In this issue of Blood, Hivert et al systematically examine the acquired reduction of von Willebrand factor (VWF) activity in patients with Waldenström macroglobulinemia (WM), but, surprisingly, they found that the high levels of VWF were a significant independent adverse prognostic factor.1
Acquired von Willebrand syndrome (AVWS) is a rare acquired bleeding disorder in which VWF levels and activity are reduced. AVWS has been described in patients with IgM gammopathy and it has been hypothesized that this could be the result of IgM-related “deactivation” of VWF due to antibody activity or other physical properties of the monoclonal IgM (mIgM).2,3 WM is characterized by the infiltration of the BM by malignant lymphoplasmacytic cells that produce mIgM, often in large quantities. The clinical presentation of WM is dependent on the extent of the BM infiltration and the physical and chemical properties of the mIgM. Hivert et al prospectively and systematically measured VWF levels and activity in 72 patients with WM (including 17 patients with asymptomatic WM). The main findings: (1) About 13% of the patients had low levels of VWF and in 60% of them (6 of 10) there was an increased bleeding tendency. Patients with low VWF levels had higher levels of IgM and among them hyperviscosity was common. Furthermore, VWF levels increased after reduction of mIgM with plasmapheresis or treatment. It is of interest to note that no antibody activity of the mIgM was identified in the cases that were studied. Thus, another type of interaction between mIgM and VWF must exist, perhaps associated with the physicochemical properties of the large and hydrophilic molecule of IgM. (2) In 59% of patients the levels of VWF were actually increased. Furthermore, the higher levels of VWF were associated with inferior survival, independently of the International Scoring System for WM, a prognostic system that includes age and factors associated with tumor burden.4 In these patients the levels of VWF did not significantly decrease over time, even after successful therapy for WM. Hivert et al proposed that an underlying chronic endothelial activation could explain the high levels of VWF and indeed the BM microvessel density was higher in patients with more elevated VWF, indicating an association between VWF levels and angiogenesis in the BM.
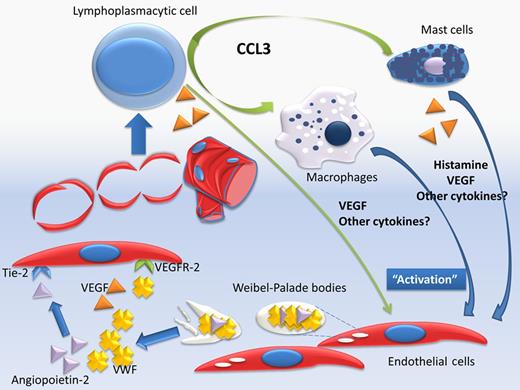
This is the first time that a mediator that is directly or indirectly associated with angiogenesis and endothelial function has prognostic significance in WM, and importantly, independently of other patient and disease-related factors. But how do these findings fit into our current knowledge? Angiogenesis represents an essential step in the evolution of several malignancies, including lymphomas and myeloma. Activation of endothelial cells (ECs) results in new vessel formation and several mediators, proangiogenic and antiangiogenic, are playing a role in this finely coordinated process. In cancer, proangiogenic cytokines and inflammatory mediators prevail and promote uncoordinated vessel formation. Our group has published data indicating that in patients with either WM or IgM–monoclonal gammopathy of unknown significance (MGUS) the serum levels of several angiogenic cytokines including VEGF, VEGF-A, angiogenin, and basic fibroblast growth factor are increased. Angiopoietin-2 was also increased and the balance of angiopoietin-1/angiopoietin-2, which antagonize for the receptor tyrosine kinase Tie-2, was proangiogenic in WM but not in IgM-MGUS.5 In the current study by Hivert et al lymphoplasmacytic cells stain positive for VEGF, indicating that these cells may also be a source of angiogenic cytokines. Other inflammatory mediators may also play a crucial role in angiogenesis in WM. C-C motif ligand 3 (CCL3, previously known as MIP1a) is a chemokine with a crucial role in inflammation, cell migration, and chemoattraction of monocytes/macrophages, neutrophils, and mast cells, and serum levels of CCL3 were also elevated in WM patients, even at remission.6 Importantly, in all WM cases, either at diagnosis or at relapse, all the neoplastic cells showed strong cytoplasmic positivity for CCL3, which indicates that these cells produce large amounts of this chemokine. CCL3 attracts macrophages, and probably mast cells, in the BM microenvironment and the number of macrophages was strongly correlated with the grade of angiogenesis.6,7 Mast cells have been recognized to support lymphoplasmacytic cells, and Hivert et al found that mast cells stain positive for VEGF and also were associated with increased microvessel density. But how do the elevated levels of VWF fit in the puzzle of angiogenesis process in WM (see figure)? VWF is produced constitutively in the megakaryocytes and ECs where it is stored in the Weibel-Palade bodies together with several other mediators, including several with angiogenic activity (such as angiopoietin-2) and others associated with inflammation, hemostasis, and vascular tone.8 VWF has a role in the trafficking and storage of these factors within ECs, including the maturation of adhesion molecules such as integrin αvβ3, while it co-localizes with molecules such as angiopoietin-2.9 The ECs release the content of Weibel-Palade bodies in response to stimuli, such as VEGF. VWF and other molecules are released simultaneously and a temporal association of VWF and angiopoietin-2 release has been shown.9,10 VWF may act as a regulator of angiogenesis and has antiangiogenic activity, that is, VWF-deficient ECs show a proangiogenic phenotype. In addition, VWF has an inhibitory activity in the constitutive VEGFR-2–dependent pathway(s) that promote EC migration; however, extracellular VWF only partially affects the previous processes.9 Thus, VWF may act as an “internal” and “external” regulator of angiogenic potential within ECs. However, these data indicate that elevated levels of VWF are rather a marker of the increased EC activity due to increased “proangiogenic” signaling rather than a major balancing factor. Thus, more questions are introduced: Do elevated levels of VWF have prognostic significance in other plasma cell dyscrasias or lymphomas? Does this imply that a “targeted” antiangiogenic therapy may have activity in patients with elevated VWF? Is the prognostic significance of elevated VWF levels treatment-specific? The results presented by Hivert et al generate more questions than answers and indicate a path for intensive research, especially considering the availability of targeted antiangiogenic therapies.
The serum levels of several angiogenic cytokines are increased in patients with Waldenström macroglobulinemia (WM). Angiopoietin-2 is also increased and the balance of angiopoietin-1/angiopoietin-2, which antagonize for the receptor tyrosine kinase Tie-2 is in favor of angiopoietin-2. Lymphoplasmacytic cells may produce VEGF, but they also produce large amounts of CCL-3 which attracts macrophages, and probably mast cells. Mast cells support lymphoplasmacytic cells and may also produce VEGF. VWF is stored in the Weibel-Palade bodies together with several other mediators, including angiopoietin-2. The endothelial cells (ECs) release the content of Weibel-Palade bodies in response to stimuli, such as VEGF and VWF and other molecules (such as angiopoitein-2) are released simultaneously. VWF may also have an inhibitory activity in the constitutive VEGFR-2–dependent pathway(s), which promote EC migration.
The serum levels of several angiogenic cytokines are increased in patients with Waldenström macroglobulinemia (WM). Angiopoietin-2 is also increased and the balance of angiopoietin-1/angiopoietin-2, which antagonize for the receptor tyrosine kinase Tie-2 is in favor of angiopoietin-2. Lymphoplasmacytic cells may produce VEGF, but they also produce large amounts of CCL-3 which attracts macrophages, and probably mast cells. Mast cells support lymphoplasmacytic cells and may also produce VEGF. VWF is stored in the Weibel-Palade bodies together with several other mediators, including angiopoietin-2. The endothelial cells (ECs) release the content of Weibel-Palade bodies in response to stimuli, such as VEGF and VWF and other molecules (such as angiopoitein-2) are released simultaneously. VWF may also have an inhibitory activity in the constitutive VEGFR-2–dependent pathway(s), which promote EC migration.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal