Abstract
It has previously been reported that VEGF-A stimulates megakaryocyte (MK) maturation in vitro. Here we show that treatment of mice with the isoform VEGF-A165 resulted in a significant increase in circulating numbers of platelets. Using specific VEGFR1 and VEGFR2 blocking mAbs and selective VEGFR1 and 2 agonists, PlGF-2 and VEGF-E, respectively, we show directly that stimulation of VEGFR1, but not VEGFR2, increases circulating platelet numbers in vivo. Using flow cytometric analysis of harvested MKs, we show that while PlGF does not change the absolute numbers of MKs present in the bone marrow and the spleen, it increases both their maturation and cell-surface expression of CXCR4 in the bone marrow. Histology of the bone marrow revealed a redistribution of MKs from the endosteal to the vascular niche in response to both VEGF-A165 and PlGF-2 treatment in vivo. Antagonism of CXCR4 suppressed both the VEGFR1-stimulated redistribution of megakyocytes within the bone marrow compartment and the VEGF-A165–induced thrombocytosis. In conclusion, we define a novel proinflammatory VEGFR1-mediated pathway that stimulates the maturation and up-regulation of CXCR4 on megakaryocytes, leading to their redistribution within the bone marrow environment, thereby enhancing platelet production in vivo.
Introduction
Under homeostatic conditions, megakaryopoiesis is driven primarily by thrombopoietin (TPO) and stem cell factor (SCF).1,2 Patients suffering from congenital amegakaryocytic thrombocytopenia (a mutation to the TPO receptor: cMPL), and mice with genetic deletions of SCF and TPO, therefore exhibit a profound thrombocytopenia.3,4 Within the bone marrow endosteal niche, TPO acts synergistically with other hematopoietic cytokines to stimulate stem cell differentiation toward the megakaryocyte lineage to create promegakaryoblasts.5 Promegakaryoblasts then begin to increase their ploidy content via the process of endomitosis. This process driven by TPO is essential for normal maturation into megakaryocytes (MKs).6,7 Mature MKs then migrate to within close proximity of the bone marrow sinusoidal endothelium to form preplatelet buds, eventually releasing platelets.8,9
Studies of megakaryopoiesis in vitro using CD34+ cell unilineage differentiation cultures have shown that the up-regulation of the chemokine receptor CXCR4 is associated with the maturation of megakaryocytes.10 Although CXCL12 (the ligand for CXCR4) acting alone does not stimulate MK development, it has been reported to act synergistically with TPO to enhance megakaryopoiesis and platelet formation in vitro and in vivo.11-13 The original studies describing the expression of CXCR4 on MKs demonstrated that CXCL12 stimulated their migration in vitro.8,14 More recent studies have shown that adenoviral delivery of CXCL12, resulting in a sustained elevation in plasma CXCL12 over 10 days, increased circulating numbers of platelets in TPO- and MPL-deficient mice. It was shown that platelet production was enhanced, as a result of CXCL12 stimulating the migration of MKs from the endosteal to the vascular niche, increasing their maturation because of interactions with the bone marrow endothelium and positioning MK to release platelets into the circulation.12
Under specific inflammatory conditions, it is likely that inflammatory mediators are released that lead to accelerated megakaryopoiesis in synergy with TPO, for example, IL-6 and IL-11.15 Increased circulating platelets may then contribute to the inflammatory process or tissue repair, for example, by enhancing the recruitment of inflammatory leukocytes or endothelial progenitor cells to the site of tissue injury and angiogenesis.16 Pertinently, a requirement for platelets to stimulate vessel growth both in vivo and in vitro has been observed, as revealed by a lack of angiogenesis in mice rendered thrombocytopenic.17 Likewise, inhibition of MK maturation upstream of platelet production has been shown to suppress angiogenesis.18
Therefore, it can be postulated that accelerated platelet production may also occur during ischemic injury because of alterations in the cytokine milieu within the bone marrow that results in platelets responsive to angiogenic signals. Indeed, we have previously shown that the mobilization of other cell types implicated in angiogenesis, for example, endothelial progenitor cells and mesenchymal stem cells can be significantly enhanced by VEGF-A conditioning of bone marrow.19 Interestingly, VEGFR1 and VEGFR2 expression has been demonstrated on cultured human MKs.20,21 Moreover, under conditions of hypoxia, MKs have been shown to secrete VEGF-A, which acts in an autocrine fashion via VEGFR1 to accelerate MK maturation in vitro.20 In addition, VEGFR2 stimulation of MK precursors has been demonstrated to control their survival and differentiation in vitro. Hitherto, effects of VEGF-A on megakaryocyte maturation and platelet production in vivo have not been studied.
Our results show that VEGF-A165 acting via VEGFR1 can indeed stimulate a significant increase in circulating numbers of platelets by contributing to MK maturation leading to their redistribution from the endosteal to the vascular niche in a CXCR4-dependent manner. Our results therefore uncover a novel VEGFR1-dependent pathway regulating platelet production that could be relevant to the angiogenic response to ischemic injury.22
Methods
Pretreatment with VEGF-A165, PlGF, or VEGF-E
Eight- to 10-week-old female BALB/c mice (Harlan) were administered VEGF-A (murine Isoform VEGF-A165; PeproTech), PIGF (murine PlGF-2; R&D Systems), VEGF-E (ProSpec), all 2.5 μg/mouse intraperitoneally, or vehicle (PBS) on 4 consecutive days, on the basis of preliminary dose-response studies. We have previously reported these dose-response studies to be of sufficient duration to alter migratory properties of stem cell subtypes residing in the bone marrow.19 Twenty-four hours after the last injection, mice were culled and blood was collected via cardiac puncture for enumeration of circulating platelet levels. The femurs were dissected for histologic analysis. In other experiments, mice were administered the CXCR4 antagonist (AMD3100, 5 mg/kg intraperitoneally) or PBS twice daily over 4 consecutive days, concomitant with VEGF-A165 administration. All studies were carried out under the United Kingdom's Animals (Scientific Procedures) Act of 1986 and local ethical approval from Imperial College (London, United Kingdom).
Administration of anti-VEGFR1 and anti-VEGFR2 Abs
In some experiments, VEGF-A165–treated mice were administered previously defined neutralizing doses of either anti-VEGFR1 Ab (50 μg/mouse IP; R&D Systems), anti-VEGFR2 Ab (50μg/mouse IP; R&D Systems), or both, 2 hours before VEGF-A165 administration on days 0 and 2.9,19 Chromopure goat IgG (Jackson ImmunoResearch Laboratories) was administered to control groups.
Enumeration of circulating platelets
Circulating platelets were quantified using stromatol as previously described.23
Bone marrow histology and morphometry
Femurs were fixed in 4% formaldehyde for 48 hours before decalcification (2.5% neutral EDTA for 7 days) to reveal bone marrow. Marrow was processed and embedded in paraffin. Sections were then stained with toluidine blue. Other sections were heated in Tris-EDTA solution (pH 9.0) for 3 × 5 minutes at 95°C to uncover surface Ags, then immunostained for the platelet-specific Ag CD41 (integrin αIIb) using a specific goat anti–mouse polyclonal IgG Ab (Santa Cruz Biotechnology), via a horseradish peroxidase (HRP)–streptavidin complex (Santa Cruz Biotechnology); sections were background stained using Gill hematoxylin. Quantification of MKs residing immediately adjacent to bone marrow sinusoidal blood vessels was performed using a ×100 objective. MKs were identified as very large cells with blue/gray cytoplasm that often had large multilobed nuclei. Sinusoidal blood vessels were identified as having a visibly clear lumen in direct contact (sinusoidal) with the parenchymal cells, with a characteristically flattened endothelial lining around the lumen.12 MKs were identified as being adjacent to the sinusoidal endothelium only if the cell boundary was in contact with the sinusoidal blood vessels. Whole bone marrow sections (12-20 high-power fields) were traversed and scanned for each femur thus incorporating both the metaphysis and distal ends. Data were enumerated by a blinded observer using a 100× objective lens (Zeiss; Achroplan, numerical aperture 1.25) and a Zeiss AxioSkop microscope. Lucia Core System 1.0 (Lucia sro) acquisition software was used.
Flow cytometric analysis of MK CXCR4 expression and determination of MK number and ploidy
For flow cytometric analysis, femurs were removed from mice and the ends removed to expose the bone marrow. This was then gently flushed out and cells resuspended in FACS buffer using a syringe connected to a 23-G needle. Spleens were excised from mice, cut into small pieces, then gently passed through a 100-μm sieve. Splenic cells were then gently flushed as with bone marrow above to create a single-cell suspension. To identify MKs in the bone marrow or from the spleen, surface expression of the thrombopoietic marker CD41 (integrin subunit: αIIb) was determined on cells with a high forward scatter.24,25 CD41+ MKs were then stained for the surface expression of CXCR4. Other samples were analyzed to determine the ploidy status of MKs using a TOPRO-3 DNA stain (Invitrogen Life Technologies). Thus, samples were fixed in ice-cold ethanol (70%) for 1 hour (to permeabilize the cell membrane and allow DNA binding). Samples were again washed with ice-cold PBS before resuspension with 500 μL of TOPRO-3 (1μM), and 50 μL of RNase (100 μg/mL) was added to minimize TOPRO-3 binding to RNA. Samples were then quantified on a FACSAria cell sorter (BD Biosciences) and analyzed using FACSDiva software. A dotplot of TOPRO-width against TOPRO-area was recorded. A TOPRO-area histogram of the gated cells showed the first peak to represent cells in G1, (ploidy number 2n) the second peak cells in 4n, with subsequent peaks representing ploidy numbers of 8n, 16n, 32n, 64n, and 128n.20
Statistical analysis
Data are expressed as mean ± SEM. In vivo circulating platelet data and bone marrow morphometry were analyzed using 1-way ANOVA, followed by Bonferroni multiple-comparisons test, with the exception of experiments where mice were administered CXCR4 antagonist twice daily, where 2-way ANOVA was conducted. Flow cytometric data of CXCR4 expression and MK ploidy were analyzed using the Student t test. All analyses were conducted using the GraphPad Prism statistical package (Version 4.0). P values < .05 were considered significant.
Results
VEGFR1 stimulation increases numbers of circulating platelets
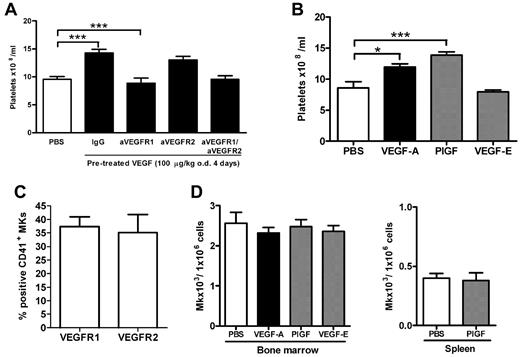
To investigate whether VEGF-A165 could impact on platelet numbers in the blood, mice were treated with VEGF-A165 over a 4-day period. As shown in Figure 1, VEGF-A165 treatment resulted in a significant increase in the number of circulating platelets compared with vehicle-treated control mice (Figure 1A control: 9.5 ± 0.5 × 108 platelets/mL vs VEGF-A165: 14.3 ± 0.6 × 108 platelets/mL; P < .05). Megakaryocytes express VEGFR 1 and 2 which exhibit high- and low-affinity binding for VEGF-A165, respectively. To assess the relative contribution of VEGF-R1 and 2 to the observed thrombocytosis, blocking Abs to either VEGFR1 or VEGFR2 were administered on days 1 and 3 to mice subjected to the 4-day VEGF-A165 treatment. The increased thrombocytosis because of VEGF-A165 administration was completely suppressed in mice administered anti-VEFGR1 Ab (VEGF-A165 + IgG: 14.3 ± 0.6 × 108 platelets /mL vs VEGF-A165 + aVEGFR1 Ab: 8.9 ± 0.9 × 108 platelets/mL; P < .05; Figure 1A). However, the blockade of VEGFR2 with anti-VEGFR2 Ab did not affect raised circulating platelet numbers induced by VEGF-A165 treatment (Figure 1A: 13.0 ± 0.6 × 108 platelets/mL). Furthermore, combined inhibition of both VEGFR1 and VEGFR2 did not further reduce circulating platelets compared with inhibition of VEGFR1 alone (Figure 1A PBS: 9.5 ± 0.5 × 108 platelets/mL vs VEGF-A165 + aVEGFR1 and aVEGFR2 Abs: 9.6 ± 0.6 × 108 platelets/mL). Because blockade of VEGFR1 did not suppress circulating levels of platelets beyond the control baseline, these data suggest that VEGFR1 activation has an inflammatory or angiogenic role in regulating platelet production rather than a homeostatic role (Figure 1A).
VEGFR1 stimulation, but not VEGFR2 stimulation, is responsible VEGF-A165–induced thrombocytosis. (A-B) Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally), PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days. (A) Some groups of mice receiving the 4-day VEGF-A165 regimen were also administered blocking Abs to either VEGFR1, VEGFR2, VEGFR1&2, or control IgG 20 minutes before VEGF-A165 administration on days 1 and 3. Enumeration of circulating platelets was then conducted 24 hours after last growth factor administration. Bone marrow or spleens were also harvested, and MKs were identified by flow cytometry via forward and side scatter and CD41 expression. (C) Percentage of MKs expressing VEGFR1 and VEGFR2 in vehicle control–treated mice. (D) The number of MKs per million cells from bone marrow or spleen; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05; ***P < .001 compared with control or as indicated.
VEGFR1 stimulation, but not VEGFR2 stimulation, is responsible VEGF-A165–induced thrombocytosis. (A-B) Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally), PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days. (A) Some groups of mice receiving the 4-day VEGF-A165 regimen were also administered blocking Abs to either VEGFR1, VEGFR2, VEGFR1&2, or control IgG 20 minutes before VEGF-A165 administration on days 1 and 3. Enumeration of circulating platelets was then conducted 24 hours after last growth factor administration. Bone marrow or spleens were also harvested, and MKs were identified by flow cytometry via forward and side scatter and CD41 expression. (C) Percentage of MKs expressing VEGFR1 and VEGFR2 in vehicle control–treated mice. (D) The number of MKs per million cells from bone marrow or spleen; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05; ***P < .001 compared with control or as indicated.
Consistent with these findings, administration of the VEGFR1 selective ligand, murine placental growth factor 2 (PlGF-2), also significantly increased circulating platelet numbers (Figure 1B control: 8.6 ± 0.9 × 108 platelets/mL vs 13.8 ± 0.5 × 108 platelets/mL; P < .001), whereas the VEGFR2 selective ligand, VEGF-E, did not significantly raise circulating platelet numbers above baseline (8.0 ± 0.3 × 108 platelets/mL; Figure 1B). Neither VEGF-A165 nor PlGF-2 had any effect on circulating leukocyte numbers (data not shown).
Effect of VEGFR1 and VEGFR2 on MK numbers
Cultured human MKs express both VEGFR1 and VEGFR2, and it has previously been reported that VEGFR2 is expressed at low levels on early quiescent MKs, but is selectively re-expressed on mature terminal MKs.20,26 In vitro VEGFR2 stimulation of cultured human MKs has been shown to promote MK proliferation (mitosis) and survival21 while VEGFR1 activation potentiates maturation of hematopoietic progenitor cell–derived MKs as measured by increases in DNA ploidy content (endomitosis).20 Therefore, we next examined the effect of VEGF on MK proliferation and maturation in vivo. Initially, we confirmed that murine MKs harvested from the bone marrow express both VEGFR1 and 2 (Figure 1C). Treatment of mice with VEGF-A165, PlGF-2, or VEGF-E had no impact on the absolute numbers of MKs in the bone marrow (Figure 1D). Because it is recognized that megakaryocyte progenitors can traffic from the bone marrow to the spleen, it is possible that the increase in circulating platelet numbers represents an increase in MK numbers in the spleen.27 However, we were unable to identify an increase in splenic MK numbers after PlGF-2 treatment (Figure 1D).
Effect of VEGFR1 and VEGFR2 on MK maturation
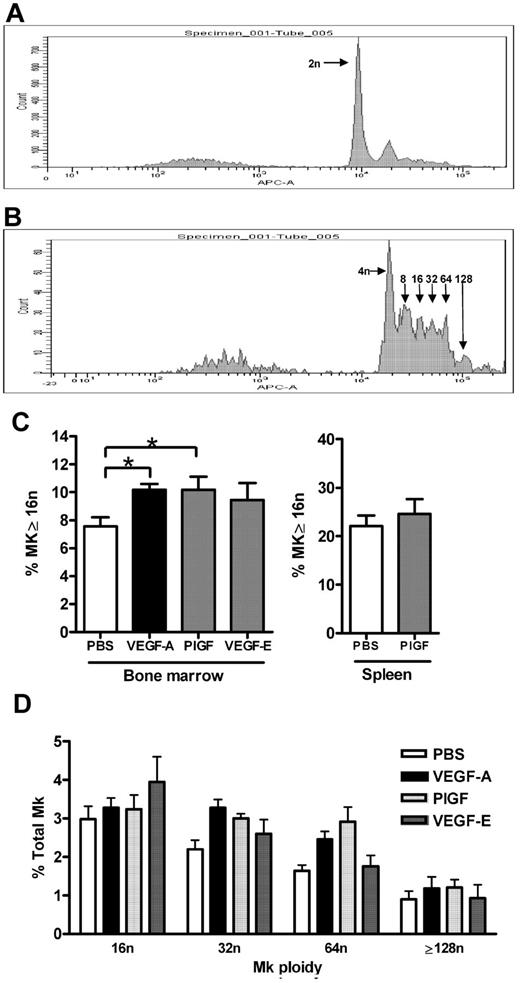
MK maturation status was measured as ploidy content by flow cytometry, representative histograms revealing successive peaks of arithmetic increases in ploidy content on CD41+ MKs via TOPRO-3 staining are shown in Figure 2A and B. Analysis of these data revealed that VEGF-A165 and PlGF-2 treatment of mice induced a 25% increase in the percentage of bone marrow MKs with a ploidy content the same or greater than 16n compared with control mice (Figure 2C vehicle: 7.5% ± 0.7% vs VEGF-A165: 10.2% ± 0.4%; PlGF-2: 10.2% ± 0.9%; P < .05). Plots of individual ploidy (16n-128n) reveal that this increase in ploidy content after VEGF-A165 or PlGF-2 treatment is spread evenly across MK populations with a high ploidy number (Figure 2D). In contrast, VEGF-E administration did not significantly affect MK ploidy content (9.4% ± 1.2%; Figure 2C). Given that the spleen is also a site of megakaryopoiesis, we investigated whether VEGFR1 stimulation affected splenic MK maturation. Notably, while the proportion of mature MKs (≥ 16n) is higher in the spleen compared with the bone marrow, PlGF-2 treatment did not affect splenic MK ploidy content (Figure 2C). Thus, VEGFR1 stimulation of MKs via VEGF-A165 or PlGF-2 administration led to increased bone marrow MK maturation in vivo.
Effect of VEGFR1 and VEGFR2 stimulation on MK maturation. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally), PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days. (A-B) Representative histograms of CD41+ MKs stained with TOPRO-3 revealing respective peaks of increasing DNA ploidy number. (A) CD41+ MKs with 2n, and (B) 2n peak subtracted to reveal 4n to 128n. (C) Percentage of bone marrow and splenic MKs with a ploidy number of 16n or greater in mice administered either VEGF-A165, PlGF-2, or VEGF-E. (D) Histogram revealing spread of bone marrow MK ploidy above 16n in mice administered either VEGF-A165, PlGF-2, or VEGF-E; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05 compared with control.
Effect of VEGFR1 and VEGFR2 stimulation on MK maturation. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally), PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days. (A-B) Representative histograms of CD41+ MKs stained with TOPRO-3 revealing respective peaks of increasing DNA ploidy number. (A) CD41+ MKs with 2n, and (B) 2n peak subtracted to reveal 4n to 128n. (C) Percentage of bone marrow and splenic MKs with a ploidy number of 16n or greater in mice administered either VEGF-A165, PlGF-2, or VEGF-E. (D) Histogram revealing spread of bone marrow MK ploidy above 16n in mice administered either VEGF-A165, PlGF-2, or VEGF-E; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05 compared with control.
VEGFR1 stimulation redistributes MKs around vascular niche
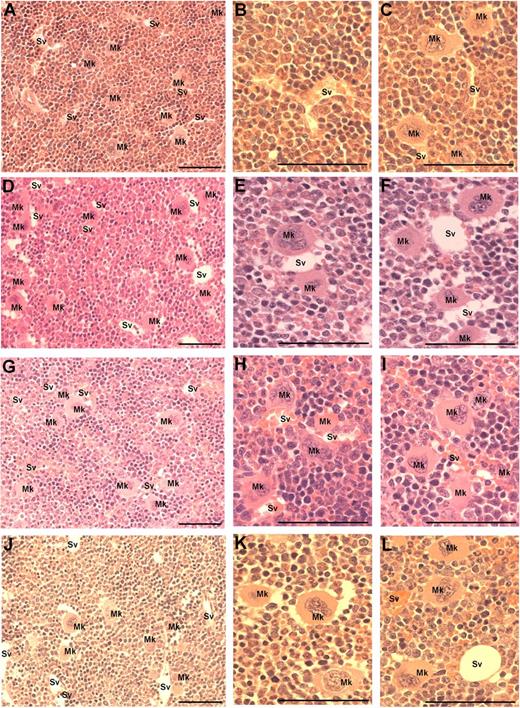
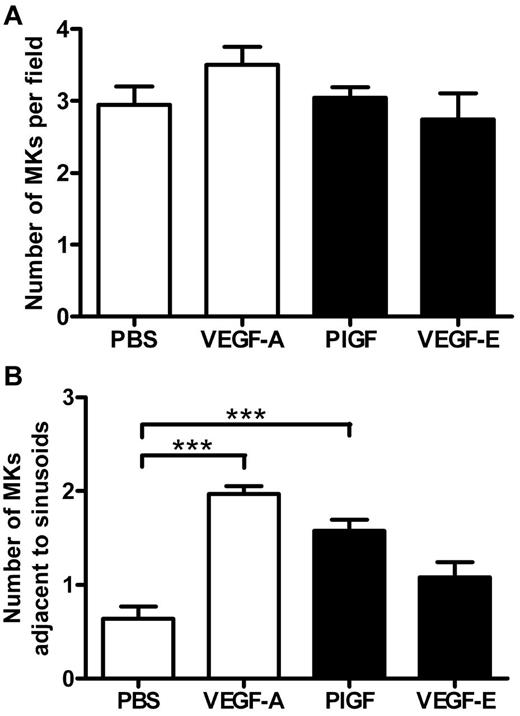
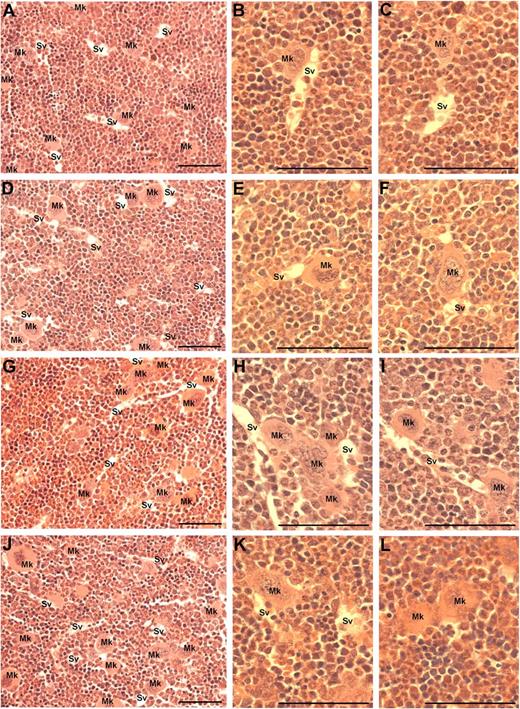
Because MK migration to sinusoidal vessels is a requisite step for platelet production and release into the circulation, we investigated whether the administration of VEGF-A165, PlGF-2, or VEGF-E altered MK localization within the bone marrow. The femurs of mice were processed to allow the detection of MKs and sinusoidal vessels in relation to bone marrow morphology (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Importantly, serial sections were immunostained with the MK Ag CD41 to confirm the correct identification and enumeration of MKs (supplemental Figure 1A-B). MKs can be clearly observed in standard toluidine blue–stained sections as very large, often with multilobed nuclei with blue/gray cytoplasm (supplemental Figure 1C-F). Representative photomicrographs at both ×40 and ×100 magnification reveal the distribution of MKs in murine bone marrow treated with PBS (Figure 3A-C), VEGF-A165 (Figure 3D-F), PlGF-2 (Figure 3G-I), and VEGF-E (Figure 3J-L) Quantification of the number of MKs per high-power field revealed no difference in the number of MKs per field section in either vehicle, VEGF-A165–, PlGF-2–, or VEGF-E–treated mice (Figure 4A). However, when we assessed the localization of MKs, we observed a significant increase in the number of MKs adjacent to sinusoidal vessels in VEGF-A165– and PlGF-2–treated mice compared with vehicle-treated controls (Figure 4B vehicle: 0.6 ± 0.1 MKs adjacent to sinusoids/field; VEGF-A165: 2.0 ± 0.1 MKs adjacent to sinusoids/field; PlGF: 1.6 ± 0.1 MKs adjacent to sinusoids/field; P < .001). No difference was observed between VEGF-E–treated mice or vehicle control (Figure 4B VEGF-E: 1.0 ± 0.2 MKs adjacent to sinusoids/field). Importantly, we have recently reported that VEGF-A165 administration does not alter the number of sinusoidal vessels in the bone marrow and therefore increased MK localization to sinusoidal vessels in mice conditioned with VEGF-A165 or PlGF-2 was not because of increased vessel density.19 These results suggest that VEGFR1 stimulation of MKs was responsible for their enhanced clustering around sinusoidal vessels.
Visualization of MKs in bone marrow sections from mice treated with VEGF-A165, PlGF-2, VEGF-E, or vehicle. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) daily for 4 consecutive days before femurs were excised for bone marrow histology. (A) Representative photomicrograph of bone marrow stained with toluidine blue reveal the distribution of sinusoidal vessels (Sv) and MKs (Mk) from a PBS-treated mouse. (B-C) Higher-power magnification of femurs from PBS-treated mice showing localization of MKs in relation to sinusoidal vessels and the stroma. (D) Representative photomicrograph of bone marrow from VEGF-A165–treated mouse reveals a more pronounced localization of MKs to sinusoidal vessels. (E-F) Higher-power confirmation of MK localized directly adjacent to sinusoidal vessels. (G-I) Representative photomicrographs of bone marrow from PlGF-2–treated mouse reveals a similar distribution pattern of MKs adjacent to sinusoidal vessels to that of VEGF-A165–treated mice. (J-L) Representative photomicrographs of bone marrow from VEGF-E–treated mouse reveals MKs distributed within the bone marrow stroma, with the occasional MK adjacent to a sinusoidal vessel. (A,D,G,J) Magnification:×40. (B,C,E,F,H,I,K,L) Magnification: ×100. Photomicrographs at ×100 magnification have been turned 90° using Microsoft Powerpoint (to allow photomicrographs to fit inside figure). Bar represents 50 μm.
Visualization of MKs in bone marrow sections from mice treated with VEGF-A165, PlGF-2, VEGF-E, or vehicle. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) daily for 4 consecutive days before femurs were excised for bone marrow histology. (A) Representative photomicrograph of bone marrow stained with toluidine blue reveal the distribution of sinusoidal vessels (Sv) and MKs (Mk) from a PBS-treated mouse. (B-C) Higher-power magnification of femurs from PBS-treated mice showing localization of MKs in relation to sinusoidal vessels and the stroma. (D) Representative photomicrograph of bone marrow from VEGF-A165–treated mouse reveals a more pronounced localization of MKs to sinusoidal vessels. (E-F) Higher-power confirmation of MK localized directly adjacent to sinusoidal vessels. (G-I) Representative photomicrographs of bone marrow from PlGF-2–treated mouse reveals a similar distribution pattern of MKs adjacent to sinusoidal vessels to that of VEGF-A165–treated mice. (J-L) Representative photomicrographs of bone marrow from VEGF-E–treated mouse reveals MKs distributed within the bone marrow stroma, with the occasional MK adjacent to a sinusoidal vessel. (A,D,G,J) Magnification:×40. (B,C,E,F,H,I,K,L) Magnification: ×100. Photomicrographs at ×100 magnification have been turned 90° using Microsoft Powerpoint (to allow photomicrographs to fit inside figure). Bar represents 50 μm.
VEGFR1 stimulation redistributes MKs to the vascular niche. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days before femurs were excised for bone marrow histology. (A) Quantification of MKs per field of view, and (B) quantification of the number of MKs adjacent to sinusoidal vessels; n = 5-6 animals per group. Data are expressed as mean ± SEM; ***P < .001 compared with control.
VEGFR1 stimulation redistributes MKs to the vascular niche. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days before femurs were excised for bone marrow histology. (A) Quantification of MKs per field of view, and (B) quantification of the number of MKs adjacent to sinusoidal vessels; n = 5-6 animals per group. Data are expressed as mean ± SEM; ***P < .001 compared with control.
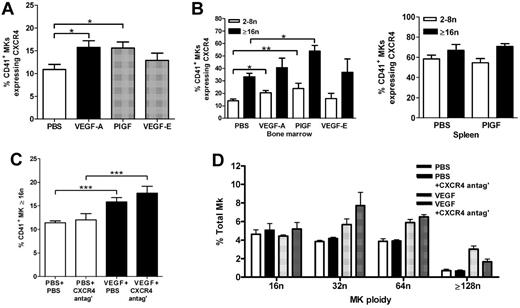
VEGFR1 increases MK CXCR4 expression
It has previously been reported that the expression of CXCR4 increases on the surface of MKs as they undergo maturation.10 Furthermore, transendothelial migration of cultured MKs in response to the CXCR4 ligand CXCL12 has been reported to enhance platelet production in vitro.9 We therefore examined whether CXCR4 expression on the surface of MKs harvested from the bone marrow is affected by either VEGFR1 or VEGFR2 treatment of mice in vivo. As shown in Figure 5A, the percentage of MKs expressing CXCR4 significantly increased in mice treated with either VEGF-A165 or PlGF-2 compared with vehicle-treated mice (Figure 5A vehicle: 10.9% ± 1.1%, VEGF-A165: 15.7% ± 1.4%, PlGF-2: 15.6% ± 1.3%, P < .05). VEGF-E treatment of mice had no effect on CXCR4 expression (VEGF-E: 12.8% ± 1.6%, Figure 5A). Thus, VEGFR1 stimulation, but not VEGFR2 stimulation, increased CXCR4 expression on the surface of MKs in vivo. We next determined whether CXCR4 expression increased on MKs with a higher ploidy number. It was apparent that while CXCR4 was expressed on a greater percentage of MKs derived from the spleen, compared with MKs derived from the bone marrow (Figure 5B), a significantly raised expression of CXCR4 on MKs with a ploidy the same or greater than 16n compared with MKs with a ploidy between 2n and 8n was only observed in MKs derived from the bone marrow and not in MKs derived from the spleen (Figure 5B bone marrow PBS 2n-8n: 13.9% ± 1.5% vs PBS ≥ 16n: 33.3% ± 2.7%; P < .001). Interestingly, there was a significant increase in the percentage of bone marrow–derived MKs expressing CXCR4 in mice treated with PIGF-2 compared with vehicle-treated control mice (Figure 5B PBS 2n-8n: 13.9% ± 1.5% vs PlGF-2 2n-8n: 23.8% ± 4.3%, P < .05; PBS ≥ 16n: 33.3% ± 2.7% vs PlGF-2 ≥ 16n: 53.9% ± 4.6%, P < .01). Treatment of mice with VEGF-E did not result in an increased expression of CXCR4 on bone marrow–derived MKs compared with vehicle-treated control animals, either with a low (2n-8n) or higher (≥ 16n) ploidy number (Figure 5B). PlGF-2 treatment did not increase the expression of CXCR4 on splenic MKs compared with vehicle-treated control mice (Figure 5B). These results show that CXCR4 expression on bone marrow–derived MKS increases with ploidy number, and that VEGFR1 stimulation, but not VEGFR2 stimulation, increases this expression further.
VEGFR1 increases MK migration to vascular niche via a CXCR4-dependent process. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days before bone marrow and spleens were harvested 24 hours later. (A) Percentage of CD41+ MKs expressing CXCR4. (B) Percentage of 2n-8n and ≥ 16n CD41+ MKs expressing CXCR4 in bone marrow and spleen. Other groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment for evaluation of MK ploidy content. (C) Bone marrow MKs analyzed for ploidy content using TOPRO-3 24 hours after the last VEGF-A165 injection. (D) Histogram revealing spread of bone marrow MK ploidy above 16n; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05, **P < .01, and ***P < .001 compared with control or as indicated.
VEGFR1 increases MK migration to vascular niche via a CXCR4-dependent process. Mice were administered either VEGF-A165, PlGF-2, VEGF-E (100 μg/kg intraperitoneally), or vehicle (PBS) each day for 4 consecutive days before bone marrow and spleens were harvested 24 hours later. (A) Percentage of CD41+ MKs expressing CXCR4. (B) Percentage of 2n-8n and ≥ 16n CD41+ MKs expressing CXCR4 in bone marrow and spleen. Other groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment for evaluation of MK ploidy content. (C) Bone marrow MKs analyzed for ploidy content using TOPRO-3 24 hours after the last VEGF-A165 injection. (D) Histogram revealing spread of bone marrow MK ploidy above 16n; n = 5-6 animals per group. Data are expressed as mean ± SEM; *P < .05, **P < .01, and ***P < .001 compared with control or as indicated.
We next determined whether CXCR4 expression was secondary to VEGF in inducing MK maturation, because it has been reported that CXCL12/CXCR4 can induce maturation and proplatelet formation in vitro.11-13 Groups of mice were treated with VEGF-A165 or vehicle over 4 days in the presence or absence of the CXCR4 antagonist and bone marrow–derived MKs subsequently analyzed for DNA ploidy content. The percentage of MKs with a ploidy content the same or greater than 16n was significantly increased in VEGF-A165–treated mice compared with vehicle controls (Figure 5C vehicle: 11.4% ± 0.4%, VEGF-A165: 15.9% ± 0.9%, P < .001.). However, there was no significant difference between respective groups with prolonged administration of the CXCR4 antagonist (Figure 5C CXCR4 antagonist: 12.0% ± 1.3%, VEGF-A165 + CXCR4 antagonist: 17.7% ± 1.5%). Plots of individual ploidy (16n-128n) reveal that this increase in ploidy content after VEGF-A165 or VEGF-A165 + CXCR4 antagonist treatment is spread evenly across MK populations with high ploidy number (Figure 5D). This suggests that enhanced CXCL12 stimulation of MKs via increased CXCR4 expression was not itself responsible for accelerated MK maturation.
VEGFR1 increases MK migration to vascular niche via a CXCR4-dependent process
Evidence from other studies reveals CXCR4 expression increases with MK maturation in vitro, and that preferential migration of polyploidy MKs compared with MKs with 2n ploidy content occurs in response to CXCL12.8,10 We therefore next examined whether VEGFR1-induced CXCR4 expression was responsible for the relocalization of MKs to the vascular niche. Representative photomicrographs at both 40× and 100× magnification reveal the distribution of MKs in murine bone marrow treated with PBS (Figure 6A-C), CXCR4 antagonist (Figure 6D-F), VEGF-A165 (Figure 6G-I), and VEGF-A165 + CXCR4 antagonist (Figure 6J-L). As shown previously, although there was no change in the total number of MKs observed per high-power field (Figure 7A), VEGF-A165 induced MK clustering around the sinusoidal vessels (Figure 7B). Importantly, we show here that VEGF-A165–induced relocalization of MKs to the vascular niche was significantly suppressed by treatment of the mice with prolonged treatment of the CXCR4 antagonist (Figure 7B VEGF-A165: 3.0 ± 0.2 MKs adjacent to sinusoids/field; VEGF-A165 + CXCR4 antagonist: 1.5 ± 0.2 MKs adjacent to sinusoids/field; P < .001). We have previously reported that CXCL12 levels are similar in vehicle and VEGF-A165–treated mice.19 Thus, we surmise that activation of VEGFR1 leads to increased expression of CXCR4 on the surface of polyploidy MKs, driving their redistribution around the sinusoidal vessels.
Visualization of MKs in bone marrow sections from mice treated with VEGF-A165 or vehicle and CXCR4 antagonist. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally) or vehicle (PBS) each day for 4 consecutive days. Some groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment. Twenty-four hours after final AMD3100 administration, femurs were excised for bone marrow histology. (A) Representative photomicrograph of bone marrow stained with toluidine blue reveal the distribution of sinusoidal vessels (Sv) and MKs (Mk) from a PBS-treated mouse. (B-C) Higher-power magnification of femurs from PBS-treated mice showing localization of MKs in relation to sinusoidal vessels and the stroma. (D-F) Representative photomicrographs of bone marrow from a PBS + CXCR4 antagonist–treated mouse reveals a similar distribution pattern of MKs within the bone marrow stroma to that of PBS-treated mice. (G) Representative photomicrograph of bone marrow from VEGF-A165 + PBS–treated mouse reveals a more pronounced localization of MKs to sinusoidal vessels. (H-I) Higher-power confirmation of MK localized directly adjacent to sinusoidal vessels. (J-L) Representative photomicrographs of bone marrow from VEGF-A165 + CXCR4 antagonist–treated mouse reveals MKs distributed within the bone marrow stroma, with the occasional MK adjacent to a sinusoidal vessel. (A,D,G,J) Magnification: ×40. (B,C,E,F,H,I,K,L) Magnification: ×100. Photomicrographs at ×100 magnification have been turned 90° using Microsoft Powerpoint (to allow photomicrographs to fit inside figure). Bar represents 50 μm.
Visualization of MKs in bone marrow sections from mice treated with VEGF-A165 or vehicle and CXCR4 antagonist. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally) or vehicle (PBS) each day for 4 consecutive days. Some groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment. Twenty-four hours after final AMD3100 administration, femurs were excised for bone marrow histology. (A) Representative photomicrograph of bone marrow stained with toluidine blue reveal the distribution of sinusoidal vessels (Sv) and MKs (Mk) from a PBS-treated mouse. (B-C) Higher-power magnification of femurs from PBS-treated mice showing localization of MKs in relation to sinusoidal vessels and the stroma. (D-F) Representative photomicrographs of bone marrow from a PBS + CXCR4 antagonist–treated mouse reveals a similar distribution pattern of MKs within the bone marrow stroma to that of PBS-treated mice. (G) Representative photomicrograph of bone marrow from VEGF-A165 + PBS–treated mouse reveals a more pronounced localization of MKs to sinusoidal vessels. (H-I) Higher-power confirmation of MK localized directly adjacent to sinusoidal vessels. (J-L) Representative photomicrographs of bone marrow from VEGF-A165 + CXCR4 antagonist–treated mouse reveals MKs distributed within the bone marrow stroma, with the occasional MK adjacent to a sinusoidal vessel. (A,D,G,J) Magnification: ×40. (B,C,E,F,H,I,K,L) Magnification: ×100. Photomicrographs at ×100 magnification have been turned 90° using Microsoft Powerpoint (to allow photomicrographs to fit inside figure). Bar represents 50 μm.
VEGF-A165 increases MK migration to vascular niche via a CXCR4-dependent process. Prolonged CXCR4 antagonism suppresses VEGF-A165–induced thrombocytosis. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally) or vehicle (PBS) each day for 4 consecutive days. Some groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment. Femurs were excised for bone marrow histology, stained with toluidine blue, and then analyzed for the number of MKs (A) per field of view and (B) adjacent to sinusoidal vessels. (C) Circulating platelet numbers were enumerated 24 hours after last growth factor administration. Data are expressed as mean ± SEM; n = 5-6 animals per group; **P < .01 and ***P < .001 compared with control or as indicated compared with control.
VEGF-A165 increases MK migration to vascular niche via a CXCR4-dependent process. Prolonged CXCR4 antagonism suppresses VEGF-A165–induced thrombocytosis. Mice were administered either VEGF-A165 (100 μg/kg intraperitoneally) or vehicle (PBS) each day for 4 consecutive days. Some groups of mice were administered a CXCR4 antagonist (AMD3100 5 mg/kg) or PBS b.i.d. (bis in die: twice-daily dosing) for the 4 days of VEGF-A165 treatment. Femurs were excised for bone marrow histology, stained with toluidine blue, and then analyzed for the number of MKs (A) per field of view and (B) adjacent to sinusoidal vessels. (C) Circulating platelet numbers were enumerated 24 hours after last growth factor administration. Data are expressed as mean ± SEM; n = 5-6 animals per group; **P < .01 and ***P < .001 compared with control or as indicated compared with control.
VEGF-stimulated platelet production is dependent on CXCR4 activity
The ability of CXCL12 (but not VEGF-A) to stimulate chemotaxis and transendothelial migration of MKs in vitro is believed to be the process by which CXCL12 enhances platelet formation.8,9,14 In this study, we show that the VEGF-A165–induced thrombocytosis is completely abrogated in mice treated with the CXCR4 antagonist, AMD3100 (Figure 7C VEGF-A165: 14.2 ± 0.1 × 108 platelets/mL, vs VEGF-A165 + CXCR4 antagonist: 10.0 ± 0.1 × 108 platelets/mL; P < .01). Notably, the administration of CXCR4 antagonist alone had no effect on MK localization compared with vehicle-treated controls (Figure 7B vehicle: 1.3 ± 0.1 MK/field; CXCR4 antagonist: 1.2 ± 0.1 MK/field) or circulating platelet numbers (Figure 7C vehicle: 10.0 ± 0.1 × 108 platelets/mL, vs vehicle + CXCR4 antagonist: 11.7 ± 0.1 × 108 platelets/mL).
Discussion
Taken together, our results suggest that activation of VEGFR1 stimulates the up-regulation of CXCR4 on mature MKs derived from the bone marrow, promoting their relocalization from the endosteal to the vascular niche, thereby enhancing platelet production. These data generated by studying platelet production in vivo identify a molecular pathway linking VEGFR1 and CXCR4 on MKs to platelet production. We propose that VEGF-A165 may represent another mediator that has the capacity to enhance platelet production in vivo. Although the spleen is a recognized organ of megakaryopoiesis and therefore platelet production after trauma, we were unable to report a role for the spleen in this VEGFR1-dependent process.27
The expression of members of the VEGF family of growth factors is well known during periods of hypoxia, and to be integral to angiogenesis, but there is also evidence that platelets are critical for the angiogenic response in wound healing because they are a most abundant source of angiogenic growth factors.17,18 Furthermore, increased thrombocytosis occurs in conditions associated with enhanced angiogenesis, suggesting that mediators of angiogenesis may influence platelet production.28-30 Because circulating platelet numbers are controlled by TPO-induced megakaryopoiesis, it has been demonstrated that angiogenic responses are suppressed in TPO-deficient mice.3 Notably in this study, the suppressive effect of thrombocytopenia on the angiogenic response was corrected by gene transfer of VEGF-A.3 Putting these observations in the context of the work presented in this manuscript, we reveal that VEGFR1 activation (via VEGF-A165 or PlGF-2 stimulation) results in enhanced MK endomitosis (maturation) in vivo. Notably, enhanced MK production was not evident, perhaps mimicking conditions of short-term hypoxia whereby VEGF-A stimulates MK maturation without an increment in MK proliferation.20,31 It has been reported that a wider role for endogenous VEGF-A and PlGF-2 is required for hematopoiesis within the adult bone marrow, whereby VEGF-A and PlGF-2 signal via VEGFR1 to regulate HSC proliferation and survival.32 Indeed, PlGF-2 administration has been shown to restore hematopoiesis after bone marrow suppression in mice, while anti-VEGFR1, but not anti-VEGFR2 Abs decreased cell cycling and survival of HSCs after engraftment.32,33 Furthermore, PlGF-2 is able to contribute to the mobilization of HSCs and hematopoietic progenitor cells from the bone marrow when combined with prolonged G-CSF administration, although PlGF does not induce mobilization when administered in isolation.19,34 Notably, under conditions of steady-state hematopoiesis, inhibition of VEGFR1 activation does not result in a profound impairment of hematopoiesis in vivo or hematopoietic progenitor cell colony formation in vitro.32,33 Similarly, here we show that inhibition of VEGF-A165 with a VEGFR1 blocking Ab did not reduce circulating platelet numbers below the vehicle control baseline (presumably steady-state thrombopoiesis). Likewise, prolonged CXCR4 antagonism led to a reversal of VEGF-A165–induced MK clustering around bone marrow sinusoidal vessels, but did not affect homeostatic localization of MKs. These data suggest the role of VEGF-A165 in MK maturation and CXCL12/CXCR4 on MK migration culminating in platelet release is confined to inflammatory and/or hypoxic conditions leading to angiogenesis rather than homeostatic release of platelets.
CXCL12 is expressed constitutively at high levels in the bone marrow and previous studies have shown that it is critical for localizing mature MKs to the vascular niche.12 We show here, that VEGF-A165, acting via VEGFR1 up-regulates CXCR4 on MKs. CXCR4 expression is further raised on MKs with a higher ploidy number, increasing their localization to the vascular niche. Similarly, it was recently reported that in a murine model of hypercholesterolemia, a condition associated with increased platelet levels, that MKs formed large clusters in close contact with bone marrow sinusoidal vessels.35
We show here that expression of CXCR4 is increased on MKs in vivo, in mice treated with VEGF-A165 or PlGF-2, but not VEGF-E (VEGFR2 stimulation). This direct link between VEGFR1 stimulation and CXCR4 expression represents the molecular pathway by which mature MKs were able to migrate from their bone marrow niche to the vascular niche via a CXCL12 gradient because antagonism of CXCR4 profoundly repressed both VEGFR1–induced MK localization and increased platelet production. This is supported by evidence from other studies revealing CXCR4 expression increases with MK maturation in vitro, and that preferential migration of polyploidy MKs compared with MKs with a 2n ploidy content occurs.8-10 CXCL12 has also been reported to increase circulating platelet numbers by enhancing thrombopoiesis and MK maturation.11-13 However, the ability of CXCL12 (but not VEGF) to stimulate chemotaxis and transendothelial migration of MKs in vitro, is believed to be the process by which CXCL12 enhances platelet formation.8,9,14 We therefore report in vivo for the first time that the role of CXCL12 and CXCR4 is confined to MK migration and is downstream of VEGF-A165 activation.
Endothelial cells also express VEGFR1 and this receptor plays a critical role in vascular development.36,37 It is possible therefore that the sinusoidal endothelium is also activated after PlGF-2 administration and this may contribute to the phenomenon of MK maturation, localization, and platelet production. However, the precise role and importance of VEGFR1 compared with VEGFR2 on mature endothelium is the subject of continued investigation, as VEGFR1 has been variously hypothesized as a “decoy” receptor to VEGFR2; or conversely to provide an amplification to VEGFR2-dependent processes during pathologic angiogenesis.36,38,39
It is not understood why VEGF-A165, or other angiogenic mediators, might induce further platelet production above a huge reservoir of circulating platelets. However, platelets have been implicated in both processes of angiogenesis and inflammation where platelet behavior is distinct from their well-understood function in thrombosis and hemostasis.16 Furthermore, recent evidence suggests distinct mediators, and in particular pro- and antiangiogenic mediators are packaged in separate platelet compartments and can be differentially secreted.40-42 It is not known whether such differences in functional characteristics, or packaging of granule proteins, occurs before MK maturation in the bone marrow. However, synthesized proteins (eg, PF4/VWF) are detected early in the differentiation of 2n MKs expressing CD41.43 It is therefore possible that differences in the platelet secretome occur at the level of MK differentiation in the bone marrow, and that the inflammatory or hypoxic environment affects this.
In summary, while previous studies have shown that VEGF-A can stimulate the maturation of MKs in vitro, this is the first study to demonstrate a VEGF-A165–dependent thrombocytosis in vivo. Moreover, at a molecular level, we have shown that VEGF-A165, acting via VEGFR1, stimulates the maturation of MKs that is associated with the up-regulation of CXCR4. Expression of CXCR4 on mature MKs is critical for their relocalization from the endosteal to the vascular niche, thereby enhancing platelet production. We propose that enhanced platelet production may contribute to the angiogenic effects of VEGF-A165 acting on VEGFR1 observed in vivo.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partly funded by The European Community INNOCHEM (LSHB-CT-2005-518167).
Authorship
Contribution: S.C.P. designed and performed the research, analyzed the data, and wrote the manuscript; T.L. designed the research and contributed to the manuscript; and S.M.R designed the research and wrote the manuscript.
Conflict-of-interest disclosure: T.L. is an employee of Genzyme Corporation. The remaining authors declare no competing financial interests.
Correspondence: Dr Simon C. Pitchford, Sackler Institute of Pulmonary Pharmacology, Pharmaceutical Sciences Division, Room 5.17 Franklin Wilkins Bldg, Waterloo Campus, King's College London, London, United Kingdom, SE9 1NH; e-mail: simon.pitchford@kcl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal