency. For older patients, with appropriate dose reduction, HiDAC can be safely administered.30,31 A dose reduction of 50% was reported for patients with a creatinine of 1.5 to 2 mg/dL or an increase of 0.5 mg/dL from the pretreatment value, with a more drastic reduction for those with a creatinine level more than 2 mg/dL.29 Using the Hyper-CVAD protocol, the dose of HiDAC was reduced by two-thirds only when the creatinine is more than 2 mg/dL.32 For patients with very high creatinine levels, an alternative to withholding therapy is the use of hemodialysis to deplete Ara-U, the major metabolite of cytarabine.33 However, the cumbersome nature of this undertaking precludes common usage. Dose reductions for hepatic function are even less clear. Some authors report higher risk when the alkaline phosphatase is 3 times the upper limit of normal23 or bilirubin more than or equal to 2 mg/dL,29 but firm guidelines have yet to be established.
When treatment is initiated, any suspicion of cerebellar dysfunction mandates prompt discontinuation.34 We deliberately examine for horizontal nystagmus, a subtle early sign of the syndrome, as part of a daily comprehensive cerebellar examination. Computerized tomography (CT) and magnetic resonance imaging (MRI) scans are usually normal, at least initially.35,36 The risk of recurrent neurotoxicity is unknown. Successful readministration of HiDAC to a patient who had previously experienced neurotoxicity associated with renal insufficiency has been reported.28
Administering HiDAC to patients with renal or hepatic dysfunction needs to be very carefully considered with dose adjustments as in Table 2. The decision to reinstitute such therapy after complete resolution of neurotoxicity, where there is a compelling rationale to do so, may be possible. However, in practice, this generates sufficiently high anxiety on the part of physicians as to preclude such an endeavor.
Suggested dose adjustment for HiDAC therapy as initial consolidation based on age, creatinine, and bilirubin
| . | Age < 55 y . | Age 55-70 y . | Age > 70 y . |
|---|---|---|---|
| Creatinine < 1.3 mg/dL and bilirubin < 2 mg/dL | 3 g/m2, 1 h, twice daily, 12 doses (100%) | 1.5 g/m2, 1 h, twice daily, 12 doses (50%) | 1.5 g/m2, 1 h, once daily, 6 doses (25%) |
| Creatinine 1.3-2 mg/dL or bulirubin 2-3 mg/dL | 1.5 g/m2, 1 h, twice daily, 12 doses (50%) | 1.5 g/m2, 1 h, once daily, 6 doses (25%) | |
| Creatinine > 2 mg/dL* or bilirubin > 3 mg/dL | 1.5 g/m2, 1 h, once daily, 6 doses (± 15%) |
| . | Age < 55 y . | Age 55-70 y . | Age > 70 y . |
|---|---|---|---|
| Creatinine < 1.3 mg/dL and bilirubin < 2 mg/dL | 3 g/m2, 1 h, twice daily, 12 doses (100%) | 1.5 g/m2, 1 h, twice daily, 12 doses (50%) | 1.5 g/m2, 1 h, once daily, 6 doses (25%) |
| Creatinine 1.3-2 mg/dL or bulirubin 2-3 mg/dL | 1.5 g/m2, 1 h, twice daily, 12 doses (50%) | 1.5 g/m2, 1 h, once daily, 6 doses (25%) | |
| Creatinine > 2 mg/dL* or bilirubin > 3 mg/dL | 1.5 g/m2, 1 h, once daily, 6 doses (± 15%) |
Doses used are based on typical regimen as used by the Eastern Cooperative Oncology Group. For other common regimens, such as that used by the CALGB96 consisting of 3 g/m2 over 3 hours, 2 times per day on days 1, 3, and 5, the suggested dose adjustments, in percentages, are indicated in parentheses.
Consider also hemodialysis.
APL differentiation syndrome
A 40-year-old man was admitted 2 weeks ago with bleeding and a WBC of 12 000/μL. A diagnosis of APL is made. Aggressive blood product support is emergently initiated, all-trans retinoic acid (ATRA) is emergently started, and anthracycline-based chemotherapy is administered. On day 18, the patient develops fever, cough, pulmonary infiltrates, and a right-sided pleural effusion. He is hypoxemic and is transferred to the intensive care unit with a presumptive diagnosis of pneumonia and associated parapneumonic effusion.
Questions
How is the diagnosis of the APL differentiation syndrome (DS) made? What is the optimal treatment? Does ATRA and/or arsenic trioxide (ATO) need to be stopped? Can either be resumed?
When a patient with APL receiving ATRA or ATO develops pulmonary infiltrates and/or pleural or pericardial effusions, making a sole diagnosis of pneumonia or fluid overload should be resisted. Because of its life-threatening nature and reliable responsiveness to dexamethasone, the patient described in “APL differentiation syndrome” is assumed to have DS. Unlike many systems of jurisprudence, such a patient is “guilty” (of having the APL DS) until proven innocent. Although initially named the retinoic acid syndrome, it is now known as DS because it also occurs in those receiving ATO. It develops in approximately 25% of patients treated with ATRA as a single agent at a median of 11 days (range, 2-47 days) of therapy.22,37 The syndrome is manifested by fever, pulmonary infiltrates, pleural and/or pericardial effusions, episodic hypotension, and occasionally renal failure.37,38 With the addition of chemotherapy for high-risk patients, the incidence is lower, approximately 15%.39 Although the syndrome generally occurs in the setting of a rising or elevated WBC, we are not dissuaded from treating for the DS in the absence of an elevated WBC. The pathogenesis may be related to the release of cytokines from the malignant promyelocytes and/or other cells40 as well as perturbed adhesive properties of APL cells by ATRA, which induces the expression of high-avidity beta2 integrins.41 The effects are rapidly reversed with the administration of dexamethasone at a dose of 10 mg twice daily.
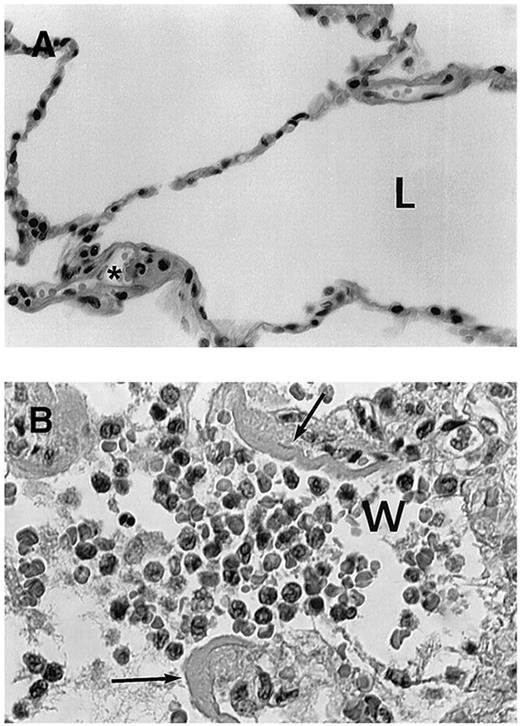
Because DS is life-threatening, we administer dexamethasone at the very earliest symptom or sign of respiratory compromise, even in the absence of objective findings. Some patients may indeed have concomitant fluid overload, congestive heart failure, pneumonia, or sepsis. Although it is not clear that prophylactic corticosteroids prevent the syndrome, they may reduce pulmonary toxicity and are associated with a low incidence of the syndrome.42 In practice, we start dexamethasone in any patient with newly diagnosed APL who presents with a WBC > 10 000/μL. Examination of the lung tissue indicates that there is no leukostasis with obstruction of the alveolae, but rather interstitial infiltration of maturing myeloid cells (Figure 1). If the patient develops sufficient dyspnea, tachypnea, or hypoxemia to a degree that warrants immediate clinical concern, or transfers to the intensive care unit, the syndrome is considered severe enough that we discontinue ATRA and/or ATO temporarily. However, if mild, we continue ATRA and/or ATO with dexamethasone until complete disappearance of all findings. We acknowledge that our threshold for holding ATRA is lower if the patient has had at least 2 weeks when, in our experience, most patients will achieve complete remission.
Histologic findings in cases with fatal retinoic acid syndrome. (A) Histologic appearance of normal lung tissue. L indicates lung alveolae in normal lung. *Normal neutrophil in microvasculature. (B) Lung tissue showing infiltration of alveolae with leukocytes from a patient who died of retinoic acid syndrome. w indicates myeloid cells in the airspace (most leukocytes to left of “w”); the lobated nuclei of myeloid cells is prominently seen. Arrows indicate fibrinous exudate resulting from vascular leak of serum fibrin. Data from Tallman et al.37
Histologic findings in cases with fatal retinoic acid syndrome. (A) Histologic appearance of normal lung tissue. L indicates lung alveolae in normal lung. *Normal neutrophil in microvasculature. (B) Lung tissue showing infiltration of alveolae with leukocytes from a patient who died of retinoic acid syndrome. w indicates myeloid cells in the airspace (most leukocytes to left of “w”); the lobated nuclei of myeloid cells is prominently seen. Arrows indicate fibrinous exudate resulting from vascular leak of serum fibrin. Data from Tallman et al.37
Thrombohemorrhagic syndrome in APL
A 59-year-old woman presents with new-onset diplopia. She has a left third nerve palsy, and CT demonstrates left occipital and thalamic infarct. A complete blood count discloses hemoglobin of 10.5 g/dL, WBC of 500/μL, and platelet count of 88 000/μL. Prothrombin time is slightly prolonged with an international normalized ratio of 1.3, and partial thromboplastin time is normal and fibrinogen is 80 mg/dL. BM and molecular and cytogenetic results established the diagnosis of APL.
Question
What is the best prophylaxis and emergent management of coagulopathy in APL?
In patients with acute leukemia, a thrombohemorrhagic syndrome is often associated with substantial activation of coagulation, which results in either localized thrombosis, widespread bleeding, or both, hence termed thrombohemorrhagic syndrome.43 In large studies, the frequency of symptomatic thrombosis is 5.2%,44 and of hemorrhage 7%.43
The coagulopathy in APL is a manifestation of not only disseminated intravascular coagulation but also primary and secondary fibrinolysis as well as fibrinogenolysis. The pathogenesis includes overexpression of annexin II, which increases plasmin generation by tissue plasminogen activator approximately 60-fold,45 and elastases from promyelocytes, which cleave fibrinogen and degrade fibrinolytic inhibitors. In addition, secondary fibrinolysis generates thrombin by aberrant tissue factor expression, resulting in tissue plasminogen activator release from the endothelium.46 The incidence of fatal hemorrhage in APL is 5% in large series.47 It occurs mainly during induction and is almost exclusively intracranial (65%) and pulmonary (32%). The associated risk factors include high WBC at diagnosis, presence of coagulopathy, age, performance status, and high creatinine. Despite the introduction of ATRA, leading to degradation of the PML-RAR∝, differentiation of APL cells, the risk of bleeding remains high. In the Swedish registry, hemorrhage accounted for early death in 11.5% of all patients, of which 59% were treated with ATRA,48 and in a recent large United States population-based study the early death rate, mostly from hemorrhage, was 17%.49 Use of unfractionated heparin, a mainstay of therapy in the pre-ATRA era, has not been studied since the introduction of ATRA.
Although thrombosis is a less known complication of APL, its frequency is 4.5% to 5.6% and may be underappreciated.50 It presents as either deep vein thrombosis (57%) or cerebral stroke (22%) and is associated with low fibrinogen, or the hypogranular variant.
We transfuse platelets to maintain the level at or more than 50 000/μL for the first several days. We transfuse cryoprecipitate, even several times a day, until the fibrinogen is maintained at least 150 mg/dL. Early death, usually because of hemorrhage, has emerged as the major cause for treatment failure in APL.
Intracranial hemorrhage (ICH) may occur in any thrombocytopenic patient with acute leukemia but is most common in APL, monocytic leukemia, and ALL.51,52 In a large cohort of 841 AML patients, the incidence of ICH was 6.1% and occurred mainly in patients with relapse/refractory disease; and in 75%, it was intraparenchymal, 31% subarachnoid, and 20% subdural. ICH was associated with a 67% mortality.53 In a survey of 2421 patients with acute leukemia, 4 factors were identified predicting the outcome of ICH: low albumin, elevated LDH, age more than 60 years, and relapsed disease.54 The most common presenting symptom is mental status change; and at the first such clinical sign, patients need to be monitored closely by repeat radiologic imaging (CT/MRI). We maintain the platelet count more than 50 000/μL. Early neurosurgical consultation is advised; and if ICH is firmly diagnosed, the platelets should be kept above 100 000/μL, if possible, and emergent neurosurgical intervention should be considered.
L-Asparaginase–associated thrombosis
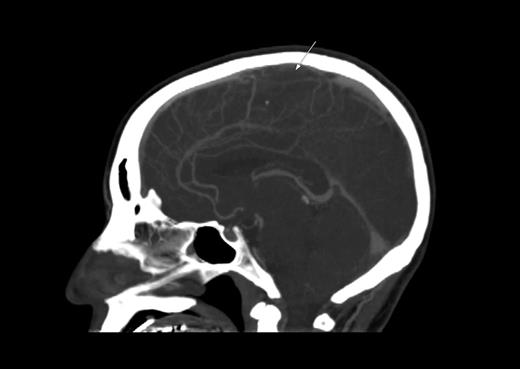
A 35-year-old man with B-lineage ALL is treated with a standard ALL protocol.55 On day 17 of induction, Escherichia coli L-asparaginase (L-ASP) is started at a dose of 10 000 units/day in combination with prophylactic subcutaneous low molecular weight heparin (LMWH). Pretreatment thrombin time, partial thromboplastin time, fibrinogen, and D-dimer levels are normal. On day 9 of L-ASP, he presents with generalized seizure, right-sided limb weakness, and slurred speech. CT scan discloses extensive sagittal sinus thrombosis (Figure 2). L-ASP treatment is discontinued and full dose heparin is initiated. There is no evidence of inherited thrombophilia.
CT scan. Sagittal plane demonstrating sagittal sinus. The arrow indicates the filling defect corresponding to sagittal sinus thrombosis.
CT scan. Sagittal plane demonstrating sagittal sinus. The arrow indicates the filling defect corresponding to sagittal sinus thrombosis.
Questions
What is the risk of thrombosis in patients treated with L-ASP? Can this complication be predicted? Is there a role for prophylaxis with anticoagulants? Is it possible to re-treat with L-ASP after a thrombotic event? What is the fibrinogen level at which replacement should occur? Is evaluation for thrombophilia mandatory before initiating treatment?
L-ASP has been a mainstay of combination chemotherapy for ALL since the 1970s. This agent selectively kills leukemic blasts by depleting circulating exogenous asparagine pools.56 Toxicity is significant, for both the native and the pegylated forms.57 Thrombosis is the more common complication, although hemorrhage can also occur. Thrombosis is caused by depletion of asparagine, which leads to hampered synthesis of proteins participating in hemostasis. These include plasminogen, fibrinogen, protein C, protein S, and, most significantly, antithrombin III (ATIII), which leads to increased thrombin generation. Thrombosis is often catheter-related but may occur in the CNS, lungs, heart, or deep veins.58 The incidence of thrombosis is 6%.59 The majority of events occur during induction.60 Inherited thrombophilia increases the risk 8-fold in pediatric patients; however, there are no data in adults.60
The treatment of thrombosis in this setting is complex and is based on administration of anticoagulants (heparin or LMWH) and of AT concentrate and cryoprecipitate (for ATIII level < 60 and fibrinogen < 100 mg/dL, respectively). We do not administer fresh frozen plasma because it repletes asparagine and may negate the antileukemia effects of L-ASP. However, if ATIII is not available, or for life-threatening thrombosis, fresh frozen plasma may be given at a dose of 20 mL/kg.
Thrombosis may be prevented by repletion of ATIII,61 although this is rarely done. In one study, prophylaxis with LMWH and ATIII completely eliminated thrombosis.62 In a small pediatric trial, the use of LMWH was found to be associated with less thrombosis.63
Some of us avoid using central venous catheters in induction of ALL, which includes L-ASP. We monitor the prothrombin time, partial thromboplastin time, and fibrinogen levels daily and correct with cryoprecipitate if fibrinogen is less than 50 mg/dL. We use this level because we want to avoid inducing thrombosis, another underappreciated risk.64 Although some of us administer prophylactic LMWH 40 mg/daily from the start of treatment and continue for one week after discontinuation of L-ASP, this strategy has not been prospectively studied and may not be ready for “prime time.” We do not routinely screen for inherited thrombophilia unless there is a suggestive history.
We treat CNS thrombosis with full-dose heparin coupled with intensive cryoprecipitate and platelet transfusion support. Sagittal sinus thrombosis is often accompanied by increased intracranial pressure and secondary hemorrhagic infarct. We continue heparin, even in the presence of such hemorrhage with blood product support and frequent CT/MRI monitoring, because the primary event is thrombosis. Importantly, for the same reason, L-ASP may be resumed at lower doses if toxicity was not greater than grade 3 and completely resolved.59,65
Leukemic meningitis
A 32-year-old woman is diagnosed with AML. Cytogenetic analysis reveals inv(16)(p13q22). She is treated with induction therapy followed by 3 cycles of HiDAC. Complete hematologic and molecular remission is achieved. Two years later, she presents with relapse in the CNS.
Questions
Can CNS relapse be predicted? Is there a role for CNS prophylaxis for any patient with AML? What is the best regimen to treat CNS disease in AML at diagnosis? Does CNS disease influence the systemic induction? What is the best treatment for isolated CNS relapse?
The incidence of CNS leukemic infiltrates in AML at diagnosis or relapse is low (3%-7%).66 However, CNS disease poses a major challenge and decreases long-term survival. The pathogenesis of leukemic infiltration in CNS is unknown but involves interruption of tight junctions of the blood-brain barrier through adhesion molecules on leukemic blasts or increased permeability from vascular endothelial growth factor.67 An increased risk of CNS involvement in AML occurs with a high WBC, high circulating blast count, high LDH, and younger age.68 It is more commonly associated with inv(16)(p13q22), chromosome 11 abnormality, or trisomy 8.68 Monocytic lineage leukemias are also associated with a 5.7-fold increased risk of CNS disease.69 In more than two-thirds of affected AML patients, CNS involvement appears at relapse, either isolated or as part of systemic disease.
The clinical presentation of CNS involvement may be subtle or may present as increased intracranial pressure (headache, nausea, vomiting, blurred vision, or altered consciousness), cranial nerve palsy, or motor deficit. Diagnosis is based on the combination of CSF examination for cytology, immunophenotyping by flow cytometry and PCR (for leukemia specific markers), and gadolinium-enhanced MRI of brain and spine.70 MRI alone is neither sensitive nor specific enough to establish the diagnosis of CNS leukemia.
In contrast to ALL, where CNS prophylaxis is mandatory, it is unknown whether prophylaxis in AML can prevent CNS relapse. Although there may be a rationale in high-risk patients, we do not routinely provide prophylaxis in AML, except for patients with high-risk APL. Such patients, or any relapsed APL patient (even molecular relapse), receive prophylactic intrathecal chemotherapy. Although it is not clear that this approach prevents CNS relapse, the apparent increased incidence among patients with high-risk APL and the low toxicity provide a reasonable rationale.
Treatment of CNS disease in AML at presentation or relapse includes intrathecal cytarabine and/or methotrexate given twice weekly until clearance of CSF and then, arbitrarily, another 2 administrations.71 After intrathecal therapy, we give either HiDAC and/or craniospinal radiation. Radiation alone has the disadvantage of increased BM relapse.72 In patients with resistant leukemic meningitis, thiotepa demonstrated favorable results,66 although the long-term survival remains poor.68
Neutropenic fever
A 60-year-old woman with newly diagnosed AML is admitted for induction chemotherapy. On the seventh day of treatment, she develops a fever of 39.6°C. Blood pressure is 70/40 and pulse 130/min. The absolute neutrophil count is less than 100/μL. She had a central line inserted 8 days previously and received fluconazole prophylaxis. She is also known to be a carrier of carbapenem-resistant enterobacteriace.
Questions
Is there a role for prophylactic antibiotics in AML? What is the optimal initial antibiotic regimen? What is the duration of treatment? Should the central line be removed? Is there a role for granulocyte transfusion?
Patients with AML undergoing induction or salvage therapy are at high risk of neutropenic fever, more so than those undergoing consolidation therapy.73 The Infectious Disease Society of America published detailed guidelines for the treatment of neutropenic fever.74 Fluroquinolones reduce all-cause mortality and infection-related mortality and have been recommended in high-risk patients.75 However, there is concern regarding acquisition of resistant bacteria in the presence of such prophylaxis, although this was not demonstrated in several meta-analyses.76,77 Initial antibiotics are directed at covering Pseudomonas aeruginosa and other Gram-negative bacteria because of higher morbidity associated with these infections, although, in the era of central venous catheters, more than 50% of bacteremias in neutropenic patients are caused by Gram-positive bacteria.78 Treatment may include single-agent β-lactam, such as cefepime, carbapenem, or piperacillin/tazobactam. There is no role for addition of aminoglycosides. In this patient, the addition of colistin or tigecycline to the initial treatment is recommended for possible carbapenem-resistant enterobacteriace infection. Vancomycin is added initially only in cases of soft tissue infection, suspected catheter-related infections, in a hemodynamically unstable patient, or in a patient who is previous colonized with Gram-positive bacteria.74 The use of granulocyte transfusion in hemodynamically unstable neutropenic patients with pneumonia or soft tissue infection, resulting from either fungal or to bacterial infection, does not improve survival.79 Although controversial, we often use daily granulocyte transfusions for deep-seated fungal infection not responding well to initial therapy.
Neutropenic enterocolitis
A 35-year-old man with AML is treated with induction therapy. On day 16, he develops fever, bloody diarrhea, and right lower abdominal pain followed a few hours later by an acute abdomen. He undergoes emergent explorative laparotomy and right hemicolectomy with temporary colostomy.
Questions
Can neutropenic enterocolitis be prevented? Should this influence the decision regarding further chemotherapy?
Neutropenic enterocolitis (NE), also termed necrotizing colitis, iliocecal syndrome, or typhlitis (from the Greek term typhlon, meaning cecum), is an ill-defined syndrome characterized by fever and abdominal pain in the setting of neutropenia. In adult patients, the reported incidence is 6.5%.80 Other clinical manifestations include nausea (75%), vomiting (67%), abdominal distension, and watery or bloody diarrhea. Examination may disclose abdominal distension and tenderness or a right lower abdominal mass.81 Bacteremia is commonly reported and often is polymicrobial.80,82 Pseudomonas spp is most frequent, whereas E coli, Klebsiella spp, Staphylococcus aureus, and α-streptococci are prevalent. Approximately 15% of patients have fungal organisms isolated in the blood, with Candida being the most common isolate.83
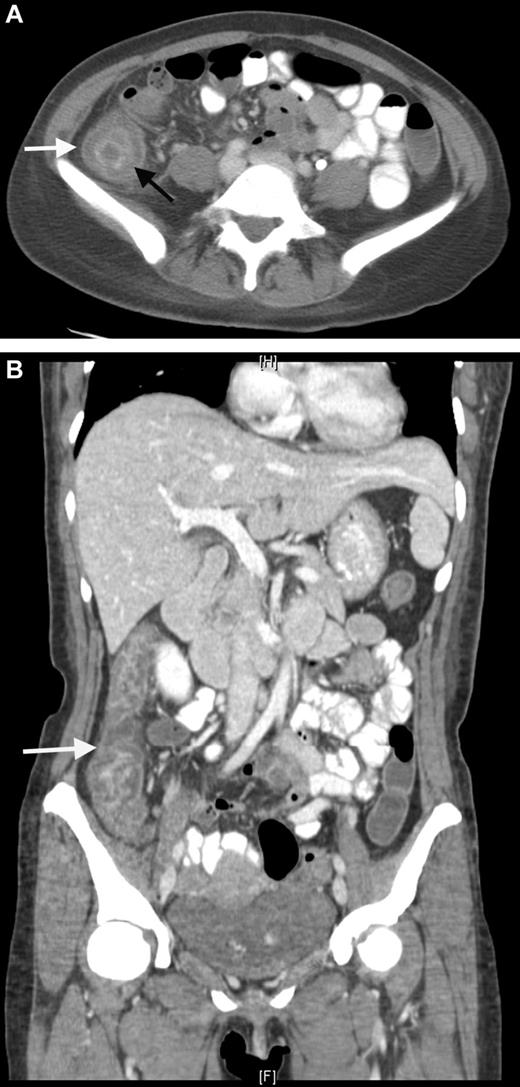
The pathogenesis of NE is linked to the damaged gastrointestinal mucosa. This is attributable to chemotherapy and neutropenia leading to intestinal dysfunction and microbial invasion with, secondarily, edema, inflammation, ulceration, transmural necrosis, and possible perforation.81,84 Abdominal CT (Figure 3) or ultrasound (US) discloses thickening of the bowel wall and intramural edema, which may be accompanied by paracolonic fluid, free air, or pneumatosis intestinalis. A more than or equal to 10-mm wall thickening was associated with 60% mortality compared with 4.2% with less thickening.85 Most authors agree that bowel thickening more than 4 mm is a mandatory criterion for establishing the diagnosis of NE.80 CT imaging is also important to rule out other possible diagnoses, such as appendicitis, diverticulitis, and ischemic colitis. Bowel rest, fluid resuscitation, nasogastric suction, parenteral nutrition, and early surgical consultation are the mainstay of treatment, together with broad-spectrum antibiotics.86
CT scan. Neutropenic enterocolitis. (A) Coronal contrast-enhanced CT image through the abdomen and pelvis. Coronal contrast-enhanced CT demonstrates bowel wall thickening with low attenuation in the submucosal layer of the bowel wall representing bowel wall edema. (B) Axial contrast-enhanced CT through the abdomen. Axial contrast-enhanced CT through the abdomen demonstrates bowel wall thickening with a “target sign” in the ascending colon. The mucosa and muscularis layers are hyperenhancing, whereas the submucosal low density represents bowel wall edema.
CT scan. Neutropenic enterocolitis. (A) Coronal contrast-enhanced CT image through the abdomen and pelvis. Coronal contrast-enhanced CT demonstrates bowel wall thickening with low attenuation in the submucosal layer of the bowel wall representing bowel wall edema. (B) Axial contrast-enhanced CT through the abdomen. Axial contrast-enhanced CT through the abdomen demonstrates bowel wall thickening with a “target sign” in the ascending colon. The mucosa and muscularis layers are hyperenhancing, whereas the submucosal low density represents bowel wall edema.
The need for surgical intervention is uncommon and is reserved for uncontrolled bleeding, bowel ischemia, and perforation. With current treatment, the mortality from NE has reportedly improved from 80% to 30% to 50%.87 However, in our experience, with contemporary supportive management and early recognition, the mortality is low.
Certain chemotherapeutic agents, although exhibiting comparable efficacy, appear to be associated with a higher risk for developing NE. Although equally efficacious as daunorubicin, Adriamycin is never used in AML following a classic study in 1982, which demonstrated significantly greater incidence of NE with Adriamycin.88
Parenteral alimentation and nasogastric suction have not proven useful and are not used in our centers.89 Antibacterial prophylaxis with quinolones in high-risk neutropenic patients, as recommended in recent guidelines,86 is an appealing approach, although it is not known if the incidence is reduced. Keratinocyte growth factors, such as palifermin90 or repifermin, have been approved (palifermin) for prevention of oral mucositis after intensive chemotherapy. However, no study has demonstrated a reduction in NE with keratinocyte growth factors, and they are not used for this purpose.
Transfusion-associated GVHD
A 58-year-old woman is transferred from another country with severe pancytopenia. Six weeks earlier, she presented with AML and received induction therapy. She has a rare red cell blood type AB+, together with anti-kell (anti-k; anti-cellano) alloantibodies. Shortly after her blood counts began to recover, and 20 days before admission, she receives 2 red blood cell transfusions from her siblings, one of whom is A+ and the other B+, and both are kell−. Two weeks later, she develops a febrile illness with extensive erythematous skin rash. This is followed by gastrointestinal pain, diarrhea, and worsening liver function tests; and a few days later, she has a precipitous decline in all her blood counts. She has a stormy hospital course and dies 2 weeks later of multiorgan failure.
Questions
Can this complication be prevented? Is there any effective therapy?
Transfusion-associated GVHD (TA-GVHD) is a rare, often overlooked, but fascinating complication of blood transfusion which can occur in patients with immune deficiencies. Although less recognized, it also can be observed in immunocompetent hosts when the person receives a transfusion of allogeneic lymphocytes from an HLA-compatible donor, particularly those with a shared haplotype.91,92 In such circumstances, the T lymphocytes may “escape” recognition as foreign by the host. These lymphocytes eventually recognize the host as foreign and mount an acute response against the target host cells. Close family members are at highest risk, but populations with less HLA disparity, such as the Japanese, are also at risk.93
The clinical spectrum of TA-GVHD (fever, hepatitis, rash, and diarrhea) mimics GVHD, commonly seen after allogeneic hemopoietic cell transplantation. A fundamental difference is the absolute aplasia in TA-GVHD, which is the hallmark of this syndrome. This occurs because cells in the bone marrow are identified as foreign by the donor lymphocytes, unlike hemopoietic transplantation where the cells in the bone marrow are of donor origin.
The reported incidence of TA-GVHD is very low; however, the true incidence is unknown as it may be undiagnosed. This is particularly true in patients with immune deficiencies, such as acute leukemia, where the clinical features are nonspecific and rapid demise may be mistaken for overwhelming sepsis.
TA-GVHD typically occurs between 1 and 4 weeks after transfusions of any cellular blood component. The overall mortality from established TA-GVHD is greater than 95%. Anecdotally, one patient diagnosed early was successfully treated with autologous peripheral blood cell transplantation.94 However, treatment is almost always unsuccessful. Irradiation of cellular blood components with a least 25 Gy is reliably effective in preventing TA-GVHD. This simple precaution for all immunocompromised patients, as well as transfusion from family members, has become the standard of care. In addition, directed donation from first-degree relatives should be discouraged. Leukodepletion, common in many blood centers, is also effective in significantly lowering TA-GVHD.95
In conclusion, emergencies in acute leukemia embrace multiple disciplines. Clinicians need to be knowledgeable, experienced, and prepared. Such emergent care often precludes exhaustive consultation. By raising these issues, it is hoped that the suggested management described herein, even if incomplete or controversial, will heighten awareness and lead to better care.
Acknowledgments
The authors thank Drs Dan Douer, Hillard Lazarus, and Aaron Rapoport for helpful review of the manuscript and Sarah Farkash and Sonia Kamenetsky for assistance in manuscript preparation.
Authorship
Contribution: T.Z., C.G., M.S.T., and J.M.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacob M. Rowe, Department of Hematology, Shaare Zedek Medical Center, 12 Shmuel Bayit Street, Jerusalem, 91031 Israel; e-mail: rowe@jimmy.harvard.edu.