Abstract
An endoplasmic reticulum transmembrane prolyl 4-hydroxylase (P4H-TM) is able to hydroxylate the α subunit of the hypoxia-inducible factor (HIF) in vitro and in cultured cells, but nothing is known about its roles in mammalian erythropoiesis. We studied such roles here by administering a HIF-P4H inhibitor, FG-4497, to P4h-tm−/− mice. This caused larger increases in serum Epo concentration and kidney but not liver Hif-1α and Hif-2α protein and Epo mRNA levels than in wild-type mice, while the liver Hepcidin mRNA level was lower in the P4h-tm−/− mice than in the wild-type. Similar, but not identical, differences were also seen between FG-4497–treated Hif-p4h-2 hypomorphic (Hif-p4h-2gt/gt) and Hif-p4h-3−/− mice versus wild-type mice. FG-4497 administration increased hemoglobin and hematocrit values similarly in the P4h-tm−/− and wild-type mice, but caused higher increases in both values in the Hif-p4h-2gt/gt mice and in hematocrit value in the Hif-p4h-3−/− mice than in the wild-type. Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mice nevertheless had increased hemoglobin and hematocrit values without any FG-4497 administration, although no such abnormalities were seen in the Hif-p4h-2gt/gt or P4h-tm−/− mice. Our data thus indicate that P4H-TM plays a role in the regulation of EPO production, hepcidin expression, and erythropoiesis.
Introduction
Erythropoiesis is a tightly controlled process, its key regulator being erythropoietin (EPO). During embryonic development most of the EPO production occurs in the liver, whereas the major EPO source in adults is the kidney, although the liver maintains a capacity for its expression.1,2 Hypoxia-inducible transcription factor (HIF) plays a pivotal role in the regulation of the transcription of the EPO gene and numerous other hypoxia-regulated genes, including many additional genes influencing erythropoiesis.1–4 The HIF-α subunit isoforms HIF-1α and HIF-2α are synthesized constitutively, and hydroxylation of 2 critical prolines generates 4-hydroxyproline residues that target HIF-α for rapid degradation in normoxia.5–7 In hypoxia, this hydroxylation is inhibited, so that HIF-α escapes degradation, translocates into the nucleus, and dimerizes with HIF-β.5–7 HIF-1α is expressed in all nucleated cells, whereas HIF-2α expression is restricted to specific cell types, including renal interstitial cells and hepatocytes.1,2 Renal and hepatic EPO production in adults is primarily regulated by HIF-2α, but HIF-1α also plays a role in several situations.1,2
Erythropoiesis requires iron. Hepcidin, a 25-amino-acid peptide secreted predominantly from hepatocytes, is the central regulator of iron metabolism. It down-regulates ferroportin and thus inhibits the absorption of dietary iron and the release of iron form erythrocytes and macrophages.8,9 Hepcidin expression is lowered by hypoxia and agents that stabilize HIF-2α leading to increased serum EPO concentration and erythropoiesis, its regulation also involving the hemojuvelin/BMP axis, hemojuvelin cleaving proteins and growth factors.8–10 Hepcidin synthesis is increased in inflammatory states and hepcidin appears to play a key role in the pathogenesis of the anemia of chronic disease.8
Hydroxylation of HIF-α is known to be catalyzed in vertebrates by 3 HIF prolyl 4-hydroxylase isoenzymes (HIF-P4Hs 1-3, also known as PHDs 1-3 and EglNs 2, 1, and 3).11–13 A fourth P4H possessing an endoplasmic reticulum transmembrane domain (P4H-TM) is also able to hydroxylate HIF-α in vitro and in cultured cells, but its roles in the regulation of HIF-α in mammalian tissues in vivo remain to be established.14,15 P4H-TM is expressed in many tissues, including the kidney,15 where the expression is seen in tubular regions of the inner cortex (M.R., P. Tiainen, J. Hyvärinen, R. Sormunen, I. Miinalainen, R. Soininen, K.I.K., J.M., and P.K., unpublished data, July 2012). All these enzymes are 2-oxoglutarate dioxygenases, which require Fe2+, 2-oxoglutarate, O2, and ascorbate.16
Hif-p4h-2 null mice die during embryonic development, whereas Hif-p4h-1 and Hif-p4h-3 null mice are viable.17 Broad-spectrum conditional inactivation of Hif-p4h-2 causes increased Epo production in the kidney, but not in the liver, and severe erythrocytosis,18–20 whereas hepatic inactivation of Hif-p4h-2 does not increase serum Epo or blood hematocrit values.21 We have generated Hif-p4h-2 hypomorphic mice (Hif-p4h-2gt/gt) that express lower amounts of wild-type Hif-p4h-2 mRNA in various tissues, approximately 35% of that in wild-type mice in the kidney and 85% in the liver.22 These mice have no increase in their serum Epo concentration, kidney Epo mRNA level or blood hemoglobin or hematocrit values.22 Hif-p4h-1−/− and Hif-p4h-3−/− mice have no increase in their serum Epo concentration, renal or hepatic Epo mRNA levels and hemoglobin or hematocrit values, whereas Hif-p4h-1/Hif-p4h-3 double-null mice show moderately increased erythropoiesis because of increased hepatic Epo expression.19,21 Hif-p4h-1/Hif-p4h-2/Hif-p4h-3 triple-null mice, with broad-spectrum Hif-p4h-2 inactivation, have markedly increased serum Epo and blood hematocrit values because of the induction of both renal and hepatic Epo synthesis.21 We have also produced P4h-tm−/− mice. These have a mild kidney defect that leads to slight late-onset proteinuria and eye abnormalities that are only seen from approximately 1 year onwards (M.R., P. Tiainen, J. Hyvärinen, R. Sormunen, I. Miinalainen, R. Soininen, K.I.K., J.M., and P.K., unpublished data, July 2012). Nothing is known about the possible role of P4H-TM in the regulation of erythropoiesis in any mammalian species, but a 3- to 4-fold increase in the epo mRNA level without any signs of polycythemia was found in p4h-tm–deficient zebrafish embryos.23
Many small-molecule compounds have been developed that inhibit HIF-P4Hs competitively with respect to 2-oxoglutarate leading to stabilization of HIF-α in cultured cells and in vivo.16,24,25 One such compound, FG-4497, has been shown to stabilize HIF-1α and HIF-2α, to increase the expression of Epo mRNA and several other hypoxia-regulated mRNAs in cultured cells and in vivo and to elevate serum Epo and blood hematocrit values in mice and rats without apparent toxicity,26–32 whereas another such compound, FG-2216 has been reported to decrease Hepcidin expression.33 We administered here FG-4497 to P4h-tm−/− mice to study its possible roles in Epo production, Hepcidin expression and erythropoiesis. We expected that if P4H-TM is involved in the regulation of these events, its null mice would be more sensitive to FG-4497 administration than wild-type mice. We also gave FG-4497 to Hif-p4h-2gt/gt and Hif-p4h-3−/− mice and compared its effects to those seen in the P4h-tm−/− mice. We also generated a Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mouse line and studied erythropoiesis in these mice without any FG-4497 administration. Our data indicate that, in addition to the 3 HIF-P4Hs, P4H-TM is a fourth P4H regulating Epo production, Hepcidin expression and erythropoiesis.
Methods
Expression, purification, and activity assays of recombinant P4H-TM and HIF-P4Hs
See supplemental Methods for description of enzyme expression, purification and activity assays (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mouse lines
Only female mice were used here. P4h-tm−/− mice were generated by targeting a LacZNeo cassette into P4h-tm exon 3, leading to a truncated transcript of exons 1-2 and a split exon 3 fused to LacZNeo (M.R., P. Tiainen, J. Hyvärinen, R. Sormunen, I. Miinalainen, R. Soininen, K.I.K., J.M., and P.K., unpublished data, July 2012). Generation of Hif-p4h-2gt/gt mice was previously described.22 Hif-p4h-3−/− mice were generated by replacing Hif-p4h-3 exon 2 with a neo-selection cassette flanked by loxP sites. Correct exon 2 deletion was verified at the cDNA level. The mice lacked 2 amino acids needed for the binding of Fe2+ at the catalytic site, which led to complete loss of the enzyme activity (A.L., J.M.M., K.I.K. and J.M., unpublished data, July 2012). All mouse lines were backcrossed to a C57BL/6 line. To obtain Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mice we crossed Hif-p4h-2+/gt mice with P4h-tm−/− mice and obtained Hif-p4h-2+/gt/P4h-tm+/− offspring. Hif-p4h-2gt/gt mice were not used in breeding because of the low number of offspring. Crossings of Hif-p4h-2+/gt/P4h-tm+/− or Hif-p4h-2+/gt/P4h-tm−/− mice with Hif-p4h-2+/gt/P4h-tm+/− or Hif-p4h-2+/gt/P4h-tm−/− mice produced a few Hif-p4h-2gt/gt/P4h-tm−/− mutants and littermates with the genotypes Hif-p4h-2+/+/P4h-tm+/+, Hif-p4h-2+/+/P4h-tm+/−, Hif-p4h-2+/gt/P4h-tm+/+, Hif-p4h-2+/gt/P4h-tm+/−, Hif-p4h-2+/gt/P4h-tm−/− and Hif-p4h-2gt/gt/P4h-tm+/−. One Hif-p4h-2gt/gt/P4h-tm−/− mouse and 4 controls (as defined in “Results”) were killed at the age of 7 weeks, whereas all the other double-gene–modified mice and their controls were killed at the age of 9 weeks.
Animal experiments
FG-4497 was dissolved in 0.5% NaCMC (Spectrum) and 0.1% Polysorbate 80 (Fluka), and the solvent was also used as a vehicle. The vehicle and inhibitor were administered orally to mice, typically at the age of 5 to 8 months with a ball-tipped needle (Popper and Sons) in a volume of 300 μL, 3 times a week (on days 1, 3, and 5) for 3 to 5 weeks, using a dosage of 100 mg/kg. All the animal experiments were performed according to protocols approved by the Provincial State Office of Southern Finland.
Blood and serum analyses
See supplemental Methods for blood and serum analyses.
Quantitative real-time RT-PCR
See supplemental Methods for quantitative real-time RT-PCR (qPCR).
Western blotting
See supplemental Methods for description of Western blotting.
Statistical analysis
See supplemental Methods for statistical analysis.
Results
Effect of FG-4497 dose
In the initial experiments we determined the FG-4497 concentration required to inhibit purified recombinant human HIF-P4Hs by 50% (IC50) by measuring the hydroxylation-coupled release of 14CO2 from 2-oxo[1-14C]glutarate with a synthetic HIF-1α peptide as a substrate.34 The IC50 obtained was 0.2 to 0.3μM for all 3 HIF-P4Hs (details not shown). As no synthetic substrate is available for P4H-TM, we determined the IC50 for this enzyme by studying the effect of FG-4497 on the uncoupled decarboxylation of 2-oxoglutarate, that is decarboxylation without any added substrate, which is also catalyzed by all these enzymes.15,35 The value obtained was 40μM, more than 100 times higher than for the 3 HIF-P4Hs. To verify that this difference was not because of a different assay, we also determined the IC50 for HIF-P4H-2 by assaying the effect of FG-4497 on its uncoupled decarboxylation and obtained a value of 0.2μM, that is identical to that obtained in the presence of the substrate. FG-4497 thus inhibits all 3 HIF-P4Hs with equal efficiency but is a much less effective P4H-TM inhibitor.
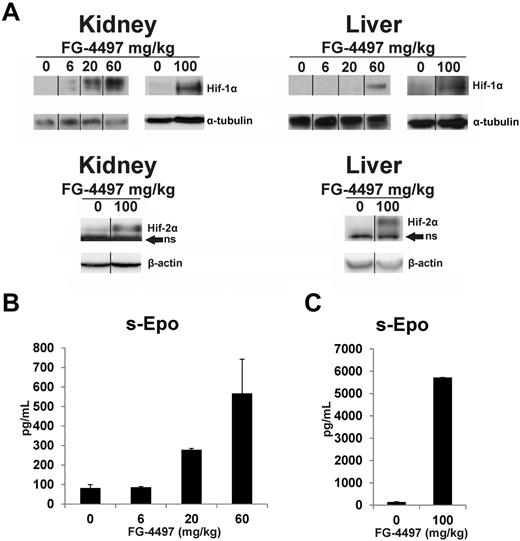
To study the effect of the FG-4497 dose in vivo, increasing amounts of the compound were given orally to wild-type mice on days 1, 3, 5, and 8, and the mice were killed 6 hours after the last dose. A slight degree of Hif-1α stabilization was seen in the kidneys of the mice receiving repeated doses of 6 mg/kg, and the stabilization increased at higher doses (Figure 1A). Stabilization of Hif-1α in the liver required a higher dose, being seen only with 60 mg/kg (Figure 1A). Serum Epo concentration increased approximately 3-fold with 20 mg/kg and 6-fold with 60 mg/kg (Figure 1B). In an additional similar experiment an oral dose of 100 mg/kg stabilized Hif-1α in the liver even more markedly than 60 mg/kg and Hif-2α was also well stabilized in the kidney and liver (Figure 1A). With a dose of 100 mg/kg serum Epo concentration increased to approximately 40 times that in the vehicle-treated animals (Figure 1C). All the experiments reported below were performed using an oral dose of 100 mg/kg.
Effect of FG-4497 dose on stabilization of Hif-1α and Hif-2α in the kidney and liver and on serum Epo concentration. Increasing doses of FG-4497 were given orally to wild-type mice on days 1, 3, 5, and 8 and they were killed 6 hours after the last dose. Anti–Hif-1α and Hif-2α Western blots in the kidney and liver (A). ns = nonspecific. The vertical black lines indicate repositioning of lanes from the same gel with the same exposure. Serum Epo concentrations with doses of 6, 20, and 60 mg/kg (B), and 100 mg/kg (C). In panel B n = 2 and in panel C n = 5 for each dose. Error bars represent SEM.
Effect of FG-4497 dose on stabilization of Hif-1α and Hif-2α in the kidney and liver and on serum Epo concentration. Increasing doses of FG-4497 were given orally to wild-type mice on days 1, 3, 5, and 8 and they were killed 6 hours after the last dose. Anti–Hif-1α and Hif-2α Western blots in the kidney and liver (A). ns = nonspecific. The vertical black lines indicate repositioning of lanes from the same gel with the same exposure. Serum Epo concentrations with doses of 6, 20, and 60 mg/kg (B), and 100 mg/kg (C). In panel B n = 2 and in panel C n = 5 for each dose. Error bars represent SEM.
Effects of FG-4497 on serum Epo concentration
The effects of FG-4497 on serum Epo concentration were studied by administering the compound either only once and sacrificing the mice 6 hours later or 3 times a week (days 1, 3, and 5) for 3 to 5 weeks and sacrificing the animals 6 hours after the last dose.
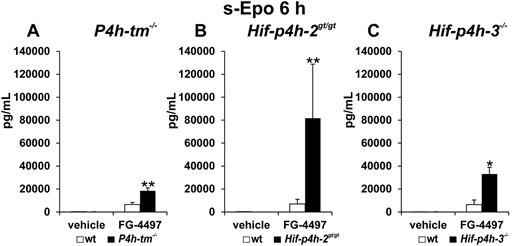
There was no difference in the serum Epo concentration between the vehicle-treated P4h-tm−/− and wild-type mice. In the single-dose 6-hour experiment the serum Epo concentration was markedly increased in both the P4h-tm−/− and wild-type mice relative to vehicle-treated controls, but the increase was much larger in the P4h-tm−/− mice (Figure 2A). In similar experiments serum Epo concentration was also much larger in the FG-4497–treated Hif-p4h-2gt/gt (Figure 2B) and Hif-p4h-3−/− mice (Figure 2C) than in the wild-type mice, although there was no difference in this concentration between the vehicle-treated Hif-p4h-2gt/gt, Hif-p4h-3−/−, and wild-type mice. The difference between the FG-4497–treated gene-modified and wild-type mice was largest (approximately 12-fold) in the Hif-p4h-2gt/gt, smaller (approximately 5-fold) in the Hif-p4h-3−/− mice and smallest (approximately 3-fold) in the P4h-tm−/− mice (Figure 2A-C).
Effect of a single FG-4497 dose on serum Epo concentration in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), Hif-p4h-3−/− (C), and wild-type mice received a single oral dose of FG-4497 (100 mg/kg), and serum Epo concentration was analyzed after 6 hours. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice (*P = .02, **P < .005). The values in the vehicle-treated wild-type and gene-modified mice were less than 3% of those in the FG-4497–treated wild-type mice. In (A) n = 3 for the 2 vehicle-treated groups, n = 7 for the FG-4497–treated wild-type mice and n = 5 for the FG-4497–treated null mice, in (B) n = 3 for each group, and in (C) n = 2 for the 2 vehicle-treated groups and n = 3 for the 2 FG-4497–treated groups. Error bars represent SEM.
Effect of a single FG-4497 dose on serum Epo concentration in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), Hif-p4h-3−/− (C), and wild-type mice received a single oral dose of FG-4497 (100 mg/kg), and serum Epo concentration was analyzed after 6 hours. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice (*P = .02, **P < .005). The values in the vehicle-treated wild-type and gene-modified mice were less than 3% of those in the FG-4497–treated wild-type mice. In (A) n = 3 for the 2 vehicle-treated groups, n = 7 for the FG-4497–treated wild-type mice and n = 5 for the FG-4497–treated null mice, in (B) n = 3 for each group, and in (C) n = 2 for the 2 vehicle-treated groups and n = 3 for the 2 FG-4497–treated groups. Error bars represent SEM.
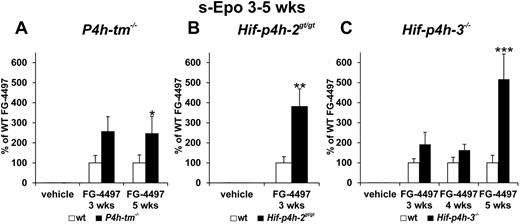
In the 3-week and 5-week experiments the mean serum Epo concentration in the FG-4497–treated P4h-tm−/− mice was approximately 2.5-fold relative to that in the treated wild-type mice (Figure 3A). In similar experiments with Hif-p4h-2gt/gt and Hif-p4h-3−/− mice the serum Epo concentration was approximately 4-fold in the FG-4497–treated Hif-p4h-2gt/gt mice relative to treated wild-type mice at 3 weeks (Figure 3B); no 5-week experiment was performed with these mice, whereas in the Hif-p4h-3−/− mice a significant increase in the serum Epo concentration to approximately 5-fold relative to wild-type was seen only at 5 weeks (Figure 3C).
Effect of repeated FG-4497 doses on serum Epo concentration in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice, the means of the latter being taken as 100% (*P < .05, **P < .01, ***P < .0001). There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C, whereas the differences between the values for the FG-4497–treated and vehicle-treated mice are highly significant in panels A through C. In panel A, n = 5 for all groups at 3 weeks, n = 13 for the FG-4497–treated wild-type mice and n = 6 for the FG-4497–treated P4h-tm−/− mice at 5 weeks; in panel B n = 6-8 for all groups; and in panel C n = 4 for each group at 3 weeks, n = 5 and n = 16-18 for the 2 FG-4497–treated groups at 4 and 5 weeks, respectively. Error bars represent SEM.
Effect of repeated FG-4497 doses on serum Epo concentration in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice, the means of the latter being taken as 100% (*P < .05, **P < .01, ***P < .0001). There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C, whereas the differences between the values for the FG-4497–treated and vehicle-treated mice are highly significant in panels A through C. In panel A, n = 5 for all groups at 3 weeks, n = 13 for the FG-4497–treated wild-type mice and n = 6 for the FG-4497–treated P4h-tm−/− mice at 5 weeks; in panel B n = 6-8 for all groups; and in panel C n = 4 for each group at 3 weeks, n = 5 and n = 16-18 for the 2 FG-4497–treated groups at 4 and 5 weeks, respectively. Error bars represent SEM.
Effects of FG-4497 on stabilization of Hif-1α and Hif-2α and levels of Epo mRNA in the kidney and liver
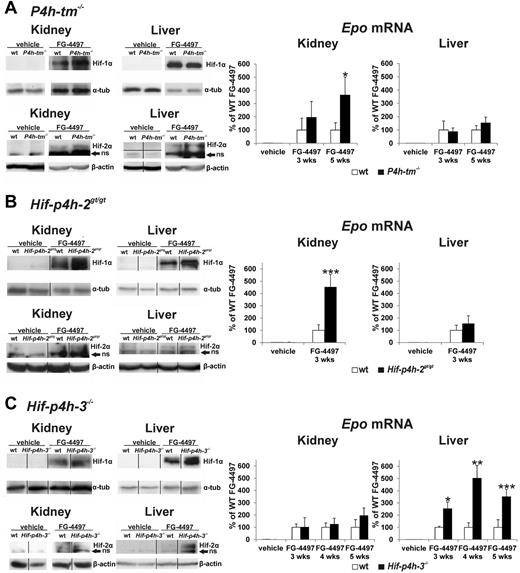
No stabilization of Hif-1α or Hif-2α was found in the kidney or liver of the vehicle-treated P4h-tm−/− mice, and no difference was seen in the Epo mRNA level in these tissues between the vehicle-treated P4h-tm−/− and wild-type mice (Figure 4A), the hepatic Epo mRNA level being so low that it could not be measured in some vehicle-treated animals in these and other experiments reported in this study. FG-4497 administration stabilized Hif-1α and Hif-2α in both tissues, the extent of stabilization of both Hif-αs in the kidney, but not in the liver, being stronger in the P4h-tm−/− than in the wild-type mice (Figure 4A). The FG-4497–induced increase in the mean Epo mRNA level in the kidney of the P4h-tm−/− mice was approximately 2-fold relative to the wild-type at 3 weeks and 4-fold at 5 weeks, whereas there was no significant difference between these mice in the liver (Figure 4A).
Effect of FG-4497 on stabilization of Hif-1α and Hif-2α and the levels of Epo mRNA in the kidney and liver of P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Anti–Hif-1α and Hif-2α Western blots are shown at 5 weeks in panels A and C and at 3 weeks in panel B (the Hif-p4h-2gt/gt mice were treated only for 3 weeks). The vertical black lines indicate repositioning of lanes from the same or separate gels of the same experiment with the same exposure. The horizontal black line indicates exact rejoining of the lanes of a blot that was originally cut in 2 halves. ns indicates nonspecific. In the case of Epo mRNA values statistical significance is shown only for comparisons between the FG-4497–treated gene-modified and wild-type mice, the means of the latter being taken as 100% (*P < .05, **P < .01, and ***P = .001 in panel B, and < .0001 in panel C). The differences in the kidney and liver Epo mRNA values between the FG-4497–treated and vehicle-treated mice are highly significant, whereas there are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice. Values of n as in Figure 3. Error bars represent SEM.
Effect of FG-4497 on stabilization of Hif-1α and Hif-2α and the levels of Epo mRNA in the kidney and liver of P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Anti–Hif-1α and Hif-2α Western blots are shown at 5 weeks in panels A and C and at 3 weeks in panel B (the Hif-p4h-2gt/gt mice were treated only for 3 weeks). The vertical black lines indicate repositioning of lanes from the same or separate gels of the same experiment with the same exposure. The horizontal black line indicates exact rejoining of the lanes of a blot that was originally cut in 2 halves. ns indicates nonspecific. In the case of Epo mRNA values statistical significance is shown only for comparisons between the FG-4497–treated gene-modified and wild-type mice, the means of the latter being taken as 100% (*P < .05, **P < .01, and ***P = .001 in panel B, and < .0001 in panel C). The differences in the kidney and liver Epo mRNA values between the FG-4497–treated and vehicle-treated mice are highly significant, whereas there are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice. Values of n as in Figure 3. Error bars represent SEM.
As previously reported,22 a slight degree of Hif-1α stabilization was seen in the kidney of the Hif-p4h-2gt/gt mice even without FG-4497 treatment, but not in the liver, whereas no difference was seen in the Epo mRNA level between the vehicle-treated Hif-p4h-2gt/gt and wild-type mice (Figure 4B). Administration of FG-4497 for 3 weeks stabilized Hif-1α and Hif-2α in the kidney and liver of the Hif-p4h-2gt/gt and wild-type mice, although to a greater extent in the kidney of the former, with a small difference between these genotypes also to be seen in the liver (Figure 4B). The FG-4497–induced increase in the Epo mRNA level in the kidney was approximately 4.5-fold in the Hif-p4h-2gt/gt mice relative to the wild-type, there being no significant difference between the genotypes in this mRNA level in the liver (Figure 4B).
No stabilization of Hif-1α or Hif-2α was seen in the kidney or liver of the Hif-p4h-3−/− mice without FG-4497 treatment and no difference was seen in the Epo mRNA level between the vehicle-treated Hif-p4h-3−/− and wild-type mice (Figure 4C). Administration of the compound stabilized Hif-1α and Hif-2α in both tissues and in both genotypes. The extent of FG-4497–induced stabilization of Hif-1α was slightly stronger and that of Hif-2α distinctly stronger in the liver of the Hif-p4h-3−/− than the wild-type mice, but not in the kidney (Figure 4C). The FG-4497–induced increase in the Epo mRNA level in the liver of the Hif-p4h-3−/− mice was approximately 2.5-fold relative to the wild-type in the 3-week experiment and 4 to 5-fold in the 4-week and 5-week experiments, whereas no significant difference in the Epo mRNA level was seen between these genotypes in the kidney (Figure 4C).
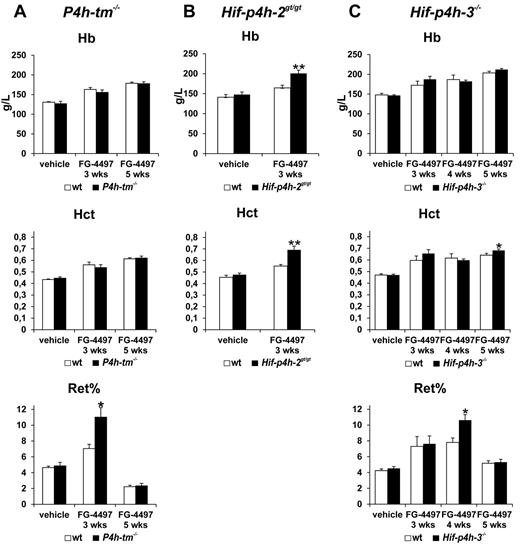
Effects of FG-4497 on blood hemoglobin, hematocrit, and reticulocyte values
There was no difference in hemoglobin and hematocrit values between the vehicle-treated P4h-tm−/− and wild-type mice (Figure 5A). Administration of FG-4497 increased these values significantly in both genotypes, no difference being seen between the treated P4h-tm−/− and wild-type mice at 3 or 5 weeks, although the reticulocyte counts at 3 weeks were significantly higher in the former than in the latter (Figure 5A). Similarly, no difference in hemoglobin and hematocrit values was seen between the vehicle-treated Hif-p4h-2gt/gt and wild-type (Figure 5B) or Hif-p4h-3−/− and wild-type mice (Figure 5C). Administration of FG-4497 increased these values in the Hif-p4h-2gt/gt mice significantly more than in the wild-type mice already at 3 weeks (Figure 5B), whereas in the Hif-p4h-3−/− mice a statistically significant increase relative to the wild-type was seen only in the hematocrit value at 5 weeks (P = .05; Figure 5C). The reticulocyte counts were significantly higher in the FG-4497–treated Hif-p4h-3−/− mice than in the wild-type mice at 4 weeks but not at 3 or 5 weeks (Figure 5C).
Effect of FG-4497 on blood hemoglobin, hematocrit, and reticulocyte values in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice (*P < .02 for reticulocytes in panel A and C, P = .05 for hematocrit in panel C, **P < .005). The differences between the values for the FG-4497–treated and vehicle-treated mice are significant or highly significant for all hemoglobin and hematocrit values in panels A through C as follows. Comparison of FG-4497–treated wild-type mice with vehicle-treated wild-type mice: in panel A hemoglobin and hematocrit at 3 and 5 weeks P < .001; in panel B hemoglobin P < .05, hematocrit P < .001; in panel C hemoglobin at 3 weeks P = .056, at 4 weeks P < .02 and at 5 weeks P < .001, hematocrit at 3 weeks P < .005 and at 4 and 5 weeks P < .001; comparison of FG-4497–treated gene-modified mice with vehicle-treated gene-modified mice: (A) hemoglobin and hematocrit at 3 weeks P < .02, at 5 weeks P < .001; (B) hemoglobin and hematocrit P < .001; (C) hemoglobin and hematocrit at 3 weeks P < .005 and at 4 and 5 weeks P < .001. Similar comparisons of reticulocyte values are as follows. Comparison of FG-4497–treated wild-type mice with vehicle-treated wild-type mice: in panel A at 3 weeks P < .005, in panel C at 3 weeks P < .05 and at 4 weeks P < .001; comparison of FG-4497–treated gene-modified mice with vehicle-treated gene-modified mice in panel A at 3 weeks P < .002, in panel C at 3 weeks P < .05 and at 4 weeks P < .001. There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C. Values of n as in Figure 3. Error bars represent SEM.
Effect of FG-4497 on blood hemoglobin, hematocrit, and reticulocyte values in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for 3 to 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown only for the comparison of the values between the FG-4497–treated gene-modified and wild-type mice (*P < .02 for reticulocytes in panel A and C, P = .05 for hematocrit in panel C, **P < .005). The differences between the values for the FG-4497–treated and vehicle-treated mice are significant or highly significant for all hemoglobin and hematocrit values in panels A through C as follows. Comparison of FG-4497–treated wild-type mice with vehicle-treated wild-type mice: in panel A hemoglobin and hematocrit at 3 and 5 weeks P < .001; in panel B hemoglobin P < .05, hematocrit P < .001; in panel C hemoglobin at 3 weeks P = .056, at 4 weeks P < .02 and at 5 weeks P < .001, hematocrit at 3 weeks P < .005 and at 4 and 5 weeks P < .001; comparison of FG-4497–treated gene-modified mice with vehicle-treated gene-modified mice: (A) hemoglobin and hematocrit at 3 weeks P < .02, at 5 weeks P < .001; (B) hemoglobin and hematocrit P < .001; (C) hemoglobin and hematocrit at 3 weeks P < .005 and at 4 and 5 weeks P < .001. Similar comparisons of reticulocyte values are as follows. Comparison of FG-4497–treated wild-type mice with vehicle-treated wild-type mice: in panel A at 3 weeks P < .005, in panel C at 3 weeks P < .05 and at 4 weeks P < .001; comparison of FG-4497–treated gene-modified mice with vehicle-treated gene-modified mice in panel A at 3 weeks P < .002, in panel C at 3 weeks P < .05 and at 4 weeks P < .001. There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C. Values of n as in Figure 3. Error bars represent SEM.
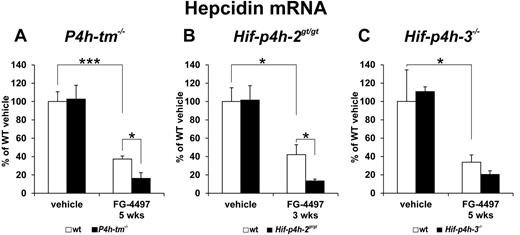
Effect of FG-4497 on hepatic Hepcidin mRNA concentration
Recent studies have indicated that Hif-2α down-regulates Hepcidin expression through an Epo-mediated increase in erythropoiesis.8,10 As FG-4497 administration increased serum Epo concentration and renal Epo mRNA levels, but not blood hemoglobin or hematocrit values, more in the P4h-tm−/− than in the wild-type mice, we studied whether the effect of FG-4497 on Hepcidin expression is similar or different in P4h-tm−/− and wild-type mice. We therefore determined liver Hepcidin mRNA levels in 5-week experiments with the P4h-tm−/− and Hif-p4h-3−/− mice and the 3-week experiment with the Hif-p4h-2gt/gt mice relative to corresponding wild-type controls.
There was no difference in the Hepcidin mRNA levels between any of these vehicle-treated gene-modified and wild-type mice (Figure 6A-C). FG-4497 administration lowered the Hepcidin mRNA level in wild-type mice by approximately 60%-65%, but the magnitude of the decrease was even larger in all 3 gene-modified mouse lines (Figure 6A-C). In the case of the FG-4497–treated P4h-tm−/− mice the Hepcidin mRNA level was approximately 40% of that in the FG-4497–treated wild-type mice (Figure 6A), while in the Hif-p4h-2gt/gt and Hif-p4h-3−/− mice the corresponding levels were approximately 30% and 60% (Figure 6B-C), the difference between the FG-4497–treated Hif-p4h-3−/− and wild-type mice not being statistically significant. It is thus obvious that the effect of FG-4497 on Hepcidin mRNA was no smaller in the P4h-tm−/− mice than in the Hif-p4h-3−/− mice even though only the latter showed a further increase in the hematocrit value compared with the FG-4497–treated wild-type.
Effect of FG-4497 on hepatic Hepcidin mRNA level in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for (B) 3 or (A-C) 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown for comparisons between the values for the vehicle-treated and FG-4497–treated wild-type mice, the means of the former being taken as 100%, and for comparisons between the FG-4497–treated gene-modified and wild-type mice (*P < .05, ***P < .0001). There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C. Values of n as in Figure 3. Error bars represent SEM.
Effect of FG-4497 on hepatic Hepcidin mRNA level in P4h-tm null, Hif-p4h-2 hypomorphic, and Hif-p4h-3 null mice. The P4h-tm−/− (A), Hif-p4h-2gt/gt (B), and Hif-p4h-3−/− (C) mice and wild-type mice received 3 oral doses of FG-4497 (100 mg/kg) per week for (B) 3 or (A-C) 5 weeks and were killed 6 hours after the last dose. Statistical significance is shown for comparisons between the values for the vehicle-treated and FG-4497–treated wild-type mice, the means of the former being taken as 100%, and for comparisons between the FG-4497–treated gene-modified and wild-type mice (*P < .05, ***P < .0001). There are no significant differences between the values for the vehicle-treated gene-modified and wild-type mice in panels A through C. Values of n as in Figure 3. Error bars represent SEM.
Increased hemoglobin and hematocrit values in Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mice
As indicated in the Introduction and in the second and the fourth paragraphs of Results, there is no difference in serum Epo concentration and erythropoiesis between Hif-p4h-2gt/gt, P4h-tm−/− and wild-type mice without FG-4497 treatment. To study further the role of P4h-tm in erythropoiesis we produced a double gene-modified Hif-p4h-2gt/gt/P4h-tm−/− mouse line.
As crossings of Hif-p4h-2+/gt/P4h-tm+/− or Hif-p4h-2+/gt/P4h-tm−/− mice with Hif-p4h-2+/gt/P4h-tm+/− or Hif-p4h-2+/gt/P4h-tm−/− mice produced only small numbers of female wild-type mice or none at all, we decided to use not only wild-type but also Hif-p4h-2+/+/P4h-tm+/−, Hif-p4h-2+/gt/P4h-tm+/+, and Hif-p4h-2+/gt/P4h-tm+/− mice as littermate controls, and thus had a control group of 21 littermates. This decision was based on our previous data indicating that there is no difference in hemoglobin and hematocrit values even between homozygous Hif-p4h-2gt/gt and wild-type mice,22 together with current data indicating that there is no difference in these values between vehicle-treated homozygous Hif-p4h-2gt/gt and wild-type mice (Figure 5B) or homozygous P4h-tm−/− and wild-type mice (Figure 5A). Analysis of hemoglobin and hematocrit values in these 21 controls indicated that there were indeed no differences between the 4 subgroups (details not shown).
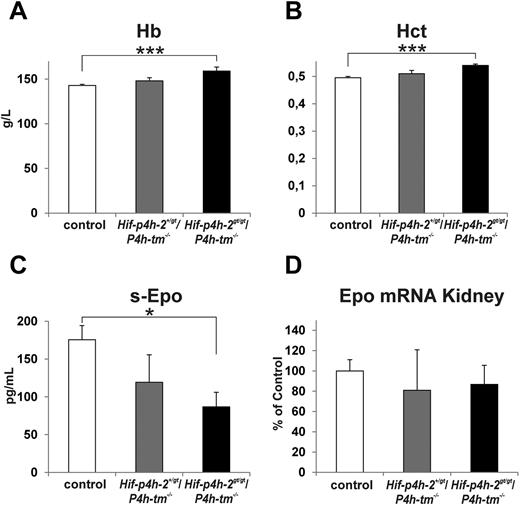
The mean hemoglobin concentration in the 21 controls was 142.9 g/L (range 131-151 g/L; Figure 7A), whereas the mean concentration in 6 Hif-p4h-2+/gt/P4h-tm−/− mice was 148.0 g/L (range 136-161 g/L; Figure 7A). The 6 Hif-p4h-2gt/gt/P4h-tm−/− mice had a mean hemoglobin concentration of 159.0 g/L (range 149-180 g/L; Figure 7A), this mean being significantly higher than the control mean (P < .000 05). Similar differences were also seen in hematocrit values between the 3 groups (Figure 7B).
Increased hemoglobin and hematocrit values in Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mice. The hemoglobin (A), hematocrit (B), serum Epo (C), and kidney Epo mRNA (D) values are shown for controls (these include wild-type, Hif-p4h-2+/+/P4h-tm+/−, Hif-p4h-2+/gt/P4h-tm+/+, and Hif-p4h-2+/gt/P4h-tm+/− mice), Hif-p4h-2+/gt/P4h-tm−/− mice, and Hif-p4h-2gt/gt/P4h-tm−/− mice. Statistical significance is shown for comparisons with control mice (*P < .05, ***P < .00005). n = 21 for controls, n = 6 for Hif-p4h-2+/gt/P4h-tm−/− mice, and n = 6 for Hif-p4h-2gt/gt/P4h-tm−/− mice. Error bars represent SEM.
Increased hemoglobin and hematocrit values in Hif-p4h-2gt/gt/P4h-tm−/− double gene-modified mice. The hemoglobin (A), hematocrit (B), serum Epo (C), and kidney Epo mRNA (D) values are shown for controls (these include wild-type, Hif-p4h-2+/+/P4h-tm+/−, Hif-p4h-2+/gt/P4h-tm+/+, and Hif-p4h-2+/gt/P4h-tm+/− mice), Hif-p4h-2+/gt/P4h-tm−/− mice, and Hif-p4h-2gt/gt/P4h-tm−/− mice. Statistical significance is shown for comparisons with control mice (*P < .05, ***P < .00005). n = 21 for controls, n = 6 for Hif-p4h-2+/gt/P4h-tm−/− mice, and n = 6 for Hif-p4h-2gt/gt/P4h-tm−/− mice. Error bars represent SEM.
Surprisingly, the serum Epo concentration was significantly lower in the Hif-p4h-2gt/gt/P4h-tm−/− mice than in the wild-type mice (Figure 7C), the lowest concentration, 20.4 pg/mL, being seen in the Hif-p4h-2gt/gt/P4h-tm−/− mouse with the highest hemoglobin value, 180 g/L. A similar situation has been found in Hif-p4h-1−/−/Hif-p4h-3−/− mice in which increased hemoglobin values were associated with a markedly decreased serum Epo concentration.19 The reasons for these decreases are unknown but it has been speculated that an increased number of erythroid progenitors and early erythrocytes may have reduced free Epo protein molecules through increased receptor binding.19 It has also been reported that several patients with increased hemoglobin values because of heterozygous HIF-P4H-2 mutations have their serum EPO concentrations within the normal range, and in some cases even close to or at the lower limit of normal.36–38
Although FG-4497 administration caused larger increases in the Epo mRNA levels in the kidneys of the Hif-p4h-2gt/gt and P4h-tm−/− mice than wild-type mice (Figure 4), the Epo mRNA level was not increased in the kidneys of the Hif-p4h-2gt/gt/P4h-tm−/− mice (Figure 7D). This finding is not entirely surprising, as the increases in the hemoglobin and hematocrit values were small, and as very small increases in the Epo mRNA levels could probably not be detected because of the wide variation of individual values. Furthermore, the increased erythropoiesis in the Hif-p4h-2gt/gt/P4h-tm−/− mice presumably persisted continuously, whereas the much larger changes seen in the FG-4497–treated mice occurred during a short period of time. The level of Epo mRNA in the liver was so small that it could not be measured in all animals, but there was no increase in the Hif-p4h-2gt/gt/P4h-tm−/− mice (controls 100 ± 20.3%, Hif-p4h-2gt/gt/P4h-tm−/− mice 90.2 ± 29.1%). It was recently reported that bone39 and brain40 can also produce Epo in quantities sufficient to increase hemoglobin values. In the case of bone, an increased Epo mRNA level was seen by augmented HIF-2 signaling, whereas this mRNA could not be detected in normal bone.39 In our study Epo mRNA was not expressed at detectable levels in either control or Hif-p4h-2gt/gt/P4h-tm−/− bone (details not shown), and it thus seems unlikely that the increased hemoglobin and hematocrit values in the Hif-p4h-2gt/gt/P4h-tm−/− mice could be because of an increased Epo production in the bone. We were able to measure Epo mRNA levels in mouse brain, but found no increase in the Hif-p4h-2gt/gt/P4h-tm−/− mice relative to wild-type (details not shown). It thus seems possible that the increased erythropoiesis in the Hif-p4h-2gt/gt/P4h-tm−/− mice was because of a small increase in the Epo production in the kidney, although we could not demonstrate this increase. However, we cannot exclude the possibility that the increased erythropoiesis was because of some Epo-independent change. Although we could not demonstrate the mechanism involved, our data on the Hif-p4h-2gt/gt/P4h-tm−/− mice support the conclusion that P4h-tm plays a role in the regulation of erythropoiesis.
The Hif-p4h-2 gene in Hif-p4h-2gt/gt mice is disrupted by a GeneTrap insertion cassette, but small amounts of wild-type Hif-p4h-2 mRNA are generated from gene-trapped alleles in various tissues because of partial skipping of the cassette.22 To verify that the P4h-tm−/− allele present in the Hif-p4h-2gt/gt/P4h-tm−/− mice did not cause any change in this splicing, we determined the wild-type Hif-p4h-2 mRNA level as a percentage of total Hif-p4h-2 mRNA in the kidney of the Hif-p4h-2gt/gt and Hif-p4h-2gt/gt/P4h-tm−/− mice. The relative level of the wild-type Hif-p4h-2 mRNA was identical in both mouse lines, being approximately 35%-40% (details not shown).
Discussion
Studies with HIF-P4H–inhibiting small-molecule compounds have indicated that pharmacologic HIF stabilization appears promising as a strategy for treating diseases associated with acute or chronic hypoxia, such as anemia, myocardial infarction, and stroke, and clinical trials are in progress to evaluate their effectiveness in the treatment of anemia.16,24,25,41–43 In an inflammation-induced anemia model HIF stabilization increased erythropoiesis even though exogenous EPO was ineffective,33,44 and in anemia of chronic disease HIF stabilization may also improve the outcomes by bypassing the effective iron deficiency and increasing the red cell mass without any supraphysiologic increases in serum EPO concentrations.25,33,41,43 It has been reported that HIF-P4Hs may also have enzyme-specific substrates other than HIF-αs3,16,45–48 and that the effects of knockout of the individual enzymes are distinctly different.17–21,49,50 These findings suggest potential applications in different therapeutic settings for compounds that differentially inhibit HIF hydroxylating enzymes. It is therefore important to understand the individual roles of the HIF hydroxylating enzymes in the regulation of erythropoiesis.
It is now well established that all 3 HIF-P4Hs play a role in the regulation of erythropoiesis. The data reported on mice with broad-spectrum conditional inactivation of Hif-p4h-2 indicate that the lack of this isoenzyme alone is sufficient to produce large increases in erythropoiesis.19–21 The detailed roles of the other 2 HIF-P4Hs are less well understood, as knockout of either isoenzyme alone caused no changes in serum Epo concentration or blood hemoglobin or hematocrit values,19 and as changes in erythropoiesis in Hif-p4h-2−/−/Hif-p4h-3−/− mice did not differ from those seen in Hif-p4h-2−/− mice, regardless of whether the Hif-p4h-2 knockout was general or liver-specific.21 In contrast, moderate increases in serum Epo concentration and erythropoiesis were seen in Hif-p4h-1−/−/Hif-p4h-3−/−, and Hif-p4h-1−/−/Hif-p4h-2−/− mice with liver-specific knockout of Hif-p4h-2,19,21 even though hepatic inactivation of Hif-p4h-2 alone did not increase serum Epo values.21 Nothing has so far been known about the roles of P4H-TM in the regulation of the response to hypoxia in general or in the regulation of EPO production and erythropoiesis in particular in any mammalian species, although expression levels of several HIF target genes, including epo, have been found to be increased in p4h-tm–deficient zebrafish embryos without any signs of polycythemia.23
Administration of the 2-oxoglutarate analog HIF-P4H inhibitor FG-4497 to P4h-tm−/− mice caused here larger increases in serum Epo concentration and the Hif-1α and Hif-2α protein and Epo mRNA levels than in wild-type mice in the kidney, but not in the liver. In addition, the level of liver Hepcidin mRNA in the FG-4497–treated P4h-tm−/− mice was less than one-half of that in the treated wild-type mice. Similar differences between the FG-4497–treated gene-modified and wild-type mice were also seen in the Hif-p4h-2gt/gt and Hif-p4h-3−/− mice, except that in the case of the FG-4497–treated Hif-p4h-2gt/gt mice there was also a slight further increase in the level of the Hif-1α and Hif-2α protein in the liver and in the case of the FG-4497–treated Hif-p4h-3−/− mice the increased extent of Hif-1α and Hif-2α stabilization and higher Epo mRNA level were seen in the liver rather than kidney. The magnitudes of the changes seen in the Hif-p4h-2gt/gt mice were distinctly larger than in the P4h-tm−/− mice and the magnitudes of those seen in the Hif-p4h-3−/− mice were in most cases slightly larger, but the decrease in the Hepcidin mRNA level in the liver was no larger in the Hif-p4h-3−/− than the P4h-tm−/− mice. Our results with the Hif-p4h-2gt/gt mice agree with previous data indicating that broad-spectrum conditional inactivation of Hif-p4h-2 increased Epo production only in the kidney.18–20 Similarly, the results with the Hif-p4h-3−/− mice agree with data indicating that Hif-p4h-1−/−/Hif-p4h-3−/− mice had increased Hif-2α protein and Epo mRNA levels only in the liver.19
Although the serum Epo concentration had increased approximately 2.5-fold at 3 and 5 weeks and the reticulocyte counts had increased approximately 1.6-fold at 3 weeks in the FG-4497–treated P4h-tm−/− mice compared with the treated wild-type, no increase was found in the hemoglobin and hematocrit values in the former relative to the latter. This finding may be because of the fact that the serum Epo concentration was approximately 100-fold in the FG-4497–treated wild-type mice compared with the vehicle-treated mice, and thus a further 2.5-fold increase may have been insufficient to give any additional increase in the already elevated hemoglobin and hematocrit values. In the case of the FG-4497–treated Hif-p4h-3−/− mice that had a 5-fold further increase in their serum Epo concentration at 5 weeks and a 1.4-fold further increase in their reticulocyte counts at 4 weeks there likewise was no significant further increase in the hemoglobin or hematocrit values relative to the treated wild-type at 3 or 4 weeks, but a significant further increase was found in the hematocrit value at 5 weeks. The possibility can thus not be excluded that a longer FG-4497 administration might have caused further increases in the hemoglobin and hematocrit values relative to the wild-type even in the P4h-tm−/− mice. In agreement with the major role of HIF-P4H-2 in the regulation of erythropoiesis,18–20 FG-4497 treatment for only 3 weeks caused a further increase in the hemoglobin and hematocrit values in the Hif-p4h-2gt/gt mice compared with the FG-4497–treated wild-type, the serum Epo concentration in the former being approximately 4-fold relative to the latter.
Recent studies have indicated that stabilization of Hif-α is not directly involved in the hypoxic down-regulation of hepcidin expression, the effect being mediated by increased Epo expression and a bone-derived systemic signal caused by the increased erythropoiesis.8,10 Our data indicating that the Hepcidin mRNA level was decreased in the liver of the FG-4497–treated P4h-tm−/− mice to less than one-half of that in the treated wild-type supports our suggestion that erythropoiesis was further increased in the FG-4497–treated P4h-tm−/− mice even though this increase was not large enough to lead to a further increase in the already highly elevated hemoglobin and hematocrit values.
The role of P4H-TM as a fourth P4H involved in the regulation of erythropoiesis was supported by data obtained from the Hif-p4h-2gt/gt/P4h-tm−/− mice without any FG-4497 administration. Although neither of the single mutant mouse lines, Hif-p4h-2gt/gt or P4h-tm−/−, showed any change in erythropoiesis, the double-mutant mice had significantly increased hemoglobin and hematocrit values.
Another 2-oxoglutarate analog, FG-2216, which, similar to FG-4497, inhibits all 3 HIF-P4Hs, increased EPO production in anephric patients with end stage renal disease as strongly as in healthy subjects, suggesting that it caused effective EPO production in the liver.41 As the present and previous18–21 data indicate that HIF-P4H-2 and P4H-TM are involved in the regulation of EPO production in the kidney but not in the liver, it is evident that a compound inhibiting several HIF-P4Hs could be desirable for the treatment of patients with advanced kidney disease. Based on the present data, a compound inhibiting P4H-TM together with some of the HIF-P4H isoenzymes may produce a stronger erythropoietic response and a larger decrease in the hepcidin level than that obtained with a HIF-P4H antagonist lacking P4H-TM inhibition.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Aatsinki, R. Juntunen, K. Kvist-Mäkelä, E. Lehtimäki, R. Polojärvi, and M. Siurua for their excellent technical assistance.
J.M. and P.K. were supported by Health Science Council grant 114344 and Center of Excellence 2012-2017 grant 251314 (to J.M.), and Biosciences and Environment Council grants 120156, 218129, and 140765 (to P.K.) from the Academy of Finland, and by grants from the S. Juselius Foundation (to J.M. and P.K.) and FibroGen, Inc (to J.M.).
Authorship
Contribution: A.L. performed research and analyzed data, E.A., J.M.M., M.R., M.H., and E.-R.S. performed research; G.W. performed preliminary experiments and participated in the design of the experiments; M.A. synthesized FG-4497; K.I.K., P.K., and J.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: K.I.K. is a scientific founder and consultant of FibroGen Inc, which develops HIF-P4H inhibitors as potential therapeutics; J.M. and K.I.K. own equity in this company, and the company has supported research in the laboratory of K.I.K. and currently supports research in that of J.M; and G.W. is a senior cell biology director and M.A. an associate director of chemistry at FibroGen Inc. The remaining authors declare no competing financial interests.
Correspondence: Johanna Myllyharju, Oulu Center for Cell-Matrix Research, Biocenter Oulu, and Dept of Medical Biochemistry and Molecular Biology, Institute of Biomedicine, University of Oulu, FIN-90014 Oulu, Finland; e-mail: johanna.myllyharju@oulu.fi.
References
Author notes
P.K. and J.M. contributed equally to this work.