Abstract
Despite major improvements in outcome over the past decades, acute myeloid leukemia (AML) remains a life-threatening malignancy in children, with current survival rates of ∼ 70%. State-of-the-art recommendations in adult AML have recently been published in this journal by Döhner et al. The primary goal of an international expert panel of the International BFM Study Group AML Committee was to set standards for the management, diagnosis, response assessment, and treatment in childhood AML. This paper aims to discuss differences between childhood and adult AML, and to highlight recommendations that are specific to children. The particular relevance of new diagnostic and prognostic molecular markers in pediatric AML is presented. The general management of pediatric AML, the management of specific pediatric AML cohorts (such as infants) or subtypes of the disease occurring in children (such as Down syndrome related AML), as well as new therapeutic approaches, and the role of supportive care are discussed.
Introduction
Childhood acute myeloid leukemia (AML) is a rare and heterogeneous disease, with an incidence of 7 cases per million children younger than 15 years. In high-income countries, intensive therapy in conjunction with effective supportive care has increased survival rates to ∼ 70%. In 1990 and 2003, expert working groups made recommendations for diagnosis, outcomes, standardization of response criteria, and reporting standards for AML.1,2 Recent improvements in identifying the molecular genetics and pathogenesis of AML have been implemented in the new World Health Organization (WHO) classification of AML.3 These changes, and the definition of new diagnostic and prognostic markers and their associated targeted therapies, have prompted the update of earlier recommendations by an international group, on behalf of the European LeukemiaNet for AML in adults in 2010.4
Despite broad overlap in the diagnostic and treatment recommendations for AML for children and adults, there are important differences in both the diagnostic criteria and disease management, which merit age-specific recommendations. The absence of published recommendations specific for pediatric AML motivated an international group of pediatric hematologists and oncologists (panel and participating groups see “Appendix”) to develop evidence- and expert opinion-based consensus recommendations for the diagnosis and management of AML in children, incorporating emerging information on the biology of the disease. The scope of the review is presented in the “Appendix.” Recommendations for specific subgroups are also included. This article discusses diagnostic procedures and initial workup, prognostic factors, response criteria, and management, and in particular focuses on differences between adults and children with AML.
WHO classification and pediatric AML
The recent WHO 2008 classification is applicable to both adult and pediatric AML3,5 and has been summarized by Döhner et al.4 The classification contains most, but not all, cytogenetic subgroups specific to children. Differences in genetic background between children and adults are given in Table 1 and discussed further in “Cytogenetics.”
Comparison of biologic properties and genetic abnormalities in pediatric (children and adolescents < 18 years of age) and adult (age < 60 years) AML
| . | Adult AML . | Pediatric AML . | ||
|---|---|---|---|---|
| Frequency/special features . | Prognosis 5-y survival (age 18-60 y) . | Frequency/special features/occurrences . | Prognosis 5-y survival (age < 18 y) . | |
| Epidemiology | 12 (20-39 y) to 30 (40-59 y) | — | 2 (< 1 y) | — |
| Incidence rate (age-adjusted/100 person-years)206 | 0.7 (5-19 y) | |||
| Biology | ||||
| De novo AML | 83% | 30%-40% | > 95% | 60%-75% |
| Secondary (MDS-related) AML207,208 | 17% | Adverse | 1% | Adverse |
| Abnormal karyotypes | 55% | Depends on subgroups | 70%-80% | Depends on subgroups |
| . | Adult AML . | Pediatric AML . | ||
|---|---|---|---|---|
| Frequency/special features . | Prognosis 5-y survival (age 18-60 y) . | Frequency/special features/occurrences . | Prognosis 5-y survival (age < 18 y) . | |
| Epidemiology | 12 (20-39 y) to 30 (40-59 y) | — | 2 (< 1 y) | — |
| Incidence rate (age-adjusted/100 person-years)206 | 0.7 (5-19 y) | |||
| Biology | ||||
| De novo AML | 83% | 30%-40% | > 95% | 60%-75% |
| Secondary (MDS-related) AML207,208 | 17% | Adverse | 1% | Adverse |
| Abnormal karyotypes | 55% | Depends on subgroups | 70%-80% | Depends on subgroups |
| WHO classification . | ||||
|---|---|---|---|---|
| AML with recurrent genetic abnormalities14,15,209–211 | ||||
| t(8;21)(q22;q22)/RUNX1-RUNX1T1 | 8% | Favorable | 12%-14% | Favorable |
| inv(16)(p13.1q22)/CBFB-MYH11 | 5% | Favorable | 8% | Favorable |
| t(15;17)(q22;q21)/PML-RARA | 5%-10% | Favorable | 6%-10% | Favorable |
| t(9;11)(p22;q23)/MLLT3-MLL | 2% | 50% | 7% | Intermediate or favorable (63%-77%) |
| t(10;11)(p12;q23)/MLLT10-MLL | 1% | Unclear | 3%, mainly infants | Adverse |
| AML with t(6;9)(p23;q34)/DEK-NUP214 | 1% | Adverse | < 2% | Adverse |
| AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2)/RPN1-EVI1 | 1% | Adverse | < 1% | Adverse |
| AML (megakaryoblastic) with t(1;22)(p13;q13)/RBM15-MKL1 | < 1% | Unclear | AMKL only; infants | Intermediate |
| Provisional entity: AML with mutated NPM133,212,213 | 35% (53% in CN) | Favorable in case of FLT3-ITD negative | 5%-10% (14%-22% in CN), type A mutation dominant, increasing by age | Favorable |
| Provisional entity: AML with mutated CEBPA212 | 10% in CN | Favorable | 5% (14% in CN) | Favorable |
| FLT3-ITD27,30,213–215 | 20%-40% (∼ 50% in CN) | Adverse | 10% (18% in CN) | Context dependent* |
| WHO classification . | ||||
|---|---|---|---|---|
| AML with recurrent genetic abnormalities14,15,209–211 | ||||
| t(8;21)(q22;q22)/RUNX1-RUNX1T1 | 8% | Favorable | 12%-14% | Favorable |
| inv(16)(p13.1q22)/CBFB-MYH11 | 5% | Favorable | 8% | Favorable |
| t(15;17)(q22;q21)/PML-RARA | 5%-10% | Favorable | 6%-10% | Favorable |
| t(9;11)(p22;q23)/MLLT3-MLL | 2% | 50% | 7% | Intermediate or favorable (63%-77%) |
| t(10;11)(p12;q23)/MLLT10-MLL | 1% | Unclear | 3%, mainly infants | Adverse |
| AML with t(6;9)(p23;q34)/DEK-NUP214 | 1% | Adverse | < 2% | Adverse |
| AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2)/RPN1-EVI1 | 1% | Adverse | < 1% | Adverse |
| AML (megakaryoblastic) with t(1;22)(p13;q13)/RBM15-MKL1 | < 1% | Unclear | AMKL only; infants | Intermediate |
| Provisional entity: AML with mutated NPM133,212,213 | 35% (53% in CN) | Favorable in case of FLT3-ITD negative | 5%-10% (14%-22% in CN), type A mutation dominant, increasing by age | Favorable |
| Provisional entity: AML with mutated CEBPA212 | 10% in CN | Favorable | 5% (14% in CN) | Favorable |
| FLT3-ITD27,30,213–215 | 20%-40% (∼ 50% in CN) | Adverse | 10% (18% in CN) | Context dependent* |
| Not included in WHO 2008 . | ||||
|---|---|---|---|---|
| Adverse karyotypes/mutations | 32% | 5%-6% | ||
| t(7;12)(q36;p13)/t(7;12)(q32;p13) | Not in adults | — | Infants | Adverse |
| t(5;11)(q35;p15.5)/NUP98/NSD122 | Not in adults | — | Mostly in CN | Adverse |
| New mutations | ||||
| N-RAS35,40,216 | 10% | No prognostic significance | 20% (3% in CN) | No prognostic significance |
| MLL-PTD45 | 3%-5% | Adverse | 3% | Not yet defined |
| c-KIT40,43,87,217 | 17% in CBF leukemia | Adverse in t(8;21) | 25% in t(8;21) | Unclear |
| WT131,36,214 | 10% in CN | Adverse combined with FLT3-ITD | 13% in CN | Adverse combined with FLT3-ITD |
| PTPN1142 | Not in adults | — | 5%-21%, infants only | Unclear |
| IDH1/251 | 16% | Context-dependent mutation | Rare, 2%-3% | No prognostic significance |
| TET2218 | 8%-17% | Unclear | Very rare | Unclear |
| DNMT3A219 | 20% | Unclear | Rare | Unclear |
| Not included in WHO 2008 . | ||||
|---|---|---|---|---|
| Adverse karyotypes/mutations | 32% | 5%-6% | ||
| t(7;12)(q36;p13)/t(7;12)(q32;p13) | Not in adults | — | Infants | Adverse |
| t(5;11)(q35;p15.5)/NUP98/NSD122 | Not in adults | — | Mostly in CN | Adverse |
| New mutations | ||||
| N-RAS35,40,216 | 10% | No prognostic significance | 20% (3% in CN) | No prognostic significance |
| MLL-PTD45 | 3%-5% | Adverse | 3% | Not yet defined |
| c-KIT40,43,87,217 | 17% in CBF leukemia | Adverse in t(8;21) | 25% in t(8;21) | Unclear |
| WT131,36,214 | 10% in CN | Adverse combined with FLT3-ITD | 13% in CN | Adverse combined with FLT3-ITD |
| PTPN1142 | Not in adults | — | 5%-21%, infants only | Unclear |
| IDH1/251 | 16% | Context-dependent mutation | Rare, 2%-3% | No prognostic significance |
| TET2218 | 8%-17% | Unclear | Very rare | Unclear |
| DNMT3A219 | 20% | Unclear | Rare | Unclear |
| WHO classification continued . | ||||
|---|---|---|---|---|
| AML with myelodysplasia-related changes220,221 | 48% | Unfavorable | Low | No prognostic significance |
| Therapy-related myeloid neoplasms213 | 6% | Adverse | 3.5% | Adverse |
| Acute myeloid leukemia, not otherwise specified (NOS)213,222;27 | ∼ 17% | Intermediate | ∼ 15% | Intermediate |
| Myeloid sarcoma (syn.: extramedullary myeloid tumor; granulocytic sarcoma; chloroma) | 1% | — | 2%-4%, leukemia cutis (infants), chloroma | Favorable in case of favorable karyotypes |
| Myeloid proliferations related to DS | ||||
| Transient abnormal myelopoiesis (syn.: transient myeloproliferative disorder)223 | Not in adults | — | In 5% of the newborns with DS | Favorable |
| Myeloid leukemia associated with Down syndrome (ML-DS) | Not in adults | — | 400-fold increased risk for AMKL | 90% |
| Blastic plasmacytic dendritic cell neoplasia | Rare | Adverse | Not seen | — |
| Acute leukemias of ambiguous lineage | — | |||
| MPAL134 | Rare | Adverse | 4.5% | 65% |
| Therapy | ||||
| Percentage in clinical trials | < 40% | — | 50%-96% | — |
| HSCT in first CR | ∼ 60% | — | 13%-30% | — |
| CNS | Not analyzed | — | Intrathecal prophylaxis | — |
| WHO classification continued . | ||||
|---|---|---|---|---|
| AML with myelodysplasia-related changes220,221 | 48% | Unfavorable | Low | No prognostic significance |
| Therapy-related myeloid neoplasms213 | 6% | Adverse | 3.5% | Adverse |
| Acute myeloid leukemia, not otherwise specified (NOS)213,222;27 | ∼ 17% | Intermediate | ∼ 15% | Intermediate |
| Myeloid sarcoma (syn.: extramedullary myeloid tumor; granulocytic sarcoma; chloroma) | 1% | — | 2%-4%, leukemia cutis (infants), chloroma | Favorable in case of favorable karyotypes |
| Myeloid proliferations related to DS | ||||
| Transient abnormal myelopoiesis (syn.: transient myeloproliferative disorder)223 | Not in adults | — | In 5% of the newborns with DS | Favorable |
| Myeloid leukemia associated with Down syndrome (ML-DS) | Not in adults | — | 400-fold increased risk for AMKL | 90% |
| Blastic plasmacytic dendritic cell neoplasia | Rare | Adverse | Not seen | — |
| Acute leukemias of ambiguous lineage | — | |||
| MPAL134 | Rare | Adverse | 4.5% | 65% |
| Therapy | ||||
| Percentage in clinical trials | < 40% | — | 50%-96% | — |
| HSCT in first CR | ∼ 60% | — | 13%-30% | — |
| CNS | Not analyzed | — | Intrathecal prophylaxis | — |
Favorable, intermediate, and adverse were defined according to the definitions given14,15,224 : favorable indicates 5-year survival > 60% in adults and > 70% in children; intermediate, 23%-60% in adults and 50%-70% in children; and adverse, < 23% and < 50%, respectively, in children and adults.
— indicates not applicable; and AMKL, acute megakaryoblastic leukemia.
Different prognosis in combination with different other mutations.
Compared with previous classifications (European Group of Immunologic Characterization of Leukemias [EGIL], WHO 2001),6 the new WHO classification introduced a stringently defined subclass of acute leukemias of ambiguous lineage (mixed phenotype acute leukemias [MPALs]), mainly on the basis of detailed immunophenotypic criteria (Table 2) or presence of t(9;22)(q34;q11.2)/BCR-ABL1 or t(v;11q23)/MLL rearrangement.3,5,6 The new classification aims to create uniform subgroups defined by unifying molecular targets, which allow selection of specific treatment.
Immunophenotyping panel for diagnosis of AML and mixed phenotype AML in children
| Diagnosis of AML* | |
| Precursor stage | CD34, CD117, CD133, HLA-DR |
| Myelomonocytic markers | CD4, CD11b, CD11c, CD13, CD14, CD15, CD33, CD36, CD64, CD65, CD184 (CXCR4), intracellular† myeloperoxidase (iMPO), i-lysozyme |
| Megakaryocytic markers | CD41, CD42, CD61 |
| Erythroid marker | CD235a |
| Leukemia specific antigen | NG2 homolog‡ |
| Lineage aberrant antigens | CD2, CD7, CD19, CD56 |
| Pan-leukocyte markers | CD11a, CD45 |
| Diagnosis of MPAL§ | |
| Myeloid lineage | iMPO or evidence of monocytic differentiation by at least two of i-lysozyme, CD11c, CD14, CD64 |
| B-lineage | CD19 (strong) with at least 1 of iCD79a, iCD22, CD10; or CD19 (weak) with at least 2 of iCD79a, iCD22, CD10 |
| T-lineage | iCD3 or surface CD3 |
| Diagnosis of AML* | |
| Precursor stage | CD34, CD117, CD133, HLA-DR |
| Myelomonocytic markers | CD4, CD11b, CD11c, CD13, CD14, CD15, CD33, CD36, CD64, CD65, CD184 (CXCR4), intracellular† myeloperoxidase (iMPO), i-lysozyme |
| Megakaryocytic markers | CD41, CD42, CD61 |
| Erythroid marker | CD235a |
| Leukemia specific antigen | NG2 homolog‡ |
| Lineage aberrant antigens | CD2, CD7, CD19, CD56 |
| Pan-leukocyte markers | CD11a, CD45 |
| Diagnosis of MPAL§ | |
| Myeloid lineage | iMPO or evidence of monocytic differentiation by at least two of i-lysozyme, CD11c, CD14, CD64 |
| B-lineage | CD19 (strong) with at least 1 of iCD79a, iCD22, CD10; or CD19 (weak) with at least 2 of iCD79a, iCD22, CD10 |
| T-lineage | iCD3 or surface CD3 |
The table provides a list of selected markers (bold type represents the mandatory minimal panel required to fulfill WHO and EGIL criteria3,6 ), differing from the adult panel reported by Döhner et al4,214 in only a few markers (italics), which may have diagnostic or therapeutic implications.
Intracellular expression is represented by prefix “i.”
Most cases with rearranged MLL gene express the NG2 homolog reacting with the monoclonal antibody 7.1.
The requirements for assigning MPAL are based on the WHO 2008 criteria.3 Note that MPAL can also be diagnosed if there are 2 or more blast populations from different lineages.
Diagnostic procedures and initial workup
The minimal diagnostic requirements in childhood AML are morphology with cytochemistry, immunophenotyping, karyotyping, FISH, and specific molecular genetics in the bone marrow, which is comparable with common practice in adults. The initial diagnostic tests may be done on peripheral blood if the patient's condition contraindicates a bone marrow aspirate.
However, investigation of CNS involvement at diagnosis is not practiced routinely in adults but is considered necessary in children because specific treatment is required in case of CNS involvement (see “CNS-directed therapy”). However, in patients with a bleeding tendency, such as those with severe thrombocytopenia and/or coagulopathy and patients with acute promyelocytic leukemia (APL), lumbar puncture should be postponed until the risk of hemorrhage has passed. CNS positivity is generally defined by > 5 × 106/L white blood cells (WBCs) in the cerebrospinal fluid, with blasts present in a nonbloody tap.
Table 3 lists the recommended procedures and diagnostic tests in the initial workup of pediatric patients with AML. In the presence of high-risk conditions or premorbid conditions, additional tests may be required.
Recommended tests and procedures in the initial workup of pediatric patients with AML
| Test/procedure . | Rationale . |
|---|---|
| Tests to establish the diagnosis | |
| Complete blood counts and differential count | Diagnosis, initial risk |
| Bone marrow aspirate | Diagnosis |
| Bone marrow trephine biopsy* | Diagnosis |
| Lumbar puncture† | Diagnosis, treatment, prognosis |
| Immunophenotyping | Diagnosis |
| Cytogenetics | Diagnosis, treatment, prognosis |
| Molecular genetics/translocations/mutations (see Table 4) | Diagnosis, treatment, prognosis |
| Additional tests/procedures at diagnosis | |
| Demographics and medical history‡ | Initial risk |
| Performance status (WHO score) | Initial risk |
| Physical examination | Diagnosis, side effects |
| Syndromes, comorbidities | Treatment, prognosis |
| Biochemistry, coagulation tests§ | Initial risk |
| Serum pregnancy test‖ | Treatment |
| Hepatitis A, B, C; CMV, EBV, VZV, blood group Rh¶ | Diagnosis, side effects |
| Chest x-ray, 12-lead, electrocardiography (ECG); echocardiography | Diagnosis, side effects |
| Test/procedure . | Rationale . |
|---|---|
| Tests to establish the diagnosis | |
| Complete blood counts and differential count | Diagnosis, initial risk |
| Bone marrow aspirate | Diagnosis |
| Bone marrow trephine biopsy* | Diagnosis |
| Lumbar puncture† | Diagnosis, treatment, prognosis |
| Immunophenotyping | Diagnosis |
| Cytogenetics | Diagnosis, treatment, prognosis |
| Molecular genetics/translocations/mutations (see Table 4) | Diagnosis, treatment, prognosis |
| Additional tests/procedures at diagnosis | |
| Demographics and medical history‡ | Initial risk |
| Performance status (WHO score) | Initial risk |
| Physical examination | Diagnosis, side effects |
| Syndromes, comorbidities | Treatment, prognosis |
| Biochemistry, coagulation tests§ | Initial risk |
| Serum pregnancy test‖ | Treatment |
| Hepatitis A, B, C; CMV, EBV, VZV, blood group Rh¶ | Diagnosis, side effects |
| Chest x-ray, 12-lead, electrocardiography (ECG); echocardiography | Diagnosis, side effects |
Mandatory in patients with a dry tap (punctio sicca) and in case of dysplasia in the peripheral blood or bone marrow, or in the differentiation from MDS.
Required in all patients. Patients with clinical symptoms of suspected CNS involvement should be evaluated by imaging study for intracranial bleeding, leptomeningeal disease, and mass lesion; lumbar puncture should be postponed or is considered optional in other settings (eg, high WBC count or APL).
Includes race or ethnicity, family history, prior exposure to toxic agents, prior malignancy, therapy for prior malignancy, and information on smoking.
Biochemistry: sodium, potassium, calcium, phosphate, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, bilirubin, and uric acid; on indication, add other tests. Coagulation tests: prothrombin time (PTT), international normalized ratio (INR) where indicated, and activated partial thromboplastin time (aPTT).
In adolescents (female) of child-bearing potential.
Additional tests may be considered (eg, HIV-1, Parvo B19, and HLA typing).
Recommendations
At diagnosis, morphology, cytochemistry, immunophenotyping, and molecular and cytogenetics of the bone marrow aspirate are required. In the event of a dry tap or of a suspected underlying myelodysplastic syndrome (MDS), a bone marrow trephine biopsy has to be performed as well. All patients should have a cerebrospinal fluid examination for evidence of CNS involvement.
Morphology
The morphologic classification of AML is based on the lineage-associated phenotype (undifferentiated, myeloid, monoblastic, erythroblastic, or megakaryoblastic) and defined according to the French-American-British (FAB) classification.7 Morphologic studies reveal the percentages of undifferentiated, granulated or atypical blasts, intracellular structures, such as Auer rods, and presence of myelodysplasia. Cytochemistry confirms lineage affiliation and classifies myeloid (myeloperoxidase [MPO]-positive) and monoblastic differentiation (nonspecific esterase-positive). In the presence of ambiguous morphology and cytochemistry, immunophenotyping may further support the lineage definition. Acute megakaryoblastic leukemia (AMKL, FAB M7) and minimally differentiated AML (FAB M0) have to be confirmed by immunophenotyping, although the former may show typical morphologic features.7 The presence of myelofibrosis frequently associated with acute megakaryoblastic leukemia, and consequent sampling problems may lead to an underestimation of blasts by both morphology and immunophenotyping. In case of a low blast count (< 20%), repeated bone marrow sampling, including biopsy, needs to be done.
Differentiation between AML and MDS.
Differentiating between AML and advanced MDS may be difficult in children with a low percentage of blasts. In these patients, a trephine biopsy is necessary. Differentiating between AML and MDS is important for treatment allocation, as MDS can only be cured by hematopoietic stem cell transplantation (HSCT). In adults, a blast threshold of 20% is used to differentiate between these diseases, but in children blast percentages between 20% and 30% may be seen in MDS (refractory anemia with excess of blasts in transformation).8 AML-specific genetics, hyperleukocytosis, extramedullary disease, and progression within a short time frame (2-4 weeks) are supportive of AML rather than MDS. Children with Down syndrome (DS) should be diagnosed with AML in case of evidence for leukemic blasts in the bone marrow, even if the blast threshold of 20% is not reached. This is not considered MDS but myeloid leukemia of Down syndrome (ML-DS). The same exception is valid for AML with low blast counts and recurrent genetic abnormalities t(15;17), t(8;21), inv(16), or t(16;16).3
Recommendation
To differentiate between AML with a low blast count and MDS, serial bone marrow aspirates and trephine biopsies are required, as well as detailed cytogenetic analyses.
Immunophenotyping
Immunophenotyping is a rapid method to determine lineage and distinguish between AML and acute lymphoblastic leukemia (ALL). It is required to classify the AML FAB subtypes M0 (negative MPO activity by cytochemistry, but positive by immunophenotyping for myeloid markers, such as MPO [proenzyme] and/or CD13, CD33, CD117) and FAB M7 (positive for platelet markers, such as CD41 and/or CD61). However, immunophenotyping does not usually substitute for morphologic classification according to FAB criteria and blast cell enumeration. According to the currently used WHO 2008 classification, only the markers MPO, lysozyme, CD11c, CD14, CD64, i(intracellular)CD3, CD19, iCD22, iCD79a, and CD10 are essential to assign lineage affiliations and to define otherwise not specified MPAL. The latter includes biphenotypic leukemia, bilineage leukemia with distinctly differentiated blast populations, and undifferentiated leukemia without any lineage commitment.
At present, there is no standardization of antibody panels used for immunophenotyping among the large trial groups. However, upcoming standards suggest the use of multicolor monoclonal antibody combinations that include CD45 to enable optimal gating and analysis of the blast population within the complex context of residual hematopoiesis.9 In addition, these procedures might improve flow cytometry techniques for assessing minimal residual disease (MRD) in pediatric AML.10
Another methodologic transition concerns the interpretation of immunophenotypic expression data. Rigid cut-off points for determining marker positivity (eg, 10% or 20%) are being replaced by biologically more meaningful semiquantitative estimates of blast population appearances compared with those of normal populations.11 Table 2 summarizes the suggested antigen panel for immunophenotypic analysis during diagnosis of children with AML, which has been modified from that recommended for adults with AML.
Recommendation
The mandatory minimal panel required to fulfill WHO and EGIL criteria for AML includes CD34, CD117, CD11b, CD11c, CD13, CD14, CD15, CD33, CD64, CD65, iMPO, i-lysozyme, CD41, and CD61; and for MPAL: CD19, iCD79a, iCD22, CD10, and iCD3.
Conventional cytogenetics and FISH
Conventional cytogenetics can detect structural and numerical cytogenetic abnormalities in 70%-80% of children with AML. Certain fusion genes, products from cryptic translocations, or loss of chromosome material can only be reliably detected using FISH.
The diagnostic cytogenetic workup in children and adults is similar.4 The most frequent chromosomal abnormalities in children with AML include t(8;21)(q22;q22), inv(16)(p13.1q22) (together referred as core binding factor [CBF]-AML), t(15;17)(q22;q21)/PML-RARA, and 11q23/MLL-rearranged abnormalities (up to 25%), which together account for ∼ 50% of pediatric AML, a much higher frequency than in adults.12–15 Additional abnormalities that are more predominant in pediatric AML are, for example, t(1;22)(p13;q13)/RBM15(OTT)-MKL1(MAL).16,17 Further types are the cryptic abnormalities t(7;12)(q36;p13)/ETV6(TEL)-HLXB9(MNX1), which are strongly associated with a +19 and t(5;11)(q35;p15.5)/NUP98-NDS1, predominantly found in cytogenetically normal AML (CN-AML).18–22 However, both translocations have not yet been included in the WHO 2008 classification (Table 1).
Importantly, the 2008 WHO classification categorizes t(9;11)(p22;q23)/MLL-MLLT3(AF9) as an entity and recommends that partners in the variant MLL translocations should be identified. Frequently, 11q23/MLL rearrangements are complex and/or cryptic. For example, the t(10;11)(p12;q23)/MLL-MLLT10(AF10) or t(6;11)(q27;q23)/MLL-MLLT4(AF6) can generate the fusion via inversions, insertions, or translocations involving other chromosomes. The best method to evaluate these cases is the sequential G-banding to FISH with a MLL probe or RT-PCR, using specific primers.
Monosomy 7, monosomy 5/5q deletions, and aberrations of 12p are rare events (seen in 3%-5% of patients) that occur in nearly all subtypes of childhood AML.14,15,23 Monosomal karyotypes, which are associated with poor prognosis in adults, are extremely rare in children.24 Trisomies 8 und 21 are often associated with additional aberrations.14,15 Cytogenetic abnormalities correlate strongly with age: ∼ 50% of infants have MLL-rearranged AML, whereas CBF-AML occur typically in older children.25
Recommendation
Routine evaluation should include the evaluation of prognostically relevant genetic aberrations by cytogenetics/FISH, including at least the following fusion genes at diagnosis: RUNX1-RUNX1T1, CBFB-MYH11, PML-RARA, and MLL rearrangements. Other rare fusion genes mentioned in Table 4 should be traced to determine adverse risk patients.
Genetically defined prognostic groups in pediatric AML
| Prognosis14,15,22,27 . | Genetics . |
|---|---|
| Favorable | t(8;21)(q22;q22)/RUNX1-RUNX1T1 |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB-MYH11 | |
| t(15;17)(q22;q21)/PML-RARA* | |
| Molecular (in CN-AML) | |
| NPM1-mutated AML | |
| CEBPA double mutation | |
| t(1;11)(q21;q23)/MLL-MLLT11(AF1Q) | |
| GATA1s† | |
| Intermediate‡ | Cytogenetic abnormalities not classified as favorable or adverse§ |
| Adverse | −7,‖ −5 or del(5q) |
| inv(3)(q21q26.2) or t(3;3)(q21;q26.2)/RPN1-MECOM(EVI1-MDS1-EAP) | |
| t(6;9)(p23;q34)/DEK-NUP214 | |
| t(7;12)(q36;p13)/ETV6(TEL)-HLXB9(MNX1) | |
| t(4;11)(q21;q23)/MLL-MLLT2(AF4) | |
| t(6;11)(q27;q23)/MLL-MLLT4(AF6) | |
| t(5;11)(q35;p15.5)/NUP98-NSD1 | |
| t(10;11)(p12;q23)/MLL-MLLT10(AF10)¶ | |
| complex karyotype# | |
| WT1mut/FLT3-ITD** | |
| t(9;22)(q34;q11.2)†† |
| Prognosis14,15,22,27 . | Genetics . |
|---|---|
| Favorable | t(8;21)(q22;q22)/RUNX1-RUNX1T1 |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB-MYH11 | |
| t(15;17)(q22;q21)/PML-RARA* | |
| Molecular (in CN-AML) | |
| NPM1-mutated AML | |
| CEBPA double mutation | |
| t(1;11)(q21;q23)/MLL-MLLT11(AF1Q) | |
| GATA1s† | |
| Intermediate‡ | Cytogenetic abnormalities not classified as favorable or adverse§ |
| Adverse | −7,‖ −5 or del(5q) |
| inv(3)(q21q26.2) or t(3;3)(q21;q26.2)/RPN1-MECOM(EVI1-MDS1-EAP) | |
| t(6;9)(p23;q34)/DEK-NUP214 | |
| t(7;12)(q36;p13)/ETV6(TEL)-HLXB9(MNX1) | |
| t(4;11)(q21;q23)/MLL-MLLT2(AF4) | |
| t(6;11)(q27;q23)/MLL-MLLT4(AF6) | |
| t(5;11)(q35;p15.5)/NUP98-NSD1 | |
| t(10;11)(p12;q23)/MLL-MLLT10(AF10)¶ | |
| complex karyotype# | |
| WT1mut/FLT3-ITD** | |
| t(9;22)(q34;q11.2)†† |
Frequencies, response rates, and outcome measures should be reported by genetic group, and, if sufficient numbers are available, by specific subsets indicated.
t(15;17)/PML-RARA is treated separately from other AMLs.
In particular in DS patients and infants with acute megakaryoblastic leukemia, analysis of GATA1s mutations should be included. Identification of GATA1s-associated leukemia in trisomy 21 mosaicism can prevent overtreatment.
Includes all AMLs with normal karyotype, except for those included in the favorable subgroup; most of these cases are associated with poor prognosis, but they should be reported separately as they may respond differently to treatment.
For most abnormalities, adequate numbers have not been studied to draw firm conclusions on their prognostic significance.
Excluding recurrent genetic aberrations, as defined in the WHO 2008 classification.
Results in t(10;11)(p12;q23) are heterogeneous; therefore, intermediate prognosis may also be adequate.
Three or more chromosome abnormalities in the absence of one of the WHO-designated recurring translocations or inversions.
There are differences in the risk allocation of FLT3-ITD considering the allelic ratio.
t(9;22) is rare, but it is included because its poor prognostic impact is known.
Molecular genetics
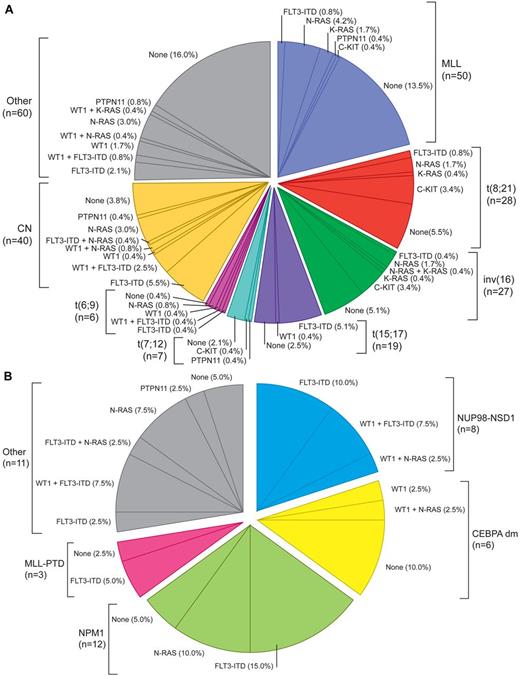
Several gene mutations and aberrantly expressed genes have been recognized in pediatric AML, further contributing to the disease's heterogeneity. AML is thought to result from at least 2 classes of cooperating mutations as hypothesized by Gilliland and supported by animal models.26 These classes can be categorized as type I mutations inducing proliferation, such as abnormalities in tyrosine kinases, and type II mutations, inducing maturation arrest, comprising most of the translocations. The frequency and nonrandom associations of type I and type II mutations in pediatric AML (Figure 1; Table 1) differ from adults, highlighting the difference in AML biology over age groups.27 In CN-AML, several mutations, such as NPM1, FLT3, WT1, and biallelic CEPBA mutations, are clinically relevant and should be included in standard diagnostics.27–31 Interestingly, the frequency and distribution of the subtypes of NPM1 mutations differ between children and adults.29,32–34 Mutations in the WT1 gene are found mainly in CN-AML and are often associated with FLT3-ITD mutations.35–37 The frequency of activating mutations of tyrosine kinase receptor genes, such as FLT3 (predominantly in CN-AML, t(15;17)(q22;q21)/PML-RARA and t(5;11)(q35;p15.5)/NUP98-NSD1), increases with age.22,30,31,35 Point mutations in the activating loop domain of the FLT3 receptor (frequency 2%-8% in children) are mutually exclusive of FLT3-ITD mutations.27,30,38
Pie chart illustrating the molecular and genetic aberrations as well as their nonrandom associations in pediatric AML. (A) Total group: Integrative analysis of recurrent cytogenetic aberrations (MLL-rearrangements, t(8;21), inv(16), t(15;17), t(7;12), and t(6;9), CN and other nonspecific cytogenetic subgroups and type I molecular aberrations (in FLT3-ITD, WT1, N-RAS, K-RAS, PTPN11, and c-KIT) based on 237 unselected de novo pediatric AML patients. Each sector indicates the percentage of patients harboring 1 or more of the aforementioned mutations. (B) CN group: Pie chart based on 40 cytogenetically normal de novo pediatric AML patients analyzed for the presence of type II molecular aberrations (in NUP98-NSD1, CEBPA dm, NPM1, MLL-PTD, and other molecular aberrations) and type I molecular aberrations (in FLT3-ITD, WT1, N-RAS, K-RAS, PTPN11, and c-KIT). Each sector indicates the percentage of patients harboring 1 or more of the aforementioned mutations. None indicates patients with only wild-type alleles of genes tested. Adapted from Hollink et al22 and Balgobind et al27 with permission.
Pie chart illustrating the molecular and genetic aberrations as well as their nonrandom associations in pediatric AML. (A) Total group: Integrative analysis of recurrent cytogenetic aberrations (MLL-rearrangements, t(8;21), inv(16), t(15;17), t(7;12), and t(6;9), CN and other nonspecific cytogenetic subgroups and type I molecular aberrations (in FLT3-ITD, WT1, N-RAS, K-RAS, PTPN11, and c-KIT) based on 237 unselected de novo pediatric AML patients. Each sector indicates the percentage of patients harboring 1 or more of the aforementioned mutations. (B) CN group: Pie chart based on 40 cytogenetically normal de novo pediatric AML patients analyzed for the presence of type II molecular aberrations (in NUP98-NSD1, CEBPA dm, NPM1, MLL-PTD, and other molecular aberrations) and type I molecular aberrations (in FLT3-ITD, WT1, N-RAS, K-RAS, PTPN11, and c-KIT). Each sector indicates the percentage of patients harboring 1 or more of the aforementioned mutations. None indicates patients with only wild-type alleles of genes tested. Adapted from Hollink et al22 and Balgobind et al27 with permission.
Mutations in genes involved in the RAS-RAF-ERK signal transduction pathway (PTPN11, NF-1, N-RAS, K-RAS) occur in 5%-21% of children with AML, more frequently in those with CBF-AML, and in young children with MLL-rearranged AML (Figure 1).27,39–42
C-KIT mutations occur in ∼ 25% of children with CBF-AML, but in only 5%-8% of those with other leukemia types.30,35,43,44 MLL-PTDs are rare in childhood AML.45
In addition to mutations, aberrant expression levels of genes have recently been reported in both adults and children; however, the biologic and clinical relevance might differ. Whereas BAALC and ERG overexpression is associated with CN-AML, EVI1 expression, which in adults is associated with monosomy 7 and inv(3), rarely occurs in children but is mainly found in association with t(6;11)(q27;q23)/MLL-MLLT4(AF6) and FAB types M6/7.46,47 Recent whole-genome sequencing studies have identified that novel somatic mutations in TET2, IDH1, IDH2, and DNMT3A are highly prevalent in adult AML but not in childhood AML.27,48–52
The detection of the different, prognostically relevant MLL fusions genes is also important; for further details concerning the impact of molecular aberrations on outcome in pediatric AML, see “Prognostic factors, Molecular genetics.”
Recommendation
Routine evaluation should include the evaluation of a prognostically relevant and potentially targetably selected set of molecular genetic markers FLT3-ITD, WT1, C-KIT, CEBPA (double mutation), NPM1, and further specific MLL-abnormalities with favorable or very poor prognosis (eg, MLL-AF1Q, AF6, AF10).
Genome-wide studies
Several gene expression profiling studies have been conducted in recent years in pediatric AML to study their diagnostic potential and to determine whether they can replace current labor-intensive diagnostic procedures that involve many different techniques. However, the value of gene expression profiling for routine diagnostics is currently limited.53
Biobanking
Biobanking of primary AML blasts, DNA, and RNA is relevant for patient care as it allows confirmation of initial findings or adding new data if required (ie, in case of relapse). It is a prerequisite for translational research. Stored material can be used to identify and characterize new prognostic markers, validate methods such as MRD, and enable experimental research of biologic mechanism, subgroups, and leukemogenesis. To discriminate between germline and somatic genetic aberrations in pediatric AML, the panel suggests storing additional material, such as buccal swabs.
Recommendation
Biobanking of leukemic blasts, DNA, and RNA as well as germline material is advised for potentially required diagnostic procedures and translational research.
Prognostic factors
Collaborative research in childhood and adult AML has identified several prognostic factors. These factors have been variably used for risk stratification. The most relevant factors are genetic abnormalities and treatment response, with substantial differences between adult and childhood AML. In the AML-Berlin/Frankfurt/Muenster (BFM) studies, age could not be used as an independent prognostic factor in infants and adolescents, but this may not be the case for other study groups, which reflects treatment dependency.25,54 Very high blast counts at diagnosis are associated with an increased risk of early death and nonresponse, but not necessarily with disease-free survival.55
Cytogenetics
As in adult AML, CBF-AML and t(15;17)(q22;q21) in children are highly predictive of a favorable outcome. Translocation t(1;11)(q21;q23)/MLL-MLLT11(AF1Q) is a newly described translocation associated with favorable outcome in childhood AML.56 However, prognosis of different MLL fusions is heterogeneous.56,57
Cytogenetics indicating an adverse outcome include −7 (excluding recurrent genetic aberrations, as defined in the WHO 2008 classification3 ), t(6;11)(q27;q23)/MLL-MLLT4(AF6), t(10;11)(p12;q23)/MLL-MLLT10(AF10), t(7;12)(q36;p13)/ETV6(TEL)-HLXB9(MNX1), t(6;9) (p23;q34)/DEK-NUP214, and t(5;11)(q35;p15.5)/NUP98-NSD1 and other rare abnormalities, such as 12p (Table 4).14,15,22 Further, adverse cytogenetics described in adult AML, such as 5q−, inv(3)(q21q26.2) or t(3;3)(q21;q26.2)/RPN1-EVI1, are very rare in children.
Results of studies on complex karyotypes (> 3 chromosomal abnormalities, excluding recurrent changes) have been inconsistent, in part because of differences in definition.14,15 Intermediate-risk factors include normal and other karyotypes. However, CN-AML has been shown to be a heterogeneous disease and the clinical outcome highly dependent on the presence of additional molecular aberrations and cryptic translocations (see “Molecular genetics”). The prognostic value of rare recurrent chromosomal aberrations remains to be elucidated and can be a future risk-stratification tool for pediatric AML.
Molecular genetics
The recognition of novel molecular subgroups in pediatric AML (see “Diagnostic procedures and initial workup, Molecular genetics”; Figure 1) has led to stratified treatment by using the identified mutated genes as treatment targets or by identifying patients eligible for myeloablative therapy.35
In CN-AML, single-gene mutations are of specific interest, especially the NPM1 and biallelic CEPBA mutations, as they are associated with favorable outcome.27–31 In contrast, a FLT3-ITD mutant to wild-type ratio (ITD allelic ratio) of > 0.4 has been associated with adverse outcome.30 Coincidentally occurring translocations, for example, cryptic translocations of t(5;11)(q35;p15.5)/NUP98-NDS1 or mutations such as WT1 or NPM1, can modify the prognostic relevance of the FLT3-ITD.22,37,58 The prognostic impact of other mutations mentioned in “Diagnostic procedures and initial workup, Molecular genetics” remains unclear.
Table 4 proposes genetically defined prognostic groups based on similar data obtained from large studies in Europe and the United States on the prognostic impact of mutations.14,15,22,27 These studies also represent the currently recommended standardized reporting for correlation of cytogenetic and molecular genetic data in pediatric AML.
Response and prognosis
Response to the first course of treatment and cytogenetics and molecular genetics are the 2 most important indicators of outcome. Both are independent prognostic factors and are usually essential elements of the risk group classification.59–61
Most study groups evaluate treatment response morphologically in the bone marrow after the first (eg, on day 15 or day 28) and second induction courses. This may be challenging in hypoplastic bone marrows. Blast cell reduction until day 15 and treatment response after the first and second induction are strongly predictive of outcome.59–61
Recommendation
Determination of treatment response after first and second induction courses is recommended for risk group stratification.
Monitoring of residual disease.
Residual disease can be monitored by morphology, immunophenotyping, and quantification of molecular aberrations and gene expression levels. Although some study groups have used MRD diagnostics for treatment stratification, its definitive clinical benefit is still under investigation and also probably treatment dependent.10,62 Depending on the method and the informative marker used, MRD monitoring can be applied in variable subsets of pediatric AML; however, a single approach may not meet the specific features of all patients.
Immunophenotyping.
MRD assessment by immunophenotyping can be done in up to 96% of children with AML. However, the heterogeneity of leukemia-associated immunophenotypes and frequent antigen shifts over time can limit the sensitivity and specificity of immunophenotypic detection of MRD. Whereas some groups failed to show an independent prognostic impact, other groups have used the technique for treatment intensification.10,62,63 Current technologic advances, such as ≥ 6-color flow cytometry, may overcome any limitations. However, method standardization and quality control are essential.
Fusion genes.
The high specificity and sensitivity (up to 10−5) of real-time quantitative PCR of AML fusion genes of RUNX1(AML1)-RUNX1T1(ETO), CBFB-MYH11, PML-RARA, and MLLT3(AF9)-MLL lend themselves to MRD monitoring but are applicable in only ∼ 35% of pediatric patients. The assessment of early response requires strict definition of time points because the MRD level may vary several logs in a short time frame.64 Low-level PCR positivity (< 10−4) of RUNX1(AML1)-RUNX1T1(ETO) or CBFB-MYH11 can be detected in patients in long-term remission; therefore, the evaluation of molecular relapse must consider the dynamics of increasing levels of fusion genes by quantitative PCR.65 Importantly, the kinetics of relapse differs between genetic subtypes with a median time from molecular to clinical relapse between 2 and 8 months.65 If confirmed to be of prognostic significance, increasing levels of MRD measured by fusion genes may indicate the need for early HSCT or restart of chemotherapy.
Mutation-specific MRD.
Recommendation
MRD measurement should be incorporated in the context of clinical trials and used, if appropriate, for treatment stratification.
Response and survival criteria
Response assessment
The response criteria for adult AML are widely used in pediatric AML, with some minor modifications (Table 5).4
Response criteria in AML
| Category . | Definition . |
|---|---|
| Complete remission (CR)* | Bone marrow blasts < 5%; absence of blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count > 1.0 × 109/L (1000/μL); platelet count > 80 × 109/L (80 000/μL); independence of red cell transfusions |
| CR with incomplete recovery (CRi)† | All CR criteria except for residual neutropenia (< 1.0 × 109/L [1000/μL]) or thrombocytopenia (< 80 × 109/L [80 000/μL]) |
| Treatment failure | |
| Resistant disease (RD) | Failure to achieve CR or CRi (general practice; phase 2/3 trials), or failure to achieve CR, CRi, or PR (phase 1 trials); only includes patients surviving ≥ 7 days after completion of initial treatment, with evidence of persistent leukemia by blood and/or bone marrow examination (this should be stated by bone marrow examination < day 42 and at the end of intensification courses) |
| Early death | |
| Death in aplasia | Death occurring ≥ 7 days after completion of initial treatment while cytopenic; with an aplastic or hypoplastic bone marrow obtained within 7 days of death, without evidence of persistent leukemia (< day 42) |
| Death from indeterminate cause | |
| Early bleeding/leukostasis | Deaths occurring before completion of therapy, or < 7 days after its completion; or deaths occurring ≥ 7 days after completion of initial therapy with no blasts in the blood, but no bone marrow examination available (< day 42) |
| Relapse‡ | Bone marrow blasts ≥ 5%; or reappearance of blasts in the blood; or development of extramedullary disease |
| Category . | Definition . |
|---|---|
| Complete remission (CR)* | Bone marrow blasts < 5%; absence of blasts with Auer rods; absence of extramedullary disease; absolute neutrophil count > 1.0 × 109/L (1000/μL); platelet count > 80 × 109/L (80 000/μL); independence of red cell transfusions |
| CR with incomplete recovery (CRi)† | All CR criteria except for residual neutropenia (< 1.0 × 109/L [1000/μL]) or thrombocytopenia (< 80 × 109/L [80 000/μL]) |
| Treatment failure | |
| Resistant disease (RD) | Failure to achieve CR or CRi (general practice; phase 2/3 trials), or failure to achieve CR, CRi, or PR (phase 1 trials); only includes patients surviving ≥ 7 days after completion of initial treatment, with evidence of persistent leukemia by blood and/or bone marrow examination (this should be stated by bone marrow examination < day 42 and at the end of intensification courses) |
| Early death | |
| Death in aplasia | Death occurring ≥ 7 days after completion of initial treatment while cytopenic; with an aplastic or hypoplastic bone marrow obtained within 7 days of death, without evidence of persistent leukemia (< day 42) |
| Death from indeterminate cause | |
| Early bleeding/leukostasis | Deaths occurring before completion of therapy, or < 7 days after its completion; or deaths occurring ≥ 7 days after completion of initial therapy with no blasts in the blood, but no bone marrow examination available (< day 42) |
| Relapse‡ | Bone marrow blasts ≥ 5%; or reappearance of blasts in the blood; or development of extramedullary disease |
Definitions of response criteria are based primarily on those given by Cheson et al2 and amended by Creutzig and Kaspers.225 Table 5 is analogous to the table of Döhner et al.4
All criteria need to be fulfilled; marrow evaluation should be based on a count of 200 nucleated cells in an aspirate with spicules; if ambiguous, consider repeating examination after 5-7 days; flow cytometric evaluation may help to distinguish between persistent leukemia and regenerating normal marrow; a marrow biopsy should be performed in cases of dry tap, or if no spicules are obtained; no minimum duration of response required. The categories morphologic leukemia-free state, partial remission (PR), cytogenetic CR (CRc), and molecular CR (CRm) may be useful in the clinical development of novel agents in phase 1 or 2 clinical trials. For definitions, see Döhner et al.4
The criterion of CRi is of value in protocols that use intensified induction or double-induction strategies, in which hematologic recovery is not awaited but intensive therapy will be continued. In such protocols, CR may even not be achieved in the course of the entire treatment plan. In these instances, the overall remission rate should include CR and CRi patients. Some patients may not achieve complete hematologic recovery, even with longer observation times. As treatment courses should be very condensed (with minimal delay) in pediatric patients, treatment is continued, even without complete platelet recovery. Therefore, the recommended cut-off value for platelets indicating CR is 80 × 109/L.
In patients with low blast percentages (5%-10%), a repeat marrow should be performed to confirm relapse. Appearance of new dysplastic changes should be closely monitored for emerging relapse. In a patient who has been recently treated, dysplasia or a transient increase in blasts may reflect a chemotherapy effect and recovery of hematopoiesis. Cytogenetics should be tested to distinguish true relapse from therapy-related MDS/AML.
Treatment failure
Early death can be defined more precisely using the time point of 42 days because response status is normally evaluated within 42 days. At later time points, death from nonresponse (relapse) or treatment-related mortality in remission should be distinguished as competing events.
Management
General aspects of treatment
All children with AML should preferably be treated within the context of well-designed clinical trials to ensure highest quality and safety of diagnostics and management. AML treatment in childhood should be risk-adapted, based on biologic factors to avoid overtreatment in patients with favorable prognosis and to improve outcome in those with unfavorable prognosis (see “Prognostic factors”). The aim of therapy is to cure the child by eradicating the leukemic clone while avoiding side effects and late effects as much as possible. Intensive polychemotherapy based mainly on anthracyclines and purine analogues should be started as soon as AML is diagnosed. Hyperleukocytosis and APL should be regarded as medical emergencies because of their early hemorrhagic risk (see “APL” and “Hyperleukocytosis”).
After induction therapy, postremission treatment is necessary to maintain remission. Additional CNS-directed intrathecal therapy is routine. In general, maintenance therapy has not been proven effective in children and adults, except in patients with APL (see “APL”).4,67,68
Several clinical trials suggest that increased intensity is associated with improved outcome in most AML subtypes, except APL, ML-DS, and inv(16). However, treatment-related toxicity, particularly cardiotoxicity, must be balanced against antileukemic efficacy to result in long-term cure (see “Induction”).
Improvements in supportive care have had a major impact on increased survival rates in childhood AML. Treatment intensity can be achieved either by high cumulative dosages of anthracyclines or purine analogues or intensively timed chemotherapy.69–71 The Medical Research Council (MRC) Study Group delivered relatively high doses of anthracyclines, whereas the Nordic Society of Pediatric Hematology and Oncology (NOPHO) Group, the Japanese Childhood AML Cooperative Study Group (JPLSG), and the St Jude AML Study Group have focused more on high-dose cytarabine (or other antimetabolites, such as cladribine). The AML-BFM study group has used both drugs in relatively high doses. Despite these different approaches, survival rates have been roughly similar. The number of treatment courses used by different groups has varied between 4 and 8, again with similar outcomes. Supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) gives an overview of the results of recent pediatric AML trials.
Recommendation
Children with AML should be treated within controlled clinical trials. Treatment of childhood AML requires an intensive anthracycline- and cytarabine-based therapy using at least 4 or 5 courses.
Induction
One or 2 courses of induction therapy are routinely used in children and adults. Standard induction therapy comprises 3 days of an anthracycline (eg, daunorubicin at least 60 mg/m2, idarubicin 10-12 mg/m2, or the anthracenedione mitoxantrone 10-12 mg/m2) and 7-10 days of cytarabine (100-200 mg/m2 continuously or twice daily intravenously; ie, “3 + 7” or “3 + 10”). With these regimens, > 85% of children and adolescents achieve complete remission (CR). Although a third drug, such as etoposide or 6-thioguanine, is commonly included in induction, their benefit has not been proven.72
Anthracyclines.
Various anthracyclines have been evaluated in prospective randomized pediatric clinical trials. There is evidence that higher doses of anthracyclines improve outcome in children and adults.73,74 However, toxicity, especially acute and late cardiotoxicity, is dose related and limits the cumulative dose.75,76 Cumulative dosages > 300 mg/m2 have been associated with significant later cardiac toxicity.77 There is no safe dose, but the antileukemic benefit of anthracyclines is such that most groups use a relatively high dose. The cumulative dose should consider host factors, such as age and sex.78 To avoid high peak serum concentrations of anthracyclines, splitting the daily dose or using prolonged drug infusions has been proposed; however, results concerning the benefit of dose scheduling are conflicting; hence, there is no consensus on the best regimen.75,79–81 Anthracyclines with a low cardiac exposure, such as liposomal anthracyclines, may be of benefit.79,81
Given an arbitrary conversion rate of 5:1 (daunorubicin 60 mg/m2 × 3 days: idarubicin 12 mg/m2 × 3 days), a better blast cell reduction at day 15 with comparable acute toxicity was achieved in the AML-BFM 93 trial by idarubicin (however, without an overall survival benefit).82 The randomized comparison between mitoxantrone (first induction 12 mg/m2, second induction 8 mg/m2, each × 3 days) and daunorubicin (50 mg/m2 × 3 days) in the MRC12 trial revealed similar results.83 The AML-BFM 2004 trial has shown that a liposomal formulation of daunorubicin allows the daunorubicin dose to be increased (80 mg/m2 × 3 days) without increasing acute and long-term cardiotoxicity thus far.
Cardioprotection with dexrazozane was another option to reduce cardiotoxicity during anthracycline exposure.84 However, the approved indications for this drug have been revised because of a possible higher rate of secondary malignancies in pediatric cancer patients (http://www.fda.gov/Drugs/DrugSafety/ucm263729).85
Recommendation
In childhood AML, the cumulative dose and application modus of anthracyclines must be considered because the risk of acute and long-term cardiotoxicity depends on both dosage and patient conditions.
Dosage of cytarabine.
The use of high-dose cytarabine (HiDAC) in first induction did not improve the CR rate or survival in adults or children.4,10,86 HiDAC may benefit CBF-AML patients when given in consolidation. The results of the AML-BFM 98 and 2004 trials in patients with t(8;21) AML demonstrated a significant favorable impact of HiDAC plus mitoxantrone in second induction.87
Additional agents.
Other drugs that have been used during induction include aclarubicin, amsacrine (adults), mitoxantrone (children and adults), and 2-chlorodeoxyadenosine (children).4,88–90 It is not clear whether these agents improve early treatment response, event-free survival, or overall survival compared with daunorubicin plus cytarabine at equivalent doses. Similarly, the benefit of gemtuzumab ozogamicin (GO), a calicheamicin conjugated to a CD33 antibody, has not been defined in children with de novo AML (see “Antibody-targeted drugs”).
Recommendation
One or 2 courses of induction therapy comprising 3 days of an anthracycline and 7-10 days of cytarabine should be applied.
Postremission strategies
Consolidation/intensification.
In most pediatric studies, 2 to 5 courses of chemotherapy with non–cross-resistant drug combinations similar to those given during induction are used to consolidate and maintain remission. There is no clear evidence that more than 3 consolidation courses (after 2 induction courses) are beneficial.72,83
High-dose cytarabine.
The Cancer and Leukemia Group B (CALGB) study in adults showed that 4 courses of HiDAC (3 g/m2 per every 12 hours on days 1, 3, and 5) were superior to 4 courses of lower-dose (100 mg/m2 continuous intravenously on days 1-5) cytarabine.91 This benefit of cytarabine dose intensification, however, was restricted to patients with CBF-AML and, to a lesser extent, to patients with CN-AML.92
Results of several pediatric trials (NOPHO AML 93, MRC AML 10, AML-BFM 2004, and AML99 of the JPLSG) and those of the adult CALGB study show that relapse rates can be reduced by introducing intensive chemotherapy courses that include HiDAC.92–97
HSCT.
HSCT is used as postremission consolidation therapy, and both autologous and allogeneic HSCT have been studied.
Autologous HSCT.
Several trials and a meta-analysis of pediatric AML trials have found no benefit for auto-HSCT compared with nonmyeloablative chemotherapy in first CR.95,98–101 In the absence of benefit, the toxicity associated with the conditioning regimen in auto-HSCT further negates against auto-HSCT. However, there is a role for auto-HSCT in relapsed APL without detectable MRD.102
Allogeneic HSCT.
The benefit of allo-HSCT as postremission consolidation treatment in first CR AML in children remains controversial. The significantly lower relapse risk associated with allo-HSCT compared with postremission chemotherapy has not consistently translated into an improvement in survival.103–105 Moreover, it has been calculated that at least 10 children must be transplanted to avoid 1 relapse.105 The risk-benefit for patients relies on intensity of prior chemotherapy, risk group allocation, CR status, donor type, predicted transplant-related mortality, and long-term toxicity. There is consensus that favorable-risk patients should not be transplanted in first CR, but for intermediate- and high-risk patients results are controversial, showing either no benefit in first CR or some benefit in intermediate- and high-risk patients.103,105,106
A meta-analysis of the comparative outcomes by risk group for HSCT and chemotherapy for 1373 children enrolled in 4 cooperative group clinical trials (Pediatric Oncology Group [POG] 8821, CCG 2891, CCG 2961, and MRC AML10) showed a benefit for allo-HSCT in intermediate-risk patients only.103 The analysis was weakened because a large number of patients could not be stratified by risk group, and this resulted in a possible selection bias. In addition, in some of the studies, the results of the chemotherapy only groups were inferior to those achieved in other pediatric trials.
In pediatric AML, conditioning regimens with myeloablative chemotherapy are preferred to total body irradiation as the latter is associated with more late effects and an increased risk of secondary malignancies.107–109 However, the optimal conditioning regimen remains to be identified and standardized.110,111
Pursuing a strategy of recommending sibling donors for standard-risk AML and reserving alternative donors for high-risk disease is in conflict with smaller family size and fewer children with matched siblings, and recently improved outcome with HSCT using unrelated matched donors.112 Whether HSCT from an alternative donor is superior to intensive chemotherapy alone as consolidation therapy in first CR remains to be determined. It has to be mentioned that high levels of MRD before HSCT have been associated with poorer outcome.113
In summary, the role of HSCT in children with AML in first CR should be reassessed as the field evolves, particularly within specific risk groups. There is consensus, however, that HSCT should be offered to all children with relapsed AML in second CR. In addition, many groups offer HSCT to children with high-risk or refractory disease and exploit the graft-versus-leukemia effect.114 There is agreement that favorable-risk patients should not be offered HSCT.105 The role of allo-HSCT in intermediate- and high-risk patients may be redefined by improving risk stratification, including the identification of new prognostic markers and improvements in MRD monitoring.
Recommendation
Auto-HSCT is not recommended for children with AML in first CR. Allo-HSCT in first CR is not beneficial in childhood AML with favorable risk factors. In other risk groups, the benefit of allo-HSCT must be balanced against toxicity. Allo-HSCT in second CR is generally considered.
CNS-directed therapy
CNS involvement at diagnosis and at relapse is seen in 5%-10% of pediatric patients with AML. Factors associated with CNS leukemia include hyperleukocytosis, monocytic leukemia [FAB M4 or M5, including M4eo with inv(16)], MLL gene rearrangement, and younger age.115
CNS treatment is given to all pediatric patients, including those who do not have detectable CNS involvement, assuming that systemic therapy has limited efficacy to eradicate hidden AML blasts in the CNS compartment. CNS treatment has varied from intrathecal chemotherapy (single-agent cytarabine or methotrexate, or triple cytarabine, methotrexate, and hydrocortisone) alone or given in combination with cranial radiotherapy. Cranial irradiation seems to be effective in preventing CNS leukemia; however, the risk of late toxicities and secondary malignancies, as well as the increasing use of HiDAC, which crosses the blood-brain barrier, and the lack of evidence of superiority for cranial irradiation, led to it being abandoned by all major study groups.116 The AML-BFM 87 trial was the only prospective study testing the benefit of cranial irradiation. Results including the nonrandomized patients showed an increased risk of CNS and/or bone marrow relapses in nonirradiated patients.117 However, other studies with comparable overall outcomes do not support a benefit from CNS irradiation. Pui and Howard recently reported that changing the intrathecal regimen from triple intrathecal therapy to cytarabine alone increased the CNS relapse rate.118 Finally, the optimal number of intrathecal treatments (range 4-12) remains unknown.
Recommendation
CNS treatment needs to consist of intrathecal treatments with cytarabine or methotrexate or in combination with steroids, although the optimal number of administrations is unknown.
Additional CNS-directed therapy in patients with CNS involvement.
CNS positivity (ie, CNS3 status) at the time of AML diagnosis necessitates intensified, CNS-directed therapy. Apparently, unlike in pediatric ALL, a low number of (sporadic) cerebrospinal fluid blasts (CNS2 status) does not influence the CNS relapse risk in pediatric AML.119 In contrast to ALL, CNS positivity is not a crucial factor within the AML risk group stratification because it does not affect overall survival.120 However, those with CNS involvement (as defined in “Diagnostic procedures and initial workup”) relapse more frequently in the CNS.119 Therefore, patients with CNS involvement require intensified intrathecal therapy to clear blasts from the CNS fluid. Although most study groups have added CNS irradiation to the regimen of these patients, recent observations suggest that frequent intrathecal chemotherapy combined with intensive systemic chemotherapy may yield similar results.119,121
Hematopoietic growth factors as priming agents
Sensitization of leukemic cells with hematopoietic growth factors (priming), such as G-CSF and GM-CSF, has been studied predominantly in adults with the aim of increasing cytotoxicity of chemotherapy.4 However, it cannot be recommended as routine practice in either adults and children, as the data are conflicting, and recent studies raise doubt about the beneficial role of G-CSF.124–126
Management of special patient groups
Children under 2 years of age
The biology of AML in children younger than 2 years is different from that of older children. Morphologic, cytogenetic, molecular genetics, and initial response show that these patients generally have features of high-risk AML.54 MLL rearrangements are frequent (∼ 50%), and some rare aberrations t(7;12)(q36;p13)/t(7;12)(q32;p13), t(7;12)(q36;p12), and t(1;22)(p13;q13)/RBM15(OTT)-MKL1(MAL) are nearly exclusive to this age group. In contrast, CBF-AML and t(15;17) are rarely seen.
In principle, management of young children is not different from that of older children. However, immaturity of organs (lung, liver, brain) in infants younger than 1 year and differences in pharmacokinetic and pharmacodynamic profiles of certain drugs (eg, cytarabine) increase their susceptibility to toxicities. Drug dosage in infants is generally calculated according to body weight (mg/kg) rather than body surface. Pharmacokinetic analysis has shown that children younger than 2 years have a reduced cytarabine clearance, which prompted the AML-BFM study group to adjust the dose of HiDAC by age.127
Compared with older children, acute toxicities in infants during induction chemotherapy are both more common and more severe, particularly sepsis, gut toxicity, and pulmonary toxicities.54 Acute and chronic cardiotoxicity is higher in infants than older children.78 Cranial irradiation should be avoided or postponed to avoid neurologic late effects (see “AML in Down syndrome and other genetic disorders”).
Congenital AML is rare and is curable when it occurs.54 A GATA1 mutation-associated leukemia based on an underlying constitutional (mosaicism) of trisomy 21 should be excluded because treatment is not required (see “AML in Down syndrome and other genetic disorders”). There are also rare reports of durable spontaneous remissions in newborns. Therefore, in clinically asymptomatic and stable newborns, intensive chemotherapy should be deferred until the hematologic course evolves or clinical symptoms requiring treatment develop.128
Recommendation
Infants tolerate intensive therapy, including HSCT, but their increased susceptibility to toxicities requires complex, sophisticated therapy concepts comprising age-adapted drug dosages and high treatment expertise.
Myeloid sarcoma
Myeloid sarcoma (MS; also termed extramedullary AML, extramedullary myeloid tumor, and granulocytic sarcoma) is a rare manifestation of AML characterized by the occurrence of 1 or more myeloid tumor masses (or infiltrations) at an extramedullary site.129 MS mainly occurs concurrently with AML but sometimes presents before bone marrow involvement as the first manifestation. Usually, the skin is affected (leukemia cutis), but other organs [chloroma, eg, in orbits (associated with t(8;21)), lymph nodes, bone, soft tissue, kidneys, testes] may also be involved. The presence of < 20% malignant bone marrow blasts distinguishes MS from frank de novo AML.130
Isolated primary MS and MS with a low blast count composes 2%-4% of all cases of childhood AML.131 MS is most frequent in young children (median age, 5 years), and its incidence decreases with age. MS can be misdiagnosed, primarily as non-Hodgkin lymphoma. Intensive AML-specific chemotherapy stratified according to general AML risk factors is recommended for all patients with MS.131 Local irradiation may be considered, but prospective studies are lacking because of the rarity of these manifestations.132
Recommendation
MS requires systemic treatment similar to that given for de novo AML.
Mixed phenotype acute leukemia
Recommendation
MPAL should be treated according to the dominant lineage defined by genetics, morphology, cytochemistry, and immunophenotyping.
AML in Down Syndrome and other genetic disorders
Children with specific inborn diseases are at increased risk of developing AML, with DS being the most frequent genetic disorder associated with increased likelihood of AML.136
Children with DS or DS mosaicism are at a 14- to 20-fold risk of developing acute leukemia. Approximately 5% of newborns with DS have transient leukemia (also referred to as transient abnormal myelopoiesis or transient myeloproliferative disorder), which usually disappears spontaneously.137 Within the first 4 years of life 10%-20% of these infants will develop myeloid leukemia with megakaryoblastic features (ML-DS). Transient leukemia and ML-DS are characterized by mutations in exon 2 of the hematologic transcription factor GATA1, which result in loss of the activation domain and leads to a truncated protein GATA1s.138,139 The leukemic blasts originate from fetal liver hematopoiesis.140 Although most newborns with DS experience spontaneous remission within 4-10 weeks, some have severe and life-threatening clinical symptoms associated with pancytopenia or organ infiltration. Hydrops fetalis is the most severe complication, and pleural effusions and ascites can be life-threatening in the unborn infant. Within the first weeks of life, some children develop fatal liver cirrhosis with hyperbilirubinemia (conjugated bilirubin > 250μmol/L).141 In patients with life-threatening symptoms resulting from hyperleukocytosis or organomegaly/organ dysfunction, intervention with exchange transfusion and/or low-dose cytarabine chemotherapy (1-1.5 mg/kg × 5-7 days) is usually applied.137,141
ML-DS can be successfully treated with intensity-reduced chemotherapy and without HSCT, resulting in event-free survival and survival rates of > 85%.142–144 As children with DS are especially susceptible to chemotherapy and have a compromised immune system, side effects and infections are frequent and treatment-related deaths are as frequent as relapse.142,145,146
Recommendation
Newborns with DS who have transient leukemia with severe clinical symptoms secondary to leukemic infiltrates should receive supportive care, and consideration should be given to the use of low-dose cytarabine.
ML-DS should receive reduced-intensive chemotherapy and particular attention given to the high risk of severe infections. HSCT is not indicated in first CR in ML-DS.
Patients with congenital syndromes, associated with increased chromosome fragility because of disturbed DNA-repair mechanisms (eg, Fanconi anemia and Bloom syndrome), as well as those with congenital diseases of the myelopoiesis (eg, Kostmann syndrome and Diamond-Blackfan anemia) are at a considerably increased risk of developing AML.147,148 Optimal therapy, including scheduling and intensity, has not yet been defined for these rare subgroups. In congenital neutropenia and Fanconi-anemia associated AML, less intensive therapy aimed at blast reduction followed by allo-HSCT may be successful. In Fanconi-anemia associated AML, cross-linking chemotherapy, high anthracycline dosages, and irradiation should be avoided.149
Little is known about AML in familial cancer syndromes, such as those involving germline CEBPA mutations. The few reported cases of pediatric AML have been successfully treated with conventional chemotherapy and allo-HSCT in first CR.150
Acute promyelocytic leukemia
APL is characterized by chromosomal rearrangements of 17q21 involving RARA, which is most commonly fused to the PML (promyelocytic leukemia) gene as a result of the t(15;17)(q22;q21) translocation. In < 5% of cases, RARA is fused to an alternative partner, causing a variable sensitivity to all-trans retinoic acid (ATRA). APL is considered a medical emergency because of the risk of life-threatening hemorrhage; therefore, treatment with ATRA should be initiated immediately.
In patients with high WBC counts (arbitrarily defined as > 10 × 109/L in children), the combined use of ATRA with chemotherapy decreases the incidence of the APL differentiation syndrome,102 which is characterized by fever, weight gain, respiratory distress, and pleural and pericardial effusions and occurs in ∼ 10% of children with APL treated with ATRA or arsenic trioxide. Pseudotumor cerebri during initial ATRA administration is common in children (11%) and can be treated with steroids.151,152
In children, a dose of 25 mg/m2 per day ATRA appears to produce outcomes equal to the higher dose of 45 mg/m2 per day commonly used in adults with a better safety profile.152–155
The use of ATRA in maintenance is generally recommended.102 Limited data obtained in children also support the use of ATRA in maintenance.155 Recent trials in adults suggest that subgroups of AML patients may not require (prolonged) maintenance treatment.156 This is also an important area for future clinical research in pediatric APL.
High cumulative dosages of anthracyclines combined with ATRA are very effective in treating APL.157 However, high doses of anthracyclines carry a risk of cardiotoxicity in children, and it would appear that a reduced cumulative dose of anthracyclines combined with HiDAC may be an equally successful approach.158
An intergroup protocol for children with APL (International Consortium for childhood APL; ICC APL; www.clinicaltrials.gov; #NCT01226303) has recently been opened. Quantitative monitoring of MRD will direct treatment of molecular relapse to prevent frank relapse. Such treatment has been shown to be beneficial, although only in sequential clinical trials in adults.102,159,160
Several studies in adults and children have demonstrated the effectiveness of ATRA combined with arsenic trioxid without conventional chemotherapy in refractory, relapsed, or newly diagnosed APL.161,162 However, more safety and efficacy data need to be generated before routinely introducing arsenic in newly diagnosed APL protocols in children.102
Recommendations
In children, ATRA at 25 mg/m2 per day should already be started if APL is suspected, as it reduces the risk of fatal hemorrhage. It should be used throughout treatment. The intensive, risk-adapted chemotherapy regimen in APL should be based on anthracyclines, cytarabine, and ATRA to avoid excessive anthracycline exposure.
Monitoring to detect molecular relapse allows earlier treatment, which might improve outcome.
Therapy-related AML
Therapy-related secondary AML (t-AML) is one of the most frequent secondary malignancies and generally follows the use of chemotherapy, including alkylating agents and topoisomerase II inhibitors, and/or radiotherapy.163
The etiology and strong association with specific translocations involving 11q23 (MLL) and less often with 5q−/−5 and 7q−/−7 in children are similar to that seen in adults.164 As in adults, the prognosis of t-AML in children is generally poor, which is because of resistance and cumulative toxicities of previous chemotherapy.4,165 Results from small studies suggest that these patients benefit from AML-type induction courses (double induction), followed by allogeneic HSCT, if remission can be achieved.165
Recommendations
Children with t-AML should participate in prospective AML trials that take into account prior chemotherapy. Allo-HSCT is recommended in first CR.
Relapsed and primary refractory AML
Approximately 5% of children with AML have refractory disease and 30% experience relapse.166 Bone marrow is the most common site of relapse, with the CNS being involved in up to 10% of cases (including combined relapses). Approximately 50% of patients have an early relapse, arbitrarily defined as within 1 year of initial diagnosis. In general, the prognosis in these children is poor.167,168
Prognostic factors in relapsed AML
Current treatment in relapsed AML
Different protocols and schedules have been used to induce a second remission, but currently antimetabolite (cytarabine, fludarabine) and anthracycline-based approaches are mainly used.171,172 With a realistic option for cure, reinduction should be offered to all children and adolescents with relapsed AML who can tolerate intensive treatment.167 Patients enrolled on the recently completed randomized international relapsed AML 2001/01 study comparing fludarabine/cytarabine/G-CSF with the addition of liposomal daunorubicin showed a second CR rate of 59% and 69%, respectively, translating to a survival rate of 38% for all patients.169
As in first-line treatment, CNS-directed therapy with several administrations of intrathecal triple chemotherapy is usual. Allo-HSCT is recommended for all children who achieve CR, using either related or unrelated HLA-matched donors, although formal evidence is lacking. If necessary, time to transplantation may be bridged with consolidation chemotherapy. The use of haploidentical or very high-risk allo-HSCT must be balanced against the potential treatment related mortality and the possibility of survival in late relapse after treatment with chemotherapy only.173
Patients who respond poorly to the first course of reinduction chemotherapy, who do not achieve a second CR and who relapse for the second time, can be offered more experimental therapy or palliation alone. Data on allo-HSCT in AML with blast persistence are scarce; however, survival rates are generally poor.167
Other options include GO (see “Antibody-targeted drugs”) alone or in combination with other agents.174,175 More intensive approaches include clofarabine monotherapy or combined with cytarabine and/or liposomal daunorubicin/cyclophosphamide and have shown moderate survival rates (26% and 38%, respectively) in these heavily pretreated patients.176,177
Recommendation
Children with relapsed AML should be treated with reinduction chemotherapy followed by allo-SCT.
New therapy approaches
The intensification of conventional chemotherapy along with improvements in supportive care has improved the prognosis in childhood AML. However, side effects and toxicity still remain a major concern. New compounds, such as epigenetically active agents, tyrosine kinase inhibitors, and antibody-mediated treatment, might be effective but less toxic approaches in AML.
Antibody-targeted drugs
Gemtuzumab ozogamicin.
Gemtuzumab ozogamicin (GO), a calicheamicin-conjugated CD33 antibody, has shown promising results in children with AML.175,178,179 GO causes considerable toxicity, especially myelosuppression with an extended thrombocytopenia, and veno-occlusive disease, but less frequently in children than in adults.175 Moreover, such toxicity is dose related and has been shown to be significantly less pronounced at GO doses of ≤ 6 mg/m2 in recent studies.180 Salvage studies demonstrated that the drug was beneficial in children who had poor chances of survival by conventional therapy.175,181,182
In adults, study MRC AML15 showed that GO was beneficial in those with favorable cytogenetics and in part of patients in the intermediate-risk group; however, there was no benefit to those in the adverse-risk group.174 In addition, other recent international studies on adult AML (by the French and United Kingdom groups) confirmed a survival benefit for AML subgroups when GO was added to induction chemotherapy.180,183–185 Recent data from a prospective nonrandomized COG trial showed that GO did not cause increased toxicity, but data on efficacy are pending.186 However, as the approved indications for GO have been revised in the United States by the FDA request, it may not be easily available for use in pediatric AML outside of clinical trials.
Tyrosine kinase inhibitors
AML patients with activating FLT3 or KIT mutations are candidates for targeted therapy. Many FLT3-targeting tyrosine kinase inhibitors (sorafenib, CEP701, PKC412, AC220) have been on trial in children or used on a compassionate-use basis.187 A recent report from the St Jude Children's Research Hospital demonstrated the feasibility of combining sorafenib and conventional chemotherapy in childhood AML, with some evidence of efficacy limited to patients with FLT3-ITD.188
Supportive care
Several aspects of supportive care deserve special consideration in children, such as approval and dosing of pharmaceutical agents and proxy-reporting of preferences and quality-of-life status. The improved outcome in children with AML over the last 10 years is probably associated with better supportive care strategies.
Hyperleukocytosis
Initial hyperleukocytosis (arbitrarily defined as WBC ≥ 100 × 109/L) is associated with a high risk of hemorrhage (mainly in the CNS) and leukostasis, which are often fatal. Patients with monocytic or myelomonocytic leukemia (FAB M4/M5) and hyperleukocytosis as well as APL patients have an increased risk of early death, and emergency strategies to reduce these leukemia-related complications are needed.189,190
In the case of hyperleukocytosis, emergency care with intensive monitoring and careful hydration with concurrent administration of rasburicase may be necessary.191 If there are also symptomatic coagulopathy and/or leukostasis, exchange transfusion or leukapheresis can be life-saving. Initiation of chemotherapy should not be delayed. A controlled but effective cell reduction (eg, low-dose cytarabine; leukapheresis) together with enforced diuresis, or even hemodialysis, may prevent the severe tumor lysis syndrome.189,190
Recommendation
For patients with initial hyperleukocytosis and symptomatic coagulopathy and/or leukostasis, emergency strategies should be initiated to reduce the risk of fatal hemorrhage and leukostasis.
Prophylactic anti-infectious treatment
Antifungal prophylaxis.
The incidence of invasive fungal infections in children with AML is ∼ 20%, which is similar to that in adults, for whom prophylaxis with an antifungal agent is recommended. In adults, prophylactic posaconazole is more effective at reducing invasive aspergillosis and thus reducing mortality than fluconazole or itraconazole.192 Unfortunately, posaconazole is not licensed for use in children younger than 13 years, and its dosage has not yet been defined for young children in the prophylactic setting; itraconazole, voriconazole, micafungin, or liposomal amphotericin B may be used instead. However, these compounds have limitations, such as variable absorption or multiple potential drug-drug interactions (eg, itraconazole and voriconazole), or can only be administered intravenously (eg, echinocandins and amphotericin B formulations).193 As in other childhood malignancies, cotrimoxazole prophylaxis should be used for Pneumocystis jirovecii.194,195
Recommendations
Antifungal prophyaxis (including trimethoprim-sulfamethoxazole for prophylaxis of P jirovecii) should be administered to all children.
Antibiotic prophylaxis.
Up to 70% of children have a microbiologically documented bacterial infection per course of AML therapy, and bacterial infections remain a common cause of toxic death.196 Importantly, the cumulative incidence of bacteremia with viridans group streptococci is > 40% and is, in particular, associated with use of HiDAC and mucositis.197 However, there are insufficient data to recommend routine vancomycin prophylaxis in this setting.198 A recent Cochrane review analyzing pediatric and adult patients with various underlying malignancies suggested that antibiotic prophylaxis in afebrile neutropenic patients significantly reduced all-cause mortality, with the most significant reduction in mortality observed in trials assessing prophylaxis with quinolones.199 Therefore, according to current guidelines, fluoroquinolone prophylaxis might be considered for high-risk patients with expected prolonged and profound neutropenia (absolute neutrophil count < 100/μL for > 7 days).199–202 This potential benefit of quinolones must be weighed against an increase in antimicrobial resistance to fluoroquinolones, which are not licensed for use in children.
Recommendation
The use of fluoroquinolones may be considered as prophylaxis against Streptococcus viridans and gram-negative sepsis.
Hematopoietic growth factors
Although a recent survey demonstrated that there is a wide variation in the prophylactic use of G-CSF in children with AML,203 the routine use of hematopoietic growth factors, such as G-CSF or GM-CSF, is not recommended in this patient population. Although G-CSF significantly decreases the duration of neutropenia in induction therapy, it does not influence the incidence of febrile neutropenia or microbiologically documented infections or affect infection-associated mortality.204,205
It has also been recently demonstrated that children who overexpress the differentiation-defective G-CSFR isoform IV and receive G-CSF have a high risk of relapse.124 However, in critically ill patients, shortening neutropenia by G-CSF may be an option.
Recommendation
Prophylactic hematopoietic growth factors, such as G-CSF, should not be used routinely.
Transfusion support is an important issue in childhood AML. Information regarding this topic is given in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Future perspectives
Diagnosis and treatment in pediatric AML has improved considerably during the past decades, with children currently achieving 5-year overall survival rates of ∼ 70%. This has resulted from the cooperative studies of national groups, leading to a stepwise improvement of treatment, including improved supportive care. The definition of risk groups has led to a more risk-adapted treatment, avoiding overtreatment in low-risk patients and including more intensive therapy in others. The current risk definition is mainly based on cytogenetics, a limited number of molecular markers, and the response to induction therapy. Future genomewide approaches, such as gene expression analysis, methylation array studies, genomic profiling, miRNA profiling, and whole-genome sequencing, will further expand the potential of diagnostics and promote the identification of genes and involved pathways, providing better insight into the leukemogenesis of pediatric AML. Knowledge of the biology may then translate into better treatment options and improved cure rates in conjunction with better risk-group adapted therapy and reduced late effects of therapy.
International cooperation is extremely important, as not only is AML rare but also a very heterogenous disease, and only international collaboration will provide adequate numbers of patients to power trial questions within subgroups as new targeted therapies become available to children.
Appendix
Panel
The panel included 21 representatives from national groups from Europe, the United States, and Japan collaborating in the AML committee of the International Berlin/Frankfurt/Muenster (I-BFM) Study Group who have clinical and research expertise in pediatric AML. Between May 2011 and February 2012, the panel met twice in person and communicated extensively via electronic discussion (mails and telephone conferences).
Participating groups
Participating groups included the following: AIDA, Associazione Italiana di Ematologia e Oncologia Pediatrica (Italy); BFM, Berlin/Frankfurt/Münster (Germany, Austria, Switzerland, Czech Republic); COG, Children's Oncology Group (United States); DCOG, Dutch Childhood Oncology Group (The Netherlands); JPLSG, Japan Pediatric Leukemia and Lymphoma Study Group (Japan); LAME, Leucémie Aiguë Myéloblastique Enfant (France); NCRI, National Cancer Research Institute (United Kingdom); NOPHO, Nordic Society of Pediatric Hematology and Oncology (Denmark, Finland, Island, Norway, Sweden); and St Jude AML Study Group (United States).
Scope of the review
The expert panel has developed diagnostic and treatment recommendations for AML in children and adolescents, motivated by recent recommendations from the adult AML panel.4 All the main pediatric AML study groups were included, and an extensive PubMed literature search (keywords = pediatric/childhood acute myeloid leukemia/AML, relapsed acute myeloid leukemia, diagnosis, risk stratification, therapy management, minimal residual disease, mutations AML, cytogenetic/karyotype AML) was performed. The majority of recommendations is based on well-planned but nonrandomized therapy optimizing studies often including randomized questions or on expert opinions with a consensus of the panel.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Hartmut Döhner (University Hospital of Ulm, Ulm, Germany) for valuable advice, constructive criticism, and extensive guidance while preparing the manuscript; Ursula Bernsmann (Medical School Hannover, Hannover, Germany) for help in preparing the manuscript; and Dr Vani Shanker (St Jude Children's Research Hospital, Memphis, TN) for editing the manuscript.
Authorship
Contribution: U.C. assembled the sections; U.C., M.M.v.d.H.-E., D.R., G.J.L.K., B.G., and C.M.Z. wrote the final version of the manuscript; and all authors reviewed the literature, wrote first drafts of specific sections, and reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ursula Creutzig, Hannover Medical School, Children's Hospital, Department of Paediatric Haematology and Oncology, Carl-Neuberg-Str 1, D-30625 Hannover, Germany; e-mail: ursula@creutzig.de.
References
Author notes
U.C. and M.M.v.d.H.-E. contributed equally to this study.
G.J.L.K. and D.R. contributed equally to this study.