Abstract
Erythropoiesis is a dynamic, multistep process whereby hematopoietic stem cells differentiate toward a progressively committed erythroid lineage through intermediate progenitors. Although several downstream signaling molecules have been identified that regulate steady-state erythropoiesis, the major regulators under conditions of stress remain poorly defined. Rho kinases (ROCKs) belong to a family of serine/threonine kinases. Using gene-targeted ROCK1-deficient mice, we show that lack of ROCK1 in phenylhydrazine-induced oxidative stress model results in enhanced recovery from hemolytic anemia as well as enhanced splenic stress erythropoiesis compared with control mice. Deficiency of ROCK1 also results in enhanced survival, whereas wild-type mice die rapidly in response to stress. Enhanced survivability of ROCK1-deficient mice is associated with reduced level of reactive oxygen species. BM transplantation studies revealed that enhanced stress erythropoiesis in ROCK1-deficient mice is stem cell autonomous. We show that ROCK1 binds to p53 and regulates its stability and expression. In the absence of ROCK1, p53 phosphorylation and expression is significantly reduced. Our findings reveal that ROCK1 functions as a physiologic regulator of p53 under conditions of erythroid stress. These findings are expected to offer new perspectives on stress erythropoiesis and may provide a potential therapeutic target in human disease characterized by anemia.
Introduction
Erythropoiesis is a multistep process in which multipotential hematopoietic stem cells (HSCs) differentiate toward a progressively committed erythroid lineage through intermediate progenitors, specifically, burst forming units-erythroid (BFU-E), followed sequentially by colony forming units-erythroid (CFU-E), proerythroblasts, erythroblasts, reticulocytes and, finally, enucleated erythrocytes.1 The regulation of erythropoiesis is stringent and maintained by the combined effects of transcription factors, GATA 1, Fog1 zinc-finger factors, forkhead factor 3a (Foxo3a), and erythroid Krüppel-like factor (Eklf), which are essential for recruitment of hematopoietic stem cells into the erythroid lineage.2,3 Hormones and cytokines, such as erythropoietin (EPO), stem cell factor (SCF), glucocorticoids, bone morphogenic protein-4 (BMP-4) are important for erythroid cell proliferation, sequential differentiation, maturation, and survival.4-8 Under normal physiologic conditions, erythropoiesis takes place primarily in the BM of humans and mice. Under erythroid stress conditions in mice, such as anemia or hypoxia, splenic erythropoietic population increases because of BMP4-dependent stress erythropoiesis resulting in extramedullary erythropoiesis.5,9,10
Efficient erythropoiesis is crucial for the survival and recovery from various pathophysiologic conditions including blood loss, anemia, and therapeutic procedures used in the treatment of hematologic malignancies including chemotherapy and stem cell transplantation. Impaired or defective stress erythropoiesis can be fatal under these conditions. To develop improved strategies to stimulate stress hematopoiesis in patients undergoing chemotherapy and stem cell transplantation, it is critical to identify the molecular mechanisms that regulate this process. Although many downstream signaling molecules and pathways have been elucidated that regulate steady-state erythropoiesis, the major regulators of stress erythropoiesis remain to be defined.
Rho GTPases are intracellular signaling proteins implicated in numerous cellular functions, including cytoskeletal reorganization, membrane trafficking and cellular proliferation.11 Rac, Cdc42, and Rho are the most frequently studied Rho GTPases.12 Rho cycles between the GDP-bound inactive form and the GTP-bound active form, which binds to specific targets to exert its biologic functions. As a downstream effector of RhoA, Rho kinases (ROCKs) are ubiquitously expressed serine/threonine kinases.13 Two closely related Rho kinases, ROCK1 and ROCK2, have been identified as key downstream effectors of Rho GTPases and contribute to multiple cytoskeleton functions in nonhematopoietic cells and inflammatory cells. ROCK contributes to the formation of focal adhesions and stress fibers, motility, proliferation, differentiation, and apoptosis.14,15 Although ROCK1 and ROCK2 share significant sequence homology in the kinase domain (> 90%), the regulatory domains at the C-terminus show significant divergence.16 Although pharmacologic inhibitor studies have contributed greatly to our understanding of the Rho and ROCK pathway, ROCK inhibitors nonspecifically inhibit both isoforms of ROCK, ROCK1, and ROCK2.17 Importantly, nonredundant physiologic functions of ROCK1 and ROCK2 in blood cells are not known.
Using mice deficient in the expression of ROCK1, we recently demonstrated that ROCK1 negatively regulates the migration of inflammatory cells in a mouse model of acute peritonitis.18 Here, we examined the physiologic role of ROCK1 in regulating stress erythropoiesis using a phenylhydrazine-induced murine model of hemolytic anemia. We demonstrate that ROCK1-deficient mice exhibit increased hematocrit recovery, elevated RBCs, and enhanced recovery from hemolytic anemia. In addition, we show that there is enhanced survival of ROCK1-deficient mice during oxidative stress conditions associated with reduced reactive oxygen species relative to wild-type mice. Finally, we show that ROCK1 interacts with p53 and regulates the expression of p53 and caspase-3 during stress.
Methods
Mice
Eight- to 10-week-old WT and ROCK1−/− mice (FVB background) were used to study stress erythropoiesis. Mice used in this study were fully backcrossed and maintained in the FVB genetic background and are viable. All mice were maintained in pathogen-free and certified animal facility at Indiana University School of Medicine (Indianapolis, IN). The studies were conducted with a protocol approved by the Indiana University Laboratory Animal Research Center.
5-fluorouracil and phenylhydrazine induced stress erythropoiesis
Age- and sex-matched mice were injected intraperitoneally with 5-flurouracil (5-FU; 150 mg/kg in sterile saline; Abraxis Pharmaceutical Products). Phenylhydrazine (PHZ; Sigma-Aldrich) was administered intraperitoneally (90 mg/kg). Control animals received sterile PBS. PHZ or 5-FU–injected mice were euthanized at different time points. Peripheral blood was collected from tail vein in EDTA-treated tubes.
Single-cell suspension from BM and spleen
BM (BM) was obtained from tibia, iliac crest, and femurs. Briefly, BM cell suspension was collected by flushing the BM with a syringe filled with Iscove modified Dulbecco medium (IMDM; Invitrogen). Spleen was placed in a petri dish and flushed with IMDM into single-cell suspension and passaged through a 200-μm nylon mesh. The cells were centrifuged and resuspended in 5 mL of IMDM. Low-density BM cells (LDBM) or splenocytes were isolated by density gradient centrifugation using Histopaque 1083 (Sigma-Aldrich).
Methylcellulose assay
LDBMs or splenocytes from WT and ROCK1−/− mice were cultured in 1 mL of methylcellulose complete medium containing 1% methylcellulose (StemCell Technologies), 30% fetal bovine serum (FBS), 2% penicillin-streptomycin, 1% BSA, 10−4M β-mercaptoethanol in the presence of 4 U/mL EPO (Amgen), and 100 ng/mL SCF (Pepro Tech). The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Colonies were counted under the inverted microscope after 2 to 10 days of culture. Stress BFU-Es were assessed as previously described.19
Flow cytometric analysis
Phycoerythrin (PE)–conjugated anti-CD71 antibody (eBioscience; clone R17217) and fluorescein isothiocyanate (FITC)–conjugated anti-Ter119 antibody (BD Pharmingen; clone Ter119) were used for cell-surface phenotyping of erythroid cells. Splenocytes or whole BM cells were blocked with PBS containing 0.2% BSA and 10% rat serum for 30 minutes at 4°C, followed by staining with PE-conjugated anti-CD71 and FITC-conjugated anti-Ter119 for 30 minutes at 4°C. Cells were washed with PBS containing 0.2% BSA and analyzed using fluorescence activated cell sorter (FACS) or LSRII (BD Pharmingen). Data were analyzed using Cell Quest Version 6.0 (BD Bioscience) or FlowJo Version 9.4 software.
Histology
Freshly isolated bones and spleens were fixed in 10% formalin. The bones were decalcified in EDTA solution. The fixed tissues were then processed in paraffin and sections were stained with hematoxylin and eosin. Photomicrographs were obtained.
ROS measurement
Red blood cells (1 × 107) were washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C and 5% CO2. Cells were washed with prewarm PBS, analyzed using fluorescence activated cell sorter (FACS). Data were analyzed using Cell Quest (BD Bioscience).
Assessment of apoptosis in erythroid cells
Apoptosis was assessed by examining the percentage of cells able to bind annexin V and 7-AAD (Apoptosis Detection kit; BD Bioscience). Apoptosis assay was performed as outlined in the manufacturer's instructions and analyzed by flow cytometry using a FACScan (Becton Dickinson).
Q-PCR
Quantitative polymerase chain reaction (Q-PCR) was performed as previously described.20,21 Briefly, total RNA was isolated using the RNeasy Minikit (QIAGEN) and single-stranded cDNA was synthesized using AccuScript High Fidelity 1st Strand cDNA Synthesis Kit (Aligent Technologies) according the manufacturer's instructions. Q-PCR was performed using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using the SYBR Green Master Mix (Applied Biosystems) and the following primers: Bmp4, forward 5′-AGCAAGAGCGCCGTCATTCC-3′, reverse 5′-GGGACGCTCCGGGTACTCAA-3′; L27, forward 5′-CGTCATCGTGAAGAACATT G-3′, reverse 5′-CATGGCAGCTGTCACTTTC-3′. For data analysis, ΔCT values were calculated using the expression of the ribosomal housekeeping gene L27 as an internal control. Expression of Bmp4 was normalized to WT controls treated with PHZ.
Generation of BM-derived erythroid progenitors
BM-derived erythroid progenitors were generated from 8- to 12-week-old WT and ROCK1−/− mice femurs, tibias, and iliac crest as previously described.22 Briefly, BM cells were flushed into a 50-mL Falcon tube using syringe-needle and IMDM. Cells were collected by centrifugation at 800g for 5 minutes (Beckman Coulter) at room temperature and RBCs were lysed with RBC lysis buffer for 5 minutes at room temperature. Erythroid progenitors were generated by isolating c-KIT–positive cells from BM using a c-KIT selection kit (StemCell Technologies). c-KIT–positive cells were cultured in StemPro-34 medium supplemented with 1× Stempro-34 nutrient, 2.5 u/mL EPO, 100 ng/mL SCF, 1μM dexamethasone, 1μM β-estradiol, 40 ng/mL insulin-like growth factor-1, 75 μg/mL transferring, 0.1mM 2-mercaptoethanol, and 0.5% BSA.
Transplants
Low density mononuclear BM cells (1 × 106) from WT and ROCK1−/− mice were injected along with 1 × 105 freshly isolated supporting mononuclear splenocytes from WT and ROCK1−/− mice, respectively, intravenously through tail vein into lethally irradiated (1000 cGY-split dose) recipient WT (FVB back ground) mice. PHZ was administered intraperitoneally (80 mg/kg) after 4 months of transplantation. Peripheral blood, BM, and spleen cells were harvested from transplanted mice after PHZ treatment for measuring ROS levels, blood counts, and to determine the frequency of CD71/Ter119-positive cells.
Immunoprecipitation and Western blot analysis
Results
Elevated serum EPO levels in ROCK1-deficient mice
Previous studies revealed a role for Rac GTPase (Rac 1/2) in erythroid cell development and regulation.24,25 In addition, Cdc42 deficiency alters in the development of early erythroid progenitor cells and results anemia.26 We explored whether ROCK1-deficient mice display altered erythroid parameters in peripheral blood under steady-state conditions. Peripheral blood was taken from WT and ROCK1−/− mice and blood counts were assessed. Our results show no statistically significant difference between ROCK1-deficient mice and their WT counterparts with respect to erythroid parameters including RBCs (M/μL), hemoglobulin (Hb; g/dL), hematocrit (HCT; %), mean cell volume (MCV; fL), mean corpuscular hemoglobin (MCH; pg), mean corpuscular hemoglobin concentration (MCHC; g/dL), and red cell distribution width (RDW; %). These results suggest that loss of ROCK1 does not alter erythroid cell parameters under steady-state conditions (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Furthermore, we also investigated the frequency and total erythroid subsets using erythroid markers (Ter119 and CD71) and forward scatter (FSC) parameter in the BM and the spleen of WT and ROCK1−/− mice under steady-state conditions. Early erythroid progenitors differentiate and lose the expression of CD71 while maintaining the expression of Ter119. Thus, these cell-surface markers are used for identifying the various stages of erythroid cell maturation. The erythroblast subpopulations were distinguished by assessing level of expression of Ter119, CD71, and in combination with FSC as previously described.27 Whole BM and splenocytes were stained with CD71 and Ter119 antibodies. Flow cytometric analysis revealed that the frequency of CD71high, Ter119high and FSChigh, CD71high, Ter119high, and FSC low and CD71low, Ter119high, and FSClow erythroblast subsets were unaltered in splenocytes of ROCK1-deficient mice compared with WT controls (supplemental Figure 1C-D). In contrast, the total number of splenic CD71low, Ter119high, and FSClow erythroid subsets was significantly less compared with WT controls (supplemental Figure 1E). In the BM, deficiency of ROCK1 results in a significant increase in the frequency and total CD71low, Ter119high, and FSClow erythroid subsets; whereas CD71high, Ter119high, and FSChigh, as well as CD71high, Ter119high, and FSClow subsets remain unaltered (supplemental Figure 1F-H). Increased CD71low, Ter119high, and FSClow subset cells in the BM could probably be because of an increase in the basal serum EPO levels in ROCK1-deficient mice (supplemental Figure 1I). Thus, ROCK1 plays a critical role in regulating EPO levels in steady-state erythropoiesis.
Enhanced recovery of Ter119-positive cells in ROCK1−/− mice after 5-FU stress
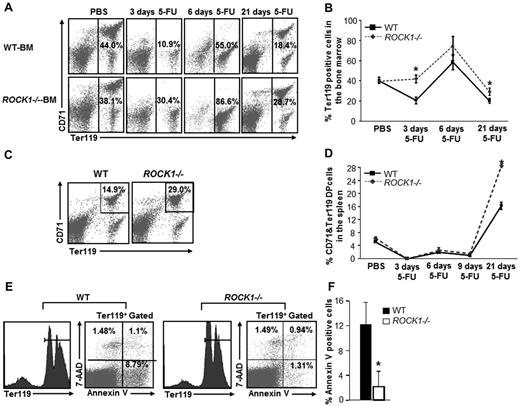
Steady-state erythropoiesis is homeostatic, which maintains RBC levels and HCTs at a constant rate. In response to stress, erythropoiesis expands into spleen, where rapidly generate large number of erythroid subsets to recover from stress.9 To test the ability of erythroid progenitor cells to recover from stress in the absence of ROCK1, we measured erythropoiesis in WT and ROCK1−/− mice during the recovery phase after treatment with 150 mg/kg of 5-FU, which selectively kills all cycling cells, thereby recruiting quiescent HSCs into cell cycle. Frequencies of Ter119/CD71 double-positive erythroblasts in BM and spleen were monitored during the recovery phases 3, 6, and 21 days after 5-FU injection using flow cytometry. Although an increase in the recovery of Ter119-positive cells because of ROCK1 deficiency was observed at all time points examined, the increase was most significant on day 3 after 5-FU treatment (Figure 1A-B). Although some erythropoiesis occurs in the spleen under steady-state conditions, spleen is the primary site for stress erythropoiesis. We thus determined the status of splenic erythropoiesis after 5-FU stress. Flow cytometry analysis of erythroid cells derived from ROCK1−/− and WT spleen showed no significant difference in the recovery of CD71/Ter119-positive cells on day 3 and 6 after 5-FU injection. In contrast, on day 21 after 5-FU injection ROCK1−/− spleens displayed significantly more CD71/Ter119 double-positive erythroid cells compared with WT spleen, suggesting higher splenic erythropoietic recovery in ROCK1-deficient mice compared with WT (Figure 1C-D).
Enhanced recovery of erythroid cells in ROCK1−/− mice in response to 5-FU. (A) Whole BM cells from WT and ROCK1−/− mice were harvested at 0, 3, 6, and 21 days after 5-FU injection. The quadrant in each dot blot indicates the percentage of Ter119-positive cells in WT and ROCK1−/− mice at 0, 3, 6, and 21 days after 5-FU treatment. (B) Line chart represents the mean ± SEM value of percent of Ter119-positive cells in WT and ROCK1−/− BM at indicated time points after 5-FU treatment (n = 8 mice per genotype for the 3 day time point; 3 mice per genotype for the 6 day and 21 day time point, *P < .05). (C) The percentage of CD71/Ter119 double-positive cells in the spleen of representative WT and ROCK1−/− mice is indicated in the top-right quadrant of each dot blot in response to 5-FU at 21 days after 5-FU treatment. (D) Line chart represents the mean ± SEM value of percentage of CD71/Ter119 double-positive cells in spleen of WT and ROCK1−/− mice at 0, 3, 6, 9, and 21 days after 5-FU treatment (n = 8 mice per genotype for the 3 day time point; 3 mice per genotype for the 6 day, 9 day, and 21 day time points, *P < .05). (E) Whole BM cells from WT and ROCK1−/− mice were harvested 6 days after 5-FU injection, stained with anti-Ter119 antibody followed by annexin V and 7-AAD staining. Left panel shows gated histogram representing Ter119-positive cells in WT and ROCK1−/− mice at 6 days after 5-FU treatment. Dot blot in the right panel represents the percentage of annexin V or 7-AAD or double-positive Ter119-positive cells in both genotypes after 5-FU treatment. (F) Bar graph represents mean ± SEM of annexin V–positive cells in the BM of WT and ROCK1−/− mice after 5-FU treatment. (n = 3 mice per genotype, *P < .05).
Enhanced recovery of erythroid cells in ROCK1−/− mice in response to 5-FU. (A) Whole BM cells from WT and ROCK1−/− mice were harvested at 0, 3, 6, and 21 days after 5-FU injection. The quadrant in each dot blot indicates the percentage of Ter119-positive cells in WT and ROCK1−/− mice at 0, 3, 6, and 21 days after 5-FU treatment. (B) Line chart represents the mean ± SEM value of percent of Ter119-positive cells in WT and ROCK1−/− BM at indicated time points after 5-FU treatment (n = 8 mice per genotype for the 3 day time point; 3 mice per genotype for the 6 day and 21 day time point, *P < .05). (C) The percentage of CD71/Ter119 double-positive cells in the spleen of representative WT and ROCK1−/− mice is indicated in the top-right quadrant of each dot blot in response to 5-FU at 21 days after 5-FU treatment. (D) Line chart represents the mean ± SEM value of percentage of CD71/Ter119 double-positive cells in spleen of WT and ROCK1−/− mice at 0, 3, 6, 9, and 21 days after 5-FU treatment (n = 8 mice per genotype for the 3 day time point; 3 mice per genotype for the 6 day, 9 day, and 21 day time points, *P < .05). (E) Whole BM cells from WT and ROCK1−/− mice were harvested 6 days after 5-FU injection, stained with anti-Ter119 antibody followed by annexin V and 7-AAD staining. Left panel shows gated histogram representing Ter119-positive cells in WT and ROCK1−/− mice at 6 days after 5-FU treatment. Dot blot in the right panel represents the percentage of annexin V or 7-AAD or double-positive Ter119-positive cells in both genotypes after 5-FU treatment. (F) Bar graph represents mean ± SEM of annexin V–positive cells in the BM of WT and ROCK1−/− mice after 5-FU treatment. (n = 3 mice per genotype, *P < .05).
Increased recovery of ROCK1-deficient mice after 5-FU treatment may be explained in part by reduced apoptosis, which was determined by annexin V and 7-AAD staining of BM and spleen-derived late erythroblasts. ROCK1-deficient Ter119-positive erythroblasts showed significantly reduced apoptosis compared with WT Ter119-positive erythroblasts (Figure 1E-F, supplemental Figure 2). These results suggest that ROCK1 negatively regulates stress erythropoiesis and survival of erythroblasts.
ROCK1-deficient mice display enhanced recovery from anemia
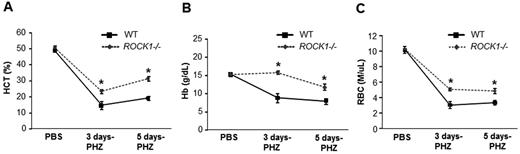
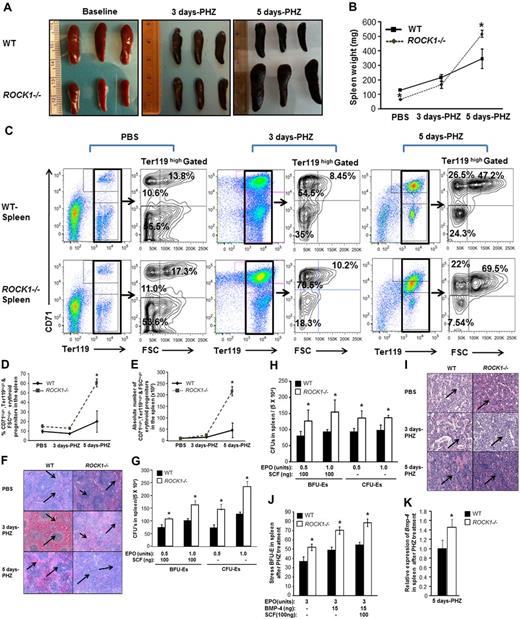
We next focused our studies on a more defined model of stress erythropoiesis. The PHZ model of hemolytic anemia provides an excellent opportunity to assess the underlying defects in stress erythropoiesis because of ROCK1 deficiency. PHZ treatment causes denaturation of hemoglobin, resulting in hemolysis leading to anemia characterized by decreased HCT and Hb levels. WT and ROCK1−/− mice were treated with PHZ (90 mg/kg on day 0). At the indicated time points (3 and 5 days), peripheral blood (20 μL/mouse) was collected from WT and ROCK1−/− mice. RBC (M/μL), Hb (g/dL), HCT (%), MCV (fL), MCH (pg), (MCHC g/dL), and RDW (%) in each sample were counted. Deficiency of ROCK1 resulted in enhanced HCT, Hb, and RBC counts, respectively, on days 3 and 5 after PHZ injection compared with WT controls (Figure 2A-C). In addition, ROCK1−/− mice support efficient erythro-splenomegaly, and exhibit a 3- and 8-fold increase in splenic weight 3 and 5 days after PHZ injection, respectively, compared with baseline (Figure 3A-B). To further explore the differences between WT and ROCK1−/− mice after PHZ treatment a complete analysis of maturation stages of erythroid cells in spleen and progenitor cell content were carried out. Flow cytometric analysis performed on days 3 and 5 post-PHZ treatment revealed increased frequency and total CD71high, Ter119high, and FSChigh erythroid subsets in ROCK1−/− spleens on day 5 after PHZ treatment (Figure 3C-E). Furthermore, histopathologic analysis of WT and ROCK1−/− spleens after PHZ treatment revealed a defined population of white pulp and red pulp in WT spleen (Figure 3F left panel section) but enhanced extramedullary erythropoiesis in ROCK1−/− spleen (Figure 3F right panel section).
Elevated hematocrits and RBCs in ROCK1-deficient mice in response to stress. WT and ROCK1−/− mice were treated with PHZ (90 mg/kg at day 0). At the indicated time points (3 and 5 days), peripheral blood (PB; 20 μL/mouse) was collected from WT and ROCK1−/− mice. RBC (M/μL), Hb (g/dL), HCT (%), MCV (fL), MCH (pg), (MCHC g/dL), and RDW (%) in each sample was counted using an automated hemovet. Mean ± SEM of blood counts are presented in the line chart. (A) HCT, (B) Hb, and (C) RBC. For each analysis, (n = 6 mice per genotype for the 3 day time point; n = 5-7 mice per genotype for the 5 day time point and 3 mice per genotype for PBS condition).
Elevated hematocrits and RBCs in ROCK1-deficient mice in response to stress. WT and ROCK1−/− mice were treated with PHZ (90 mg/kg at day 0). At the indicated time points (3 and 5 days), peripheral blood (PB; 20 μL/mouse) was collected from WT and ROCK1−/− mice. RBC (M/μL), Hb (g/dL), HCT (%), MCV (fL), MCH (pg), (MCHC g/dL), and RDW (%) in each sample was counted using an automated hemovet. Mean ± SEM of blood counts are presented in the line chart. (A) HCT, (B) Hb, and (C) RBC. For each analysis, (n = 6 mice per genotype for the 3 day time point; n = 5-7 mice per genotype for the 5 day time point and 3 mice per genotype for PBS condition).
Enhanced stress erythropoiesis in ROCK1-deficient mice. WT and ROCK1−/− mice were treated with PHZ (90 mg/kg at day 0). At the indicated time points, tissues were harvested. (A) Photomicrographs of spleens in the 2 genotypes are shown after PBS and PHZ treatment. (B) Line chart represents mean ± SEM of splenic weight of WT and ROCK1−/− mice after PHZ injection at indicated time points (n = 6 mice per genotype for the 3 day time point; n = 3 mice per genotype for 5 day and PBS time point, *P < .05). (C) Splenocytes from WT and ROCK1−/− mice were analyzed for the expression of erythroid cell surface marker Ter119 and CD71 in combination with forward scatter (FSC) parameter by flow cytometry after PHZ treatment. A representative left dot blot shows the Ter119high-positive erythroblasts in the spleen at indicated each time points. Ter119high-positive erythroblasts were further analyzed for CD71 expression and FSC parameter as seen in right contour plots at each time point. Percentages in top-right quadrant contour represents CD71high, Ter119high, and FSChigh; percentages in top-left quadrant represents CD71high, Ter119high, and FSClow; percentages in bottom-left quadrant represents CD71low, Ter119high, and FSClow erythroblast in spleen. (D) Line chart representing the mean ± SEM of CD71high,Ter119high, and FSChigh erythroid subset in the spleen of WT and ROCK1−/− mice after PHZ injection at the indicated time points. For each analysis (n = 3 mice per genotype for the 3 day time point; n = 3 mice per genotype for the 5 day and PBS time point, *P < .05). (E) Line graph representing the mean ± SEM of CD71high, Ter119high, and FSChigh erythroid subset in the spleen of WT and ROCK1−/− mice after PHZ injection at the indicated time points. For each analysis (n = 3 mice per genotype for the 3 day time point; n = 3 mice per genotype for the 5 day and PBS time point, *P < .05). (F) Histopathologic analysis of WT and ROCK1−/− spleens after PHZ treatment. Spleens were harvested at indicated time points, fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. Shown are representative spleen sections from WT and ROCK1−/− mice after PHZ treatment. Arrows in left panel indicate clearly defined architecture of white pulp and red pulp in WT spleen 3 and 5 days after PHZ treatment. Arrows in right panel indicate enhanced extramedullary erythropoiesis in ROCK1−/− spleens after PHZ treatment (n = 3 mice per genotype for each time point). (G) Splenocytes (5.0 × 104) from WT and ROCK1−/− mice were plated in a methylcellulose colony-forming assay in the presence of the indicated concentration of SCF and EPO. Colonies were enumerated on day 2 (for CFU-E) and on day 10 (for BFU-E). Bar graph represents the mean ± SD of colonies from 3 independent mice for each genotype 3 days after PHZ treatment plated in triplicates (*P < .05, ROCK−/− vs WT). (H) Bar graph indicates the mean ± SD number of colonies from WT and ROCK1−/− splenocytes on day 5 after PHZ treatment., ROCK−/− versus WT (*P < .05, n = 3 mice for each genotype). (I) Histopathologic analysis of WT and ROCK1−/− BM 5 days after PHZ treatment. Bones were harvested at indicated time points, fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. Shown are representative BM sections from WT and ROCK1−/− mice after PHZ treatment. Right panel arrows demonstrate hypercellular BM and increased red cell generation of ROCK1−/− BM compared with WT BM after PHZ treatment (n = 3 mice per genotype for each time point). (J) Splenocytes from WT and ROCK1−/− mice after were plated in a methylcellulose colony-forming assay in the presence of the indicated concentration of SCF, EPO, and BMP-4. Colonies were enumerated on day 5 for stress BFU-E. Bar graph represents the mean number ± SEM of colonies from 3 independent mice for each genotype plated in triplicates on day 5 after PHZ treatment (n = 3, *P < .05; ROCK−/− vs WT). (K) Total RNA was isolated using the RNeasy Minikit (QIAGEN) and single-stranded cDNA was synthesized using AccuScript High Fidelity 1st Strand cDNA Synthesis Kit (Aligent Technologies) according to the manufacturer's instructions. Q-PCR was performed using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using the SYBR Green Master Mix (Applied Biosystems). For data analysis, ΔCT values were calculated using the expression of the ribosomal housekeeping gene L27 as an internal control. Relative expression of Bmp4 of was normalized with WT splenocytes treated with PHZ. Bar graph represents expression of BMP-4 in ROCK1−/− splenocytes after PHZ treatment on day 5 (n = 3, *P < .05; ROCK−/− vs WT).
Enhanced stress erythropoiesis in ROCK1-deficient mice. WT and ROCK1−/− mice were treated with PHZ (90 mg/kg at day 0). At the indicated time points, tissues were harvested. (A) Photomicrographs of spleens in the 2 genotypes are shown after PBS and PHZ treatment. (B) Line chart represents mean ± SEM of splenic weight of WT and ROCK1−/− mice after PHZ injection at indicated time points (n = 6 mice per genotype for the 3 day time point; n = 3 mice per genotype for 5 day and PBS time point, *P < .05). (C) Splenocytes from WT and ROCK1−/− mice were analyzed for the expression of erythroid cell surface marker Ter119 and CD71 in combination with forward scatter (FSC) parameter by flow cytometry after PHZ treatment. A representative left dot blot shows the Ter119high-positive erythroblasts in the spleen at indicated each time points. Ter119high-positive erythroblasts were further analyzed for CD71 expression and FSC parameter as seen in right contour plots at each time point. Percentages in top-right quadrant contour represents CD71high, Ter119high, and FSChigh; percentages in top-left quadrant represents CD71high, Ter119high, and FSClow; percentages in bottom-left quadrant represents CD71low, Ter119high, and FSClow erythroblast in spleen. (D) Line chart representing the mean ± SEM of CD71high,Ter119high, and FSChigh erythroid subset in the spleen of WT and ROCK1−/− mice after PHZ injection at the indicated time points. For each analysis (n = 3 mice per genotype for the 3 day time point; n = 3 mice per genotype for the 5 day and PBS time point, *P < .05). (E) Line graph representing the mean ± SEM of CD71high, Ter119high, and FSChigh erythroid subset in the spleen of WT and ROCK1−/− mice after PHZ injection at the indicated time points. For each analysis (n = 3 mice per genotype for the 3 day time point; n = 3 mice per genotype for the 5 day and PBS time point, *P < .05). (F) Histopathologic analysis of WT and ROCK1−/− spleens after PHZ treatment. Spleens were harvested at indicated time points, fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. Shown are representative spleen sections from WT and ROCK1−/− mice after PHZ treatment. Arrows in left panel indicate clearly defined architecture of white pulp and red pulp in WT spleen 3 and 5 days after PHZ treatment. Arrows in right panel indicate enhanced extramedullary erythropoiesis in ROCK1−/− spleens after PHZ treatment (n = 3 mice per genotype for each time point). (G) Splenocytes (5.0 × 104) from WT and ROCK1−/− mice were plated in a methylcellulose colony-forming assay in the presence of the indicated concentration of SCF and EPO. Colonies were enumerated on day 2 (for CFU-E) and on day 10 (for BFU-E). Bar graph represents the mean ± SD of colonies from 3 independent mice for each genotype 3 days after PHZ treatment plated in triplicates (*P < .05, ROCK−/− vs WT). (H) Bar graph indicates the mean ± SD number of colonies from WT and ROCK1−/− splenocytes on day 5 after PHZ treatment., ROCK−/− versus WT (*P < .05, n = 3 mice for each genotype). (I) Histopathologic analysis of WT and ROCK1−/− BM 5 days after PHZ treatment. Bones were harvested at indicated time points, fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. Shown are representative BM sections from WT and ROCK1−/− mice after PHZ treatment. Right panel arrows demonstrate hypercellular BM and increased red cell generation of ROCK1−/− BM compared with WT BM after PHZ treatment (n = 3 mice per genotype for each time point). (J) Splenocytes from WT and ROCK1−/− mice after were plated in a methylcellulose colony-forming assay in the presence of the indicated concentration of SCF, EPO, and BMP-4. Colonies were enumerated on day 5 for stress BFU-E. Bar graph represents the mean number ± SEM of colonies from 3 independent mice for each genotype plated in triplicates on day 5 after PHZ treatment (n = 3, *P < .05; ROCK−/− vs WT). (K) Total RNA was isolated using the RNeasy Minikit (QIAGEN) and single-stranded cDNA was synthesized using AccuScript High Fidelity 1st Strand cDNA Synthesis Kit (Aligent Technologies) according to the manufacturer's instructions. Q-PCR was performed using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using the SYBR Green Master Mix (Applied Biosystems). For data analysis, ΔCT values were calculated using the expression of the ribosomal housekeeping gene L27 as an internal control. Relative expression of Bmp4 of was normalized with WT splenocytes treated with PHZ. Bar graph represents expression of BMP-4 in ROCK1−/− splenocytes after PHZ treatment on day 5 (n = 3, *P < .05; ROCK−/− vs WT).
To determine whether the enhanced extramedullary erythropoiesis noted in ROCK1−/− mice was because of specific expansion of cells of the erythroid lineage, the number of BFU-Es and CFU-Es in the spleens of ROCK1−/− and WT mice were determined. In vitro colony forming assays demonstrated ROCK1−/− splenocytes generated more CFU-Es than WT cells and eventually generated more BFU-Es than WT splenocytes in response to different concentrations of EPO and EPO/SCF combined days 3 and 5 after PHZ treatment (Figure 3G-H). The frequency and total numbers of CD71high, Ter119high, and FSChigh erythroid subsets were unaltered in ROCK1−/− BM compared with controls after PHZ treatment (supplemental Figure 3A-C). Whereas, the frequency of CD71high, Ter119high, and FSClow erythroid progenitors in the BM was modestly but significantly enhanced 3 days after PHZ treatment but there was no significant difference in CD71high, Ter119high, and FSClow-positive cells 5 days after PHZ treatment (supplemental Figure 3D). Total number of CD71high, Ter119high, and FSClow-positive erythroid progenitors in the BM were comparable in both genotype after PHZ stress (supplemental Figure 3E). Histopathologic analysis demonstrated increased cellularity and red cell regeneration in ROCK1−/− BM compared with WT BM 3 and 5 days after PHZ treatment (Figure 3I right panel section). To examine whether enhanced stress erythropoiesis was because of specific expansion of stress BFU-Es. We performed in vitro colony forming assay in the presence of EPO alone and in the combination with, BMP-4. Our results revealed that deficiency of ROCK1 results in significantly more stress BFU-Es compared with controls on day 5 after PHZ treatment (Figure 3J). In contrast, stress BFU-Es were comparable in both the genotypes at steady-state conditions (supplemental Figure 4).
We further determined the response of ROCK1-deficient mice to another form of physiologic stress by injecting a single intraperitoneal injection of EPO (300 units/25 gm). Peripheral blood was collected from EPO treated WT and ROCK1−/− mice after 2, 4, and 6 days and HCT, Hb, and RBC counts were analyzed. Consistent with our previous observations with PHZ induced stress, ROCK1-deficient mice also show a significant increase in hematocrits, hemoglobin and RBC counts after 4 days of EPO treatment compared with WT controls. Although comparable levels of HCT, Hb, and RBC counts were observed after 2 days of EPO injection in WT and ROCK1−/− mice, both genotypes recovered on day 6 after EPO injection with respect to erythroid parameters (supplemental Figure 5A-C).
Furthermore, to investigate whether ROCK1 regulates BMP-4 expression, we performed real-time Q-PCR analysis to determine BMP-4 expression levels after PHZ treatment. As seen in Figure 3K, deficiency of ROCK1 results in increased BMP-4 mRNA levels on day 5 in PHZ treated splenocytes compared with controls. These results are consistent with previously published studies demonstrating that enhanced BMP4 levels result in more stress BFU-Es and faster recovery from anemia.5,6 Taken together, these data indicate that deficiency of ROCK1 results in enhanced stress erythropoiesis in spleen and protects mice from PHZ induced hemolytic anemia.
ROCK1-deficient mice demonstrate greater survival and reduced ROS levels in a model of hemolytic anemia
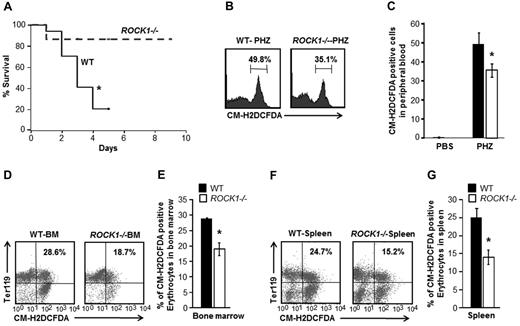
To further examine the cause of enhanced stress erythropoiesis in ROCK1-deficient mice, survival of WT and ROCK1-deficient mice was determined after treatment with PHZ. As seen in Figure 4A, ROCK1-deficient mice have a significantly higher capacity to recover from a severe anemic crisis after PHZ treatment. In contrast WT mice were unable to withstand severe anemic stress and succumb to death within 5 days of PHZ treatment. These results demonstrate that ROCK1 deficiency allows improved survival and accelerated recovery from PHZ-induced anemia.
ROCK1-deficient mice display higher survivability and reduced ROS levels in response to stress. Kaplan Meier survival curve of WT and ROCK1−/−mice after PHZ treatment. (A) Seventeen WT mice and 15 ROCK1−/− mice treated with a single intraperitoneal injection of PHZ (90 mg/kg). Data shown is pooled from 3 independent experiments (*P < .05). (B) PB (20 μL) was washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C. A representative histogram shows percentage of ROS probe-positive cells in PB of both genotypes. (C) Bar graph representing the mean ± SD of ROS levels in the peripheral blood of WT and ROCK1−/− mice at 5 days after PHZ treatment (n = 3 mice for each genotype, *P < .05). (D) BM cells (5 × 106) were immunostained for 30 minutes at 4°C with PE-conjugated anti-Ter119 antibody washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C, 5% CO2. Top right quadrant in each dot blot indicates percentage of ROS probe-positive erythroid cells in the BM of both genotypes. (E) Bar graph represents the mean ± SEM of ROS levels in erythroid cells in the BM of WT and ROCK1−/− mice at 5 days after PHZ treatment (n = 3 per genotype, *P < .05). (F) Splenocytes (5 × 106) were immunostained for 30 minutes at 4°C with PE-conjugated anti-Ter119 antibody washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C, 5% CO2. Top right quadrant in each dot blot indicates percentage of ROS probe-positive erythroid cells in spleen of both genotypes. (G) Bar graph represents the mean ± SEM of ROS levels in erythroid cells in the spleen of WT and ROCK1−/− mice, 5 days after PHZ treatment. (n = 3 mice per genotype, *P < .05).
ROCK1-deficient mice display higher survivability and reduced ROS levels in response to stress. Kaplan Meier survival curve of WT and ROCK1−/−mice after PHZ treatment. (A) Seventeen WT mice and 15 ROCK1−/− mice treated with a single intraperitoneal injection of PHZ (90 mg/kg). Data shown is pooled from 3 independent experiments (*P < .05). (B) PB (20 μL) was washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C. A representative histogram shows percentage of ROS probe-positive cells in PB of both genotypes. (C) Bar graph representing the mean ± SD of ROS levels in the peripheral blood of WT and ROCK1−/− mice at 5 days after PHZ treatment (n = 3 mice for each genotype, *P < .05). (D) BM cells (5 × 106) were immunostained for 30 minutes at 4°C with PE-conjugated anti-Ter119 antibody washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C, 5% CO2. Top right quadrant in each dot blot indicates percentage of ROS probe-positive erythroid cells in the BM of both genotypes. (E) Bar graph represents the mean ± SEM of ROS levels in erythroid cells in the BM of WT and ROCK1−/− mice at 5 days after PHZ treatment (n = 3 per genotype, *P < .05). (F) Splenocytes (5 × 106) were immunostained for 30 minutes at 4°C with PE-conjugated anti-Ter119 antibody washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C, 5% CO2. Top right quadrant in each dot blot indicates percentage of ROS probe-positive erythroid cells in spleen of both genotypes. (G) Bar graph represents the mean ± SEM of ROS levels in erythroid cells in the spleen of WT and ROCK1−/− mice, 5 days after PHZ treatment. (n = 3 mice per genotype, *P < .05).
To uncover the mechanism for enhanced survival of ROCK1-deficient mice under oxidative stress, we sought to determine ROS levels in these mice. Previous studies have shown that Foxo3a-deficient mice are unable to survive and recover from PHZ-induced anemia because of excessive ROS production.28 Accordingly, we hypothesized that ROCK1-deficient mice may regulate ROS levels. To this end, the concentration of ROS in ROCK1-deficient mice was determined by flow cytometric analysis using 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate, a peroxide-sensitive ROS level detection probe. ROS levels were found to be significantly lower in peripheral blood, spleen, and BM of ROCK1-deficient mice 5 days after PHZ challenge compared with WT controls (Figure 4B-G). In addition, treatment with ROS scavenger N-acetyl cysteine (NAC) significantly increased the survival of PHZ-treated WT mice similar to ROCK1−/− mice (supplemental Figure 6). These results demonstrate that improved survival of ROCK1-deficient mice after phenylhydrazine treatment could be because of reduced ROS levels.
Enhanced stress erythropoiesis in ROCK1−/− mice is cell autonomous
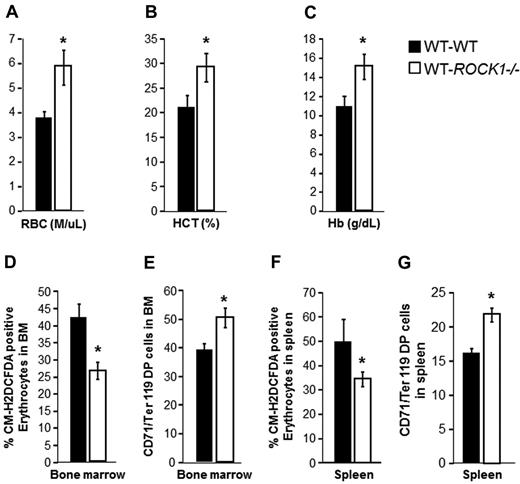
The enhanced stress erythropoiesis observed in the ROCK1−/− mice could result from a hematopoietic cell–autonomous defect or because of loss of an extrinsic component of the microenvironment. To distinguish these possibilities, we transplanted BM cells from WT or ROCK1−/− mice into lethally irradiated WT recipient mice. After 4 months, recipient mice were injected with PHZ and blood counts, ROS levels, and CD71/Ter119 expression was determined. As seen in Figure 5A through C, WT recipient mice transplanted with ROCK1−/− BM showed increased RBCs, Hb content, and elevated HCT in peripheral blood after PHZ treatment compared with WT recipient mice transplanted with WT BM. In addition, WT recipient mice transplanted with ROCK1−/− BM showed increase frequency of CD71/Ter119 double-positive cells and reduced ROS levels after PHZ treatment compared with WT recipient mice transplanted with WT BM (Figure 5D-G). Taken together, enhanced stress erythropoiesis in ROCK1−/− mice is because of a cell autonomous defect.
Enhanced stress erythropoiesis in ROCK1−/− mice is cell autonomous. PHZ was administered intraperitoneally (80 mg/kg) to WT recipient mice transplanted with WT or ROCK1−/− BM. Recipient mice were harvested 4 days after PHZ injection, PB (20 μL/mouse) was collected from the tail vein in an EDTA-coated capillary tube from recipient mice. (A) Bar graph represents the mean value ± SD of RBCs (M/μL) in each genotype (n = 3 mice per genotype, *P < .05). (B) Bar graph represents the mean value ± SD of HCT (%) in each genotype (n = 3 mice per genotype, *P < .05). (C) Bar graph represents the mean value ± SD of Hb (g/dL), in each genotype (n = 3 mice per genotype, *P < .05). (D) BM was harvested from transplanted mice 4 days after PHZ treatment. Whole BM cells were washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C. Bar graph representing the mean ± SEM of ROS levels in the WT and ROCK1−/− erythrocytes (n = 3 mice for each genotype, *P < .05). (E) Bar graph represents the mean ± SEM of Ter119/CD71-positive cells in the BM of WT and ROCK1−/− BM recipient mice after PHZ injection. (F) Spleen was harvested from transplanted mice 4 days after PHZ treatment. Splenocytes were washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C. Bar graph represents the mean ± SEM of ROS levels in the WT and ROCK1−/− BM recipient mice (n = 3 mice for each genotype, *P < .05). (G) Bar graph representing the mean ± SEM of Ter119/CD71 double-positive cells in the spleen of WT and ROCK1−/− recipient mice after PHZ injection (n = 3 mice for each genotype, *P < .05).
Enhanced stress erythropoiesis in ROCK1−/− mice is cell autonomous. PHZ was administered intraperitoneally (80 mg/kg) to WT recipient mice transplanted with WT or ROCK1−/− BM. Recipient mice were harvested 4 days after PHZ injection, PB (20 μL/mouse) was collected from the tail vein in an EDTA-coated capillary tube from recipient mice. (A) Bar graph represents the mean value ± SD of RBCs (M/μL) in each genotype (n = 3 mice per genotype, *P < .05). (B) Bar graph represents the mean value ± SD of HCT (%) in each genotype (n = 3 mice per genotype, *P < .05). (C) Bar graph represents the mean value ± SD of Hb (g/dL), in each genotype (n = 3 mice per genotype, *P < .05). (D) BM was harvested from transplanted mice 4 days after PHZ treatment. Whole BM cells were washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C. Bar graph representing the mean ± SEM of ROS levels in the WT and ROCK1−/− erythrocytes (n = 3 mice for each genotype, *P < .05). (E) Bar graph represents the mean ± SEM of Ter119/CD71-positive cells in the BM of WT and ROCK1−/− BM recipient mice after PHZ injection. (F) Spleen was harvested from transplanted mice 4 days after PHZ treatment. Splenocytes were washed and resuspended in prewarmed PBS and loaded with 10μM CM-H2DCFDA in the dark for 30 minutes at 37°C. Bar graph represents the mean ± SEM of ROS levels in the WT and ROCK1−/− BM recipient mice (n = 3 mice for each genotype, *P < .05). (G) Bar graph representing the mean ± SEM of Ter119/CD71 double-positive cells in the spleen of WT and ROCK1−/− recipient mice after PHZ injection (n = 3 mice for each genotype, *P < .05).
ROCK1 interacts with p53
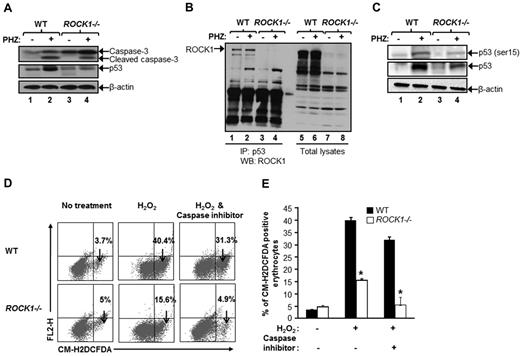
Previous studies have shown that p53 deficient mice show enhanced recovery from PHZ-induced anemia.29 It has been suggested that there is a close relationship between p53 function and ROS levels, and in particular, there is evidence that intracellular ROS levels regulate cell fate through p53, thus demonstrating the response of p53 to ROS stimulation under different stress conditions and subsequent events downstream of p53 activation. To determine whether ROCK1 regulates p53 expression levels in vivo, we analyzed p53 and caspase-3 levels from WT and ROCK1−/− splenocytes treated with PHZ. As seen in Figure 6A, loss of ROCK1 in these cells not only resulted in reduced expression of total p53 protein but also reduced cleavage of caspase-3 after oxidative stress. However, no significant difference in p53 mRNA levels was observed in ROCK1−/− cells compared with WT cells in steady-state as well as under stress condition (supplemental Figure 7).
ROCK1 interacts with p53 and regulates p53 levels in splenocytes. (A) PHZ was administered intraperitoneally (80 mg/kg) to WT and ROCK1−/− mice. Spleens were harvested 4 days after PHZ injection and freshly isolated splenocytes were lysed in lysis buffer. Cell lysates were subjected to WB analysis using antibodies against p53 and caspase-3. Arrows show the expression of indicated proteins in WT and ROCK1−/− spleen after PHZ treatment. Bottom panel shows β-actin levels in each lane (n = 3). (B) Freshly isolated splenocytes after PHZ injection were lysed in lysis buffer, and equal amount of lysates derived from untreated and PHZ-treated splenocytes were subjected to immunoprecipitation with an anti-p53 antibody. Western blot analysis was performed using an anti-ROCK1 antibody. Lanes 1 and 2 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 3 and 4 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the interaction of ROCK1 with p53 in WT splenocytes. Total lysates were subjected to Western blot analysis with an anti-ROCK1 antibody. Lanes 5 and 6 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 7 and 8 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the expression of ROCK1 in WT splenocytes (n = 3). (C) Splenocytes harvested after PHZ injection were lysed in lysis buffer, and equal amount of lysates derived from untreated and PHZ-treated spleens were subjected to Western blot analysis with an anti-phospho p53 (ser15) antibody. Lanes 1 and 2 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 3 and 4 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the phospho-p53 (ser15) levels in WT and ROCK1−/− splenocytes; middle panel arrow indicates total p53 levels in both genotypes, and bottom panel indicates β-actin controls in each line before and after PHZ stress. (D) Erythroid progenitor cells derived from the BM of WT and ROCK1−/− mice were cultured for 7 days and treated with 50μM hydrogen peroxide or 2.5μM of caspase inhibitor for 1 hour. Cells were washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C, 5% CO2. Down arrow in each dot blot indicates percentage of ROS probe-positive cells in both genotypes. (E) Bar graph represents the mean ± SD of ROS levels in the WT and ROCK1−/− erythrocytes (n = 6 mice per genotype, *P < .05).
ROCK1 interacts with p53 and regulates p53 levels in splenocytes. (A) PHZ was administered intraperitoneally (80 mg/kg) to WT and ROCK1−/− mice. Spleens were harvested 4 days after PHZ injection and freshly isolated splenocytes were lysed in lysis buffer. Cell lysates were subjected to WB analysis using antibodies against p53 and caspase-3. Arrows show the expression of indicated proteins in WT and ROCK1−/− spleen after PHZ treatment. Bottom panel shows β-actin levels in each lane (n = 3). (B) Freshly isolated splenocytes after PHZ injection were lysed in lysis buffer, and equal amount of lysates derived from untreated and PHZ-treated splenocytes were subjected to immunoprecipitation with an anti-p53 antibody. Western blot analysis was performed using an anti-ROCK1 antibody. Lanes 1 and 2 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 3 and 4 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the interaction of ROCK1 with p53 in WT splenocytes. Total lysates were subjected to Western blot analysis with an anti-ROCK1 antibody. Lanes 5 and 6 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 7 and 8 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the expression of ROCK1 in WT splenocytes (n = 3). (C) Splenocytes harvested after PHZ injection were lysed in lysis buffer, and equal amount of lysates derived from untreated and PHZ-treated spleens were subjected to Western blot analysis with an anti-phospho p53 (ser15) antibody. Lanes 1 and 2 consist of lysates derived from untreated and PHZ-treated splenocytes from WT mice, respectively; lanes 3 and 4 consist of lysates derived from untreated and PHZ-treated splenocytes from ROCK1−/− mice, respectively. The arrow indicates the phospho-p53 (ser15) levels in WT and ROCK1−/− splenocytes; middle panel arrow indicates total p53 levels in both genotypes, and bottom panel indicates β-actin controls in each line before and after PHZ stress. (D) Erythroid progenitor cells derived from the BM of WT and ROCK1−/− mice were cultured for 7 days and treated with 50μM hydrogen peroxide or 2.5μM of caspase inhibitor for 1 hour. Cells were washed and resuspended in prewarmed PBS and loaded with 10μM 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 minutes at 37°C, 5% CO2. Down arrow in each dot blot indicates percentage of ROS probe-positive cells in both genotypes. (E) Bar graph represents the mean ± SD of ROS levels in the WT and ROCK1−/− erythrocytes (n = 6 mice per genotype, *P < .05).
To further elucidate the mechanism of p53 regulation by ROCK1 in vivo, we examined the binding of p53 and ROCK1 in PHZ primed splenocytes. As seen in Figure 6B, immunoprecipitation of lysates using an anti-p53 antibody followed by Western blot analysis using an anti-ROCK1 antibody revealed a significant association between p53 and ROCK1 in baseline condition as well as in response to PHZ induced stress. Importantly, this association did not occur in splenocytes deficient in the expression of ROCK1. Consistent with reduced p53 expression in ROCK1-deficient splenocytes, p53 phosphorylation (ser15) was also impaired in ROCK1−/− PHZ treated splenocytes compared with WT controls (Figure 6C). These results suggest that ROCK1 regulates p53 levels in response to PHZ-induced stress in erythroblasts.
We further investigated ROS levels in in vitro BM-derived erythroid progenitors from WT and ROCK1−/− mice in response to H2O2 stress or in the presence of a caspase inhibitor. As seen in Figure 6D and E, deficiency of ROCK1 in erythroid progenitors resulted in reduced production of ROS levels after treatment with H2O2 and was further reduced in the presence of the caspase inhibitor. Taken together, these results demonstrate that ROCK1 plays a significant role in regulating the level of p53 in response to oxidative stress, which probably contributes to the enhanced stress erythropoiesis and protection from anemia assault.
Discussion
ROCKs are involved in a variety of important physiologic functions including cytoskeletal reorganization, migration, adhesion, survival, and proliferation. Rho kinases phosphorylate various substrates, such as myosin light chain (MLC) phosphatase, LIM kinase 2, phosphatase, and tensin homolog deleted from chromosome 10 (PTEN) and ezrin/radixin/moesin (ERM) proteins and influence physiologic functions in diverse cell types.18,30-33 To date, most of the conclusions with regard to the function of Rho kinases have involved the use of pharmacologic inhibitors as well as dominant negative approaches. Recently, Gabet et al demonstrated the involvement of ROCK1 in terminal maturation of erythroid cells using ROCK inhibitors and by knockdown of ROCK1.34 Although useful, these studies do not distinguish between the physiologic functions of ROCK1 and ROCK2, thereby underscoring the importance of these related family members in regulating different functions. Importantly, the physiologic role of ROCK in blood cells is poorly defined.
Our results demonstrate an unexpected role of ROCK1 in negatively regulating stress erythropoiesis. We show that deficiency of ROCK1 in a PHZ-induced oxidative stress model results in enhanced recovery from hemolytic anemia as well as enhanced survival compared with WT mice. We also demonstrate in BM transplantation studies that the enhanced stress erythropoiesis in ROCK1-deficient mice is stem cell intrinsic. Biochemically, our results show a unique role for ROCK1 in regulating the expression of p53 in response to oxidative stress by regulating its phosphorylation. Furthermore, we show physical association of ROCK1 with p53 in WT splenocytes. In absence of this interaction, we observed reduced caspase-3 cleavage and reduced level of ROS. Prior studies have shown that loss of p53 in p53-deficient mice also results in enhanced erythroid cell recovery after oxidative challenge.29 Taken together, we define a novel mechanism of p53 regulation in stress erythropoiesis.
Previous studies have shown that inhibition of ROCK promotes cell survival in various cell types and disease models. Pharmacologic inhibition of ROCK results in enhanced survival of human embryonic stem cells (hES) and enhanced survival of ROCK1 heterozygous mice in response to UVB-induced stress compared with WT mice.35,36 Inhibition of Rho kinases results in reduced apoptosis of cardiomyocytes during ischemia-reperfusion injury.37 Similarly, pharmacologic inhibition of ROCK activity by ROCK inhibitor results in enhanced recovery of cryopreserved hES cells as well as induced pluripotent stem (iPS) cells.35,38 Furthermore, prolong treatment of ROCK inhibitor also results in an increase in the colony number and size of hES cells compared with shorter treatment time of ROCK inhibitors.39 Our findings demonstrating enhanced survival of ROCK1-deficient mice in response to oxidative stress are consistent with these results and implicate ROCKs in regulating cell survival.
Previous studies have shown that the activation of p53 results in an increased levels of ROS in part by interfering with the mitochondrial function and integrity, which results in cell death.40 In addition, higher levels of ROS appear to be part of a feedback loop that stabilizes p53 resulting in more p53 activity.40 In response to stress, increased mitochondrial ROS generation and increased p53 protein levels have been observed in human breast carcinoma MCF-7 cells and in human diploid fibroblast IMR-90 cells.41 Importantly, antioxidant NAC and the Cu/Zn SOD inhibitor abolish stress-induced ROS and p53 levels in these cell lines.41 These results indicate that ROS plays a critical role in the induction of p53 activity followed by p53-dependent apoptosis. Consistent with these findings, our results show a significant reduction in ROS levels in ROCK1-deficient erythroblasts on PHZ challenge, which is associated with reduced phosphorylation and expression of p53.
ROS often acts as a second messenger in cellular signaling, which is mediated by specific cysteine proteases, known as caspases.42 Studies have shown that ROS damages mitochondrial membrane, releases cytochrome c and activates caspase family protease caspase-9. Activated caspase-9 further cleaves the inactive form of caspase-3 into active caspase-3 cleaved form followed by the induction of apoptosis. Caspase family protease activation and the generation of ROS are considered key steps in the induction of cell death.42,43 Our findings with respect to reduced ROS levels and caspase-3 cleavage in ROCK1-deficient erythroblasts are consistent with those of cells from Fanconi anemia (FA) patients who demonstrate hypersensitivity to DNA cross-linking agents, oxidative stress, and apoptosis associated with increased ROS levels and caspase-3 activation.44 In response to hydrogen peroxide (H2O2) treatment, Fancc-deficient primary hematopoietic progenitors and murine embryonic fibroblasts (MEFs) show hypersensitivity to oxidative stress. Furthermore, pretreatment with antioxidants selenomethionine or N-acetylcysteine (NAC) preferentially enhances the survival of Fancc deficient cells.45 These results demonstrate that ROS and caspase-3 regulate survival in response to oxidative stress. Therefore, ROS has a positive feedback effect on p53 and caspase-3 expression. Our results point to an essential role for ROCK1 in regulating this process in stress-induced erythroblasts.
In response to stress, p53 activity and stability are controlled by posttranslational modifications that take place in N and C-terminal regions of p53. Phosphorylation of p53 results in increased p53 stability, accumulation, and enhanced transcriptional activation of p53 target genes responsible for apoptosis.46,47 Phosphorylation of p53 at ser15, ser20, and Thr18, which are located in the MDM-2 binding site prevent the binding of MDM2 and p53 leading to accumulation and activation of p53.48,49 Our results demonstrate reduced p53 phosphorylation and expression in ROCK1-deficient splenocytes in response to oxidative stress, which is associated with increased survival.
The enhanced recovery of ROCK1−/− mice in response to anemic stress could be because of enhanced EPO and BMP4 signaling in addition to reduced ROS and p53 levels. Previous studies have shown that serum EPO levels increase above normal levels in response to stress.50 We also observed a significant increase in serum EPO levels under steady-state as well as under stress conditions in ROCK1−/− mice. Because ROCK1−/− mice have 2-fold more EPO levels under steady-state conditions, we believe that the increase in the frequency of Ter119-positive cells in the BM and enhanced recovery might in part be because of increased response to stress via EPO. Furthermore, previous work suggested that increased serum EPO levels can result in enhanced mobilization of cells from the BM to the spleen where they can expand and differentiate in unique splenic microenvironment supported by BMP-4 in response to stress.5,9,10 Similar to the increase in EPO levels, deficiency of ROCK1 also resulted in increase in BMP4 mRNA expression in response to stress. Taken together, our findings suggest that enhanced recovery of splenic erythroblasts could be because of reduced ROS and p53 levels as well as because of increased serum EPO levels and BMP4 mRNA levels in ROCK1−/− spleen in response to stress.
In summary, our studies reveal that ROCK1 functions as a physiologic regulator of p53 level under conditions of stress. Our findings are expected to offer new perspectives on stress erythropoiesis and may provide a potential therapeutic target in human disease characterized by anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marilyn Wales for her administrative support.
This work was supported in part by the National Institutes of Health (NIH) grants R01HL075816 and R01HL077177 (to R.K.).
National Institutes of Health
Authorship
Contribution: S.V. and R.S.M. designed and performed research and wrote the paper; J.S. performed research and provided reagent; P.M., P.H., and K.R.K. performed research; E.H., L.W., and Y.L. provided reagent; and R.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reuben Kapur, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Cancer Research Institute, 1044 W Walnut St, Rm 168, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal