Abstract
BLT1 is a high-affinity receptor for leukotriene B4 (LTB4) that is a potent lipid chemoattractant for myeloid leukocytes. The role of LTB4/BLT1 axis in tumor immunology, including cytokine-based tumor vaccine, however, remains unknown. We here demonstrated that BLT1-deficient mice rejected subcutaneous tumor challenge of GM-CSF gene-transduced WEHI3B (WGM) leukemia cells (KO/WGM) and elicited robust antitumor responses against second tumor challenge with WEHI3B cells. During GM-CSF–induced tumor regression, the defective LTB4/BLT1 signaling significantly reduced tumor-infiltrating myeloid-derived suppressor cells, increased the maturation status of dendritic cells in tumor tissues, enhanced their CD4+ T-cell stimulation capacity and migration rate of dendritic cells that had phagocytosed tumor-associated antigens into tumor-draining lymph nodes, suggesting a positive impact on GM-CSF–sensitized innate immunity. Furthermore, KO/WGM mice displayed activated adaptive immunity by attenuating regulatory CD4+ T subsets and increasing numbers of Th17 and memory CD44hiCD4+ T subsets, both of which elicited superior antitumor effects as evidenced by adoptive cell transfer. In vivo depletion assays also revealed that CD4+ T cells were the main effectors of the persistent antitumor immunity. Our data collectively underscore a negative role of LTB4/BLT1 signaling in effective generation and maintenance of GM-CSF–induced antitumor memory CD4+ T cells.
Introduction
For many cancers refractory to conventional therapies, gene-modified tumor vaccines have emerged as a promising treatment. Among numerous immunostimulatory cytokines used for tumor vaccines, GM-CSF has been the most intensively investigated and widely studied for use in clinical cancer vaccine trials.1-3 Immunization with irradiated tumor cells engineered to secrete GM-CSF stimulates potent tumor-associated antigen (TAA)-specific antitumor immunity in preclinical4,5 and clinical settings, including solid tumors and acute myeloid leukemia.6,7 We have recently shown that the GM-CSF gene-transduced murine monocytic leukemia of WEHI3B cells lose their tumorigenicity when subcutaneously administered into wild-type (WT) BALB/c mice.8 It is thought that the effective induction of antitumor immunity triggered by GM-CSF is mainly the result of augmented processing and presentation of TAAs by dendritic cells (DCs), followed by both CD4+ and CD8+ T-cell responses.4,9,10 However, the efficacy of this therapy alone is not durable, partially because of its failure to maintain antitumor memory immunity by TAA-primed T cells. Therefore, there is an imminent need for elaborate studies to improve antitumor memory responses generated by GM-CSF gene-transduced tumor cells.
Leukotriene B4 (LTB4) is known to be an extremely potent lipid inflammatory mediator derived from membrane phospholipids by the sequential actions of 5-lipoxygenase and LTA4 hydrolase.11 The major activities of LTB4 include the recruitment and activation of myeloid leukocytes, such as neutrophils.12 Recently, Del Prete et al have reported a novel role of LTB4 in regulating migration of DCs that precede adaptive immune responses.13 Lipid LTB4 mediates its functions through the G protein-coupled 7 transmembrane domain receptor superfamily,14 namely, 2 distinct receptors, BLT1 and BLT2. On the other hand, GM-CSF stimulates the production and function of neutrophils, eosinophils, and monocytes, and activate maturation of DCs.15,16 However, the underlying significance of the LTB4/BLT1 lipid chemo-attractant pathway in the field of tumor immunology, including GM-CSF-induced immunity, remains elusive.
In this context, we investigated the influence of the defective LTB4/BLT1 axis on antitumor memory responses induced by subcutaneous administration of WGM cells using WT and BLT1-knockout (KO) mice. Intriguingly, in vivo experiments showed that marked tumor rejection was reproduced only in BLT1-KO mice when WEHI3B cells were subcutaneously inoculated into WT or BLT1-KO mice that had rejected the outgrowth of WGM cells, implying that that the loss of the LTB4/BLT1 signaling may confer effective generation of TAA-specific memory T cells with retained antitumor effects triggered by GM-CSF. Therefore, the current study explored the role of the LTB4/BLT1 signaling pathway in the induction of the persistent GM-CSF–induced antitumor memory responses.
Methods
Mice
BLT1-KO mice were established using a gene-targeting strategy, as previously described.17 BLT1-KO mice and the corresponding WT mice have been backcrossed onto a BALB/c genetic background > 10 times. All animal experiments were carried out under the Guidelines for Animal Experiments of Kyushu University and Law 105 Notification 6 of the Japanese Government.
Tumor cells
WEHI3B cells obtained from Dr D. Metcalf (University of Melbourne) and recombinant mouse GM-CSF gene-transduced WEHI3B cell clones, established as previously described18 were cultured in RPMI 1640 medium (Nacalai Tesque) supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin solution (complete medium [CM]).
In vitro cell proliferation assay
WEHI3B and WGM cells were cultured independently in 48-well microplates at 5 × 104 cells/well for 2, 4, and 8 days in CM. On day 2, cells were transferred in 6-well plates and repeated every 2 days thereafter. The number of viable cells was determined by trypan blue dye exclusion assay (Invitrogen).
Tumor models
For the tumorigenicity assay, 5 × 105 cells/100 μL HBSS (Invitrogen) of WEHI3B cells or WGM cells were subcutaneously inoculated into the right flank of WT and BLT1-KO mice, respectively, as first tumor challenge (FTC; n = 6-8 per group). For the second tumor challenge (STC) assay, on day 50 after the subcutaneous inoculation of 5 × 105 cells of WEHI3B cells or WGM cells, 5 × 105 WEHI3B cells were subcutaneously inoculated into the left flank of mice that had completely rejected the challenged WEHI3B cells or WGM cells. Two bisecting diameters of each tumor were measured with calipers, and calculated using the formula V = 0.4ab2, where “a” was the larger diameter and “b” was the smaller diameter of each tumor. Changes in tumor growth were monitored 3 times a week. Mice were killed for ethical reasons when the tumor diameter exceeded 15 mm. The explanatory scheme for all of the following in vivo experiments was illustrated in Figure 1B.
Analyses for tumor-infiltrating leukocytes
Approximately 5 × 106 WGM cells/100 μL were subcutaneously inoculated into the right flank of WT or BLT1-KO mice (n = 3-5 per group). One day after the FTC, the site of tumor inoculation was dissected as described previously.5 Briefly, tumor tissue was finely minced and digested in RPMI 1640 containing collagenase (Invitrogen) for 20 minutes at 37°C, filtered through a 70-μm strainer, and washed in a fluorescent antibody buffer (FAB) consisting of 0.1% BSA in PBS. Supernatants from tumor tissue were collected, and homogenized cells were blocked with anti-FcR mAb for 15 minutes and followed by staining with either FITC– or PE/Cy7–anti-CD86, PE–anti-CD40, Alexa488– or peridinin-chlorophyll-protein (PerCP)–anti-CCR7, and allophycocyanin (APC)–anti-CD11c mAb either FITC–anti-CD11b, PE–anti-Gr-1, and APC–anti-F4/80, or FITC–anti-DX5, PE–anti-CD107a, and APC– or APC/Cy7–anti-CD3 mAb in FAB for 30 minutes, and subjected to multiparameter flow cytometry, a FACSCalibur or FACSVerse (BD Biosciences). Dead cells were excluded by 7-AAD staining and forward scatter/side scatter FSC/SCC profiles. Analyses of data were performed using FlowJo Version 8.8.6 software (Tree-Star). Concentrations of VEGF, TGF-β, and IL-10 in supernatants from tumor tissues were measured using Quantikine (R&D Systems) or mouse Th1/Th2 ELISA Ready-Set-Go (eBioscience).
MLR
One day after FTC, tumor-draining lymph nodes (TDLNs) and erythrocyte-depleted splenocytes were harvested from WT/WGM or KO/WGM mice as described in “Analyses for tumor-infiltrating leukocytes.” DCs as stimulators (S) were purified from splenocytes or TDLNs using CD11c MicroBeads (Miltenyi Biotec). T cells as responders (R) were prepared from isolated splenocytes of C57BL/6 mice using Pan T cell isolation kit II (Miltenyi Biotec), suspended in CM supplemented with 50μM β-mercaptoethanol (Sigma-Aldrich), and cocultured with 30 Gy irradiated DCs at indicated R/T ratios on 96-well U-bottom plate for 4 days. Sixteen hours before the end of culture, 1 μCi [3H]-thymidine (Moravek Biochemicals) was added to each well. Thymidine uptake was quantified in a TopCount NXT (PerkinElmer Life and Analytical Sciences). For CFSE-labeled mixed lymphocyte reaction (MLR) assay, splenic CD11c+ DCs were isolated from WT/WGM or KO/WGM mice using CD11c MicroBeads (Miltenyi Biotec), irradiated with 30 Gy and cocultured with CD3+ T cells as described in this section. After 6 days of coculture, the proliferation rate of CD3+CD4+ or CD3+CD8+ T cells labeled with 2.0μM CFSE (Invivogen) in the coculture was assessed by FACSVerse.
In vivo DC migration assay
Approximately 1 × 107 WGM cells were resuspended in 100 μL diluent C and incubated for 10 minutes with 1 × 10−6 M PKH26 (Sigma-Aldrich). After washing, 5 × 105 PKH26-labeled cells were subcutaneously inoculated into the right flank of WT and BLT1-KO mice. Two days after the FTC, TDLNs were homogenized by mechanical dissociation. Cell suspensions were pretreated with Fc-receptor (FcR) block and stained with FITC–anti-CD86 and APC–anti-CD11c mAb in FAB for 30 minutes. Cells were subjected to FACSCalibur or FACSVerse and analyzed using FlowJo Version 8.8.6 software.
Cytometric bead assay
Cytokine production profile from splenocytes was measured as previously described.5 Briefly, 1 million splenocytes were harvested from WT or BLT1-KO mice challenged with WEHI3B cells or WGM cells on day 10, depleted of erythrocytes with ammonium chloride, and cocultured with or without 4 × 105 irradiated WEHI3B cells in CM for 20 hours. Production of IL-2, IL-4, IL-5, IFN-γ, and TNF-α in each supernatant was measured using Cytometric Bead Array mouse Th1/Th2 Cytokine kit (BD Biosciences), according to the manufacturer's instructions. Data were analyzed using FCAP Array Version 1.0.1 software (BD Biosciences).
Flow cytometric analysis for intracellular cytokines
On day 46 after the FTC, TDLNs or spleen were harvested from WT/WGM or KO/WGM mice (n = 3-5 per group). Homogenized cells were cultured in CM containing 50μM β-mercaptoethanol, phorbol myristate acetate (10 ng/mL), ionophore (250 mg/mL), and brefeldin A (1 ng/mL) for 4-5 hours. After washing, cells were pretreated with FcR block followed by staining with PerCP-anti–mouse CD4 mAb for 30 minutes. Subsequently, cells were fixed with 2% paraformaldehyde and stained intracellularly with FITC–anti-IFN-γ, PE–anti-IL-4, and APC–anti-IL-17A mAb in permeabilization buffer (eBioscience) for 30 minutes and subjected to FACSCalibur or FACSVerse.
Phenotypic analysis by flow cytometric analysis for diverse immune subpopulations
For mature DCs, TDLNs were harvested from the 4 groups of mice (n = 3 or 4 per group) on days 2 and 4 after the FTC. Obtained cells were blocked with FcR and stained with either FITC–anti-CD86, PE–anti-CD80, and APC–anti-CD11c mAb, or PE–anti-CD40 and APC–anti-CD11c mAb in FAB for 30 minutes. For diverse helper T subsets, TDLNs and splenocytes were harvested from WT/WGM or KO/WGM mice (n = 3-5 per group) on day 46 after the FTC. For memory T subsets, cells were stained with PE–anti-CD44, PerCP–anti-CD4, and APC–anti-CD62L mAb. For regulatory T cells, after staining with FITC–anti-CD3, PE–anti-CD25, and PerCP/Cy5.5–anti-CD4, TDLNs cells were resuspended with 100 μL Fixation/Permeabilization solution (BD Biosciences) and washed with BD Perm/Wash buffer (BD Biosciences) for 20 minutes, followed by the addition of APC–anti–FoxP3 (FJK-16; eBioscience) mAb, and incubated for 30 minutes. For other regulatory T cells, TDLNs and splenocytes were costained with FITC–anti-CD3, PE–anti-GITR, and PerCP–anti-CD4 mAb in FAB for 30 minutes, and subjected to FACSCalibur.
In vivo depletion experiments
GK1.5 and 2.43 hybridoma cells obtained from the Cell Resource Center for Biomedical Research (Institute of Development, Aging and Cancer Tohoku University) were used for the production of anti–mouse CD4 mAb and anti–mouse CD8 mAb, respectively, as previously described.19 Briefly, these mAbs were purified with centrifugation at 53 000g for 20 minutes, and subjected to affinity chromatography using MAb Trap Kit (GE Healthcare). Effective depletion of CD4+ and CD8+ T cells was confirmed by flow cytometric analysis using splenocytes (data not shown). Mice received peritoneal injections of anti–mouse CD4 mAb, anti–mouse CD8 mAb (50 μg per mouse), or PBS for 3 days, and once every 3 days thereafter. For depletion of NK cells, mice received peritoneal injections of rabbit anti-asialo GM1 antiserum (diluted 1:20 in 200 μL PBS; Wako), on 1 day before the STC, and every 7 days thereafter.20
Adoptive T-cell transfer experiments
For Th17 adoptive cell transfer (ACT) therapy, Th17 cell preparation was performed as described previously21 with minor modification. Briefly, splenic CD4+ T cells from WT/WGM or KO/WGM mice on day 46 were magnetic cell sorting (MACS)-sorted using CD4+T cell Isolation kit II(Miltenyi Biotec) and stimulated with plate-bound 1.0 μg/mL anti-CD3 (BD Biosciences) and 1.0 μg/mL anti-CD28 (BD Biosciences) under Th17 conditions with 1.0 ng/mL TGF-β (R&D Systems), 10 ng/mL IL-6 (R&D Systems), 5.0 μg/mL anti–IL-4 (clone 11B11), and 5.0 μg/mL anti–IFN-γ (clone XMG1.2). On day 4 after stimulation with phorbol myristate acetate and ionomycin, Th17 induction rate was confirmed to be ∼ 65% among IL-17A–, IL-4–, or IFN-γ–producing T cells, and 1 × 106 cells were then intravenously injected into recipient syngeneic BALB/c mice. On the next day, they received subcutaneous challenge with 2 × 105 WEHI3B cells in the right flank. For CD4+ T-cell ACT therapy, 5 × 105 splenic CD4+CD44low T or CD4+CD44hi T cells harvested from WT/WGM or KO/WGM mice were flow cytometrically (FACSAria, BD Biosciences)-sorted on day 3 after STC and injected intravenously into recipient syngeneic BLAB/c mice. They received subcutaneous challenge with 2 × 105 WEHI3B cells in the right flank.
Statistical analyses
The 2-tailed Student t test was used to evaluate P values between experimental groups. P < .05 was considered statistically significant. Survival was plotted using Kaplan-Meier curves, and statistical relevance was determined by a log-rank comparison using Prism 5 (GraphPad). All experiments were repeated at least twice.
Results
Absence of LTB4/BLT1 axis in GM-CSF–triggered immunity induces potent antitumor effects against secondary tumor challenge with WEHI3B cells
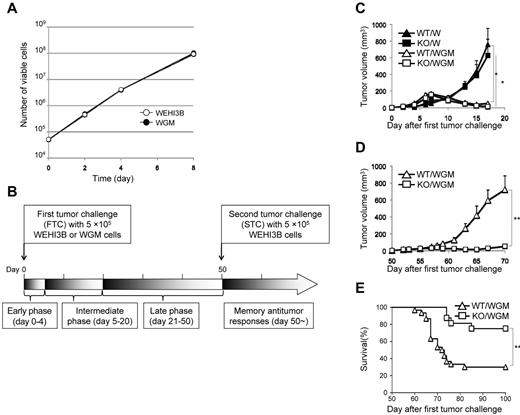
The GM-CSF production from WEHI3B cells was below detectable levels, whereas that from WGM cells was 136 ng/24 h/106 cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) enough to induce substantial antitumor effects.4,8 We compared in vitro proliferative ability between parental WEHI3B and WGM cells. Both cells exhibited an equal proliferation rate in a time-dependent manner (Figure 1A).
The absence of LTB4/BLT1 signaling induces potent antitumor effects against the second tumor challenge of WEHI3B cells after marked regression of WGM cells in mice model. (A) WEHI3B and WGM cells were cultured separately at 1 × 104 cells/well for 2, 4, 6, and 8 days in CM. At each time point, the number of viable cells was determined by trypan blue dye exclusion assay. (B) The explanatory scheme for all of the following in vivo experiments. The early phase, intermediate phase, and late phase in tumorigenicity assay are defined as spanning from days 0-4, 5-20, and 21-50 after the FTC, respectively. (C) In vivo tumorigenicity assay. The FTC consisted of 5 × 105 WEHI3B or WGM cells subcutaneously inoculated into the right flank of WT or BLT1-KO mice (n = 6–8). (D-E) In vivo STC assay. On day 50 after the FTC, 5 × 105 WEHI3B cells were subcutaneously inoculated into the left flank of mice that completely rejected the challenged tumor cells. The tumor volumes (mm3) in 4 indicated mice groups were assessed on day 17. Kaplan-Meier survival curves are shown after the STC. Bars represent mean ± SEM. Significant difference: *P < .05, **P < .01. Representative data from 2-6 independent experiments with similar results are shown.
The absence of LTB4/BLT1 signaling induces potent antitumor effects against the second tumor challenge of WEHI3B cells after marked regression of WGM cells in mice model. (A) WEHI3B and WGM cells were cultured separately at 1 × 104 cells/well for 2, 4, 6, and 8 days in CM. At each time point, the number of viable cells was determined by trypan blue dye exclusion assay. (B) The explanatory scheme for all of the following in vivo experiments. The early phase, intermediate phase, and late phase in tumorigenicity assay are defined as spanning from days 0-4, 5-20, and 21-50 after the FTC, respectively. (C) In vivo tumorigenicity assay. The FTC consisted of 5 × 105 WEHI3B or WGM cells subcutaneously inoculated into the right flank of WT or BLT1-KO mice (n = 6–8). (D-E) In vivo STC assay. On day 50 after the FTC, 5 × 105 WEHI3B cells were subcutaneously inoculated into the left flank of mice that completely rejected the challenged tumor cells. The tumor volumes (mm3) in 4 indicated mice groups were assessed on day 17. Kaplan-Meier survival curves are shown after the STC. Bars represent mean ± SEM. Significant difference: *P < .05, **P < .01. Representative data from 2-6 independent experiments with similar results are shown.
As myeloid cells, such as granulocytes, express abundant BLT1 and have direct antitumor effect,22,23 we speculated that the magnitude of antitumor effect provoked by WGM cells would be attenuated when administered into BLT1-KO mice. To verify our speculation, WEHI3B cells or WGM cells (FTC) were subcutaneously inoculated into the right flank of female WT or BLT1-KO mice. WT mice challenged with WGM cells (WT/WGM mice) rejected tumor growth, whereas WT or BLT1-KO mice challenged with WEHI3B cells (WT/W or KO/W) died of tumor burden. Unexpectedly, BLT1-KO mice challenged with WGM cells (KO/WGM mice) also significantly rejected tumor growth (P < .05; Figure 1C). We next compared antitumor responses against secondary tumor challenge with WEHI3B cells long after the FTC rejection. On day 50 after the FTC, parental WEHI3B cells (STC) were subcutaneously inoculated into the opposite (left) flank of WT/WGM and KO/WGM mice that had completely rejected the FTC. Intriguingly, KO/WGM mice significantly again rejected the outgrowth of STC (P < .01; Figure 1D) and survived for significantly longer periods compared with WT/WGM mice (P < .01; Figure 1E; Table 1). Similar results were observed in experiments using male mice, showing no sexual difference in the STC-rejection mechanism (Table 1).
Comparison of the number of WGM cell-injected mice that rejected the second tumor challenge with WEHI3B cells between WT and BLT1-KO mice
| . | Mice that rejected first tumor challenge, no. (%)* . | Mice that rejected second tumor challenge, no. (%)† . |
|---|---|---|
| Female groups‡ | ||
| WT/WGM | 30/35 (85.7) | 9/30 (30.0)§ |
| KO/WGM | 16/16 (100) | 13/16 (81.3)§ |
| Male groups | ||
| WT/WGM | 0/6 (0) | — |
| KO/WGM | 6/6 (100) | 5/6 (83.3)§ |
| . | Mice that rejected first tumor challenge, no. (%)* . | Mice that rejected second tumor challenge, no. (%)† . |
|---|---|---|
| Female groups‡ | ||
| WT/WGM | 30/35 (85.7) | 9/30 (30.0)§ |
| KO/WGM | 16/16 (100) | 13/16 (81.3)§ |
| Male groups | ||
| WT/WGM | 0/6 (0) | — |
| KO/WGM | 6/6 (100) | 5/6 (83.3)§ |
— indicates not applicable.
Assessed at day 50 after the first tumor challenge with WEHI3B or WGM cells.
Assessed at day 50 after the second tumor challenge with WEHI3B cells.
Shown are combined pooled data from at least 3 independent experiments with similar results.
χ2 test (P < .05).
Previous studies have reported that LTB4 antagonists inhibit cell proliferation of several cancer cells.24,25 To exclude the possibility that the rejection of STC was attributable to blocking of the LTB4/BLT1 axis-mediated direct antiproliferative effects, we examined BLT1 mRNA levels in WEHI3B and WGM cells by RT-PCR analysis. Both cells expressed undetectable levels of BLT1 mRNA (supplemental Figure 2), showing that the STC rejection was not induced by inhibition of LTB4/BLT1 signaling pathway.
Absence of LTB4/BLT1 axis facilitates activation of tumor-infiltrating innate immune subpopulations with mitigated immune tolerance in GM-CSF-induced antitumor immunity
We hypothesized that the rejection of STC could be induced by memory antitumor immunity. As GM-CSF–producing tumor cell vaccines not only induce the potent antitumor immunity by enhanced maturation of DCs26 but also simultaneously recruit abundant immune-regulatory myeloid-derived suppressor cells (MDSCs),27 we investigated the effect of blockade of LTB4/BLT1 signaling on tumor-infiltrating GM-CSF–sensitized diverse subsets of innate immune cells in early phase. The number of MDSCs and macrophages in tumors from KO/WGM mice were significantly decreased compared with those from WT/WGM mice (P < .05; Figure 2A-B). In contrast, the cell numbers of tumor-infiltrating NK cells, natural killer-like T cells (NKT), and cytolytic NK cells harvested from KO/WGM mice were significantly increased compared with those from WT/WGM mice (Figure 2C-E). In addition, the defective LTB4/BLT1 axis enhanced maturation of tumor-infiltrating DCs in tumors from KO/WGM mice compared with those from WT/WGM mice, as demonstrated by significantly increased expression of DC maturation markers, such as CD40 and CCR7 (P < .05; Figure 2F). Similar results were obtained for CD86+CD40+DCs, CD40+CCR7+DCs, CCR7+CD86+DCs, and CD86+CD40+CCR7+DCs isolated from tumors (P < .05; Figure 2G-H). We subsequently compared the expression of the immune-regulatory cytokines VEGF, TGF-β, and IL-10 at the tumor injection site that might account for various DC maturation. Concentrations of VEGF, TGF-β, and IL-10 in the supernatants derived from single-cell suspensions of excised tumors from KO/WGM mice were significantly lower than those of WT/WGM mice (P < .05; Figure 2I). Lastly, to examine the effect of the defective LTB4/BLT1 axis on GM-CSF–primed DCs for T-cell activation, we performed MLR assay using allogeneic T cells from C57BL/6 mice and splenic- or TDLN-derived CD11c+ DCs. The results showed that both splenic- (Figure 2J left panel) and TDLN- (Figure 2J right panel) derived DCs harvested from KO/WGM mice stimulated CD3+ T cells more efficiently than those from WT/WGM mice. When we compared the stimulation capacity of DCs harvested from KO/WGM mice for CD4+ and CD8+ T cells, we observed a superior proliferation of CD4+ T cells but not CD8+ T cells (Figure 2K).
Absence of LTB4/BLT1 signaling promotes activation of various innate immune subpopulations in tumor tissues, accompanied with attenuated immune tolerance in KO/WGM mice. (A-E) One day after the FTC with WGM cells, tumors were excised from WT/WGM or KO/WGM mice, minced finely, and treated with collagenase for tissue dissociation. For assessment of innate immunity subpopulations, tumor infiltrating cells were subsequently analyzed by flow cytometry as described in “Analyses for tumor-infiltrating leukocytes.” The numbers of viable cells are shown. (F-H) For detection of mature DCs, cells were stained with anti-CD11c, anti-CD40, anti-CD86, and anti-CCR7 mAb and subjected to flow cytometric analysis. Results shown are representative 2-dimensional dot plots (top panel, F-G) and bar graphs depicting the rates of indicated tumor infiltrating mature DCs (bottom panel, F-G). Numbers in 2-dimensional dot plots reflect the frequencies of (F) CD40+ or CCR7+CD11c+cells, (G) CD40+CD86+, CD40+CCR7+, or CCR7+CD86+CD11c+cells, and (H) CD40+CD86+CCR7+CD11c+ cells relative to the total CD11c+ cells. (I) The concentrations of VEGF (n = 5 or 6), TGF-β (n = 3), and IL-10 (n = 9 or 10) in supernatants derived from smashed tumor tissue of WT/WGM or KO/WGM mice were measured by ELISA assay. (J) MLR assay evaluated by [3H]thymidine incorporation. Various numbers of MACS-sorted splenic (left panel)- or TDLNs (right panel)-derived CD11c+ DCs from WT/WGM or KO/WGM mice in early phase were 30 Gy irradiated as stimulator cells and incubated with 2 × 105 MACS-sorted splenic CD3+ T cells (responder) harvested from splenic C57BL/6 mice at responder to stimulator (R:S) ratios as indicated for 4 days in triplicate. [3H]Thymidine was added 16 hours before the cells were harvested, and thymidine incorporation was assessed using TopCount NXT microplate scintillation counter. Cultures in the absence of DCs (T cells only) were used as a negative control. (K) CFSE-labeled MLR assay. A total of 100 000 MACS-sorted splenic CD11c+ DCs were harvested from WT/WGM or KO/WGM mice, irradiated with 30 Gy, and cocultured with CD3+ T cells at an R:S ratio of 2:1. After 6 days of coculture, the proliferation rate of CD3+CD4+ T cells (left panel) or CD3+CD8+ T cells (right panel) labeled with 2.0μM CFSE in the coculture was assessed by flow cytometric analysis. The histograms are gated on CD3+CD4+ or CD3+CD8+ T cells. Cultures in the absence of DCs (T cells only) were used as a negative control. Bar graphs represent mean ± SEM. *Significant difference (P < .05). **Significant difference (P < .01). Representative data from 3 independent experiments or combined data from 2 independent experiments (D-E) with similar results are shown.
Absence of LTB4/BLT1 signaling promotes activation of various innate immune subpopulations in tumor tissues, accompanied with attenuated immune tolerance in KO/WGM mice. (A-E) One day after the FTC with WGM cells, tumors were excised from WT/WGM or KO/WGM mice, minced finely, and treated with collagenase for tissue dissociation. For assessment of innate immunity subpopulations, tumor infiltrating cells were subsequently analyzed by flow cytometry as described in “Analyses for tumor-infiltrating leukocytes.” The numbers of viable cells are shown. (F-H) For detection of mature DCs, cells were stained with anti-CD11c, anti-CD40, anti-CD86, and anti-CCR7 mAb and subjected to flow cytometric analysis. Results shown are representative 2-dimensional dot plots (top panel, F-G) and bar graphs depicting the rates of indicated tumor infiltrating mature DCs (bottom panel, F-G). Numbers in 2-dimensional dot plots reflect the frequencies of (F) CD40+ or CCR7+CD11c+cells, (G) CD40+CD86+, CD40+CCR7+, or CCR7+CD86+CD11c+cells, and (H) CD40+CD86+CCR7+CD11c+ cells relative to the total CD11c+ cells. (I) The concentrations of VEGF (n = 5 or 6), TGF-β (n = 3), and IL-10 (n = 9 or 10) in supernatants derived from smashed tumor tissue of WT/WGM or KO/WGM mice were measured by ELISA assay. (J) MLR assay evaluated by [3H]thymidine incorporation. Various numbers of MACS-sorted splenic (left panel)- or TDLNs (right panel)-derived CD11c+ DCs from WT/WGM or KO/WGM mice in early phase were 30 Gy irradiated as stimulator cells and incubated with 2 × 105 MACS-sorted splenic CD3+ T cells (responder) harvested from splenic C57BL/6 mice at responder to stimulator (R:S) ratios as indicated for 4 days in triplicate. [3H]Thymidine was added 16 hours before the cells were harvested, and thymidine incorporation was assessed using TopCount NXT microplate scintillation counter. Cultures in the absence of DCs (T cells only) were used as a negative control. (K) CFSE-labeled MLR assay. A total of 100 000 MACS-sorted splenic CD11c+ DCs were harvested from WT/WGM or KO/WGM mice, irradiated with 30 Gy, and cocultured with CD3+ T cells at an R:S ratio of 2:1. After 6 days of coculture, the proliferation rate of CD3+CD4+ T cells (left panel) or CD3+CD8+ T cells (right panel) labeled with 2.0μM CFSE in the coculture was assessed by flow cytometric analysis. The histograms are gated on CD3+CD4+ or CD3+CD8+ T cells. Cultures in the absence of DCs (T cells only) were used as a negative control. Bar graphs represent mean ± SEM. *Significant difference (P < .05). **Significant difference (P < .01). Representative data from 3 independent experiments or combined data from 2 independent experiments (D-E) with similar results are shown.
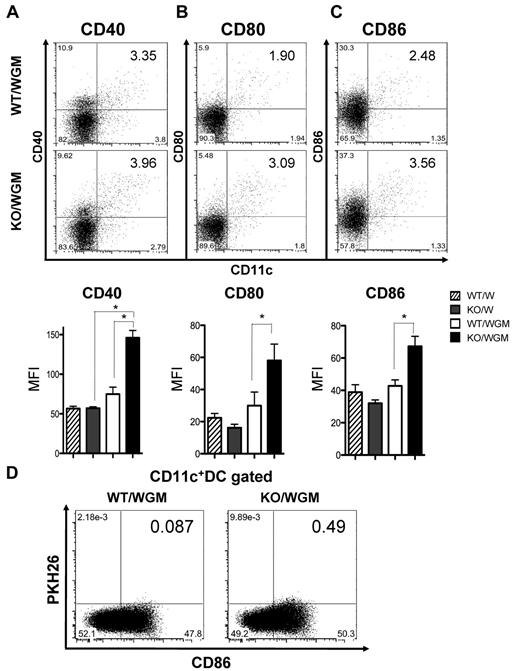
Absence of LTB4/BLT1 axis augments maturation and migration capacity of phagocytosed TAAs-DCs in GM-CSF-induced antitumor immunity
We next evaluated the impact of the defective LTB4/BLT1 axis on maturation of DCs in TDLNs on day 2 or 4 after FTC. As shown in Figure 3A-C and supplemental Figure 3, the mean fluorescence intensity of each CD40, CD80, and CD86 expression on DCs in TDLNs from KO/WGM mice was significantly more increased than that from WT/WGM mice (P < .05). To determine the influence of the defective LTB4/BLT1 axis on the migration capacity of mature DCs that had engulfed TAAs into TDLNs, we compared a proportion of CD86+PKH26+ DCs in TDLNs between WT/WGM and KO/WGM mice on day 2 after the FTC. The frequency of CD86+PKH26+ DCs in TDLNs harvested from KO/WGM mice was > 5-fold higher than that from WT/WGM mice (Figure 3D).
Absence of LTB4/BLT1 axis enhances maturation of DCs and recruitment of TAA-phagocytosed DCs into TDLNs in KO/WGM mice in early phase. Two left axillary TDLNs were harvested from WT/W, KO/W, WT/WGM, or KO/WGM mice on day 2 (CD80 and CD86) or day 4 (CD40) after the tumor challenge (n = 3-5 per group). Data are representative dot plots (top panel) and the averages of mean fluorescence intensity (bottom panel) for CD11c+ cells expressing the following markers of (A) CD40, (B) CD80, or (C) CD86 in TDLNs harvested from 4 indicated experimental groups. Bar graphs represent mean ± SEM. *Significant difference (P < .05). (D) Migration of DCs that had phagocytosed TAAs to TDLNs in WT/WGM or KO/WGM mice on day 2 after the subcutaneous inoculation with PKH26-labeled WGM cells. Numbers in 2-dimensional dot plots reflect the positive ratio of PKH26+CD86+ cells relative to total CD11c+ cells. Representative data from at least 3 independent experiments with similar results are shown.
Absence of LTB4/BLT1 axis enhances maturation of DCs and recruitment of TAA-phagocytosed DCs into TDLNs in KO/WGM mice in early phase. Two left axillary TDLNs were harvested from WT/W, KO/W, WT/WGM, or KO/WGM mice on day 2 (CD80 and CD86) or day 4 (CD40) after the tumor challenge (n = 3-5 per group). Data are representative dot plots (top panel) and the averages of mean fluorescence intensity (bottom panel) for CD11c+ cells expressing the following markers of (A) CD40, (B) CD80, or (C) CD86 in TDLNs harvested from 4 indicated experimental groups. Bar graphs represent mean ± SEM. *Significant difference (P < .05). (D) Migration of DCs that had phagocytosed TAAs to TDLNs in WT/WGM or KO/WGM mice on day 2 after the subcutaneous inoculation with PKH26-labeled WGM cells. Numbers in 2-dimensional dot plots reflect the positive ratio of PKH26+CD86+ cells relative to total CD11c+ cells. Representative data from at least 3 independent experiments with similar results are shown.
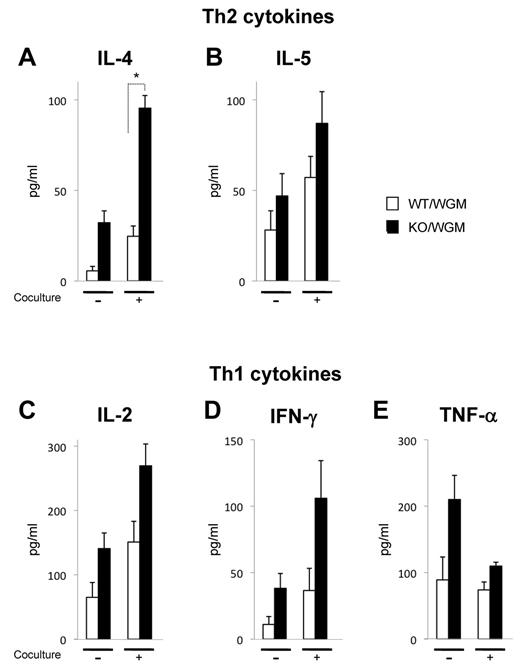
Absence of LTB4/BLT1 axis in GM-CSF–induced antitumor immunity systemically enhances TAAs-specific Th1 and Th2 responses in intermediate phase
To characterize the immune-modulatory effects of the defective LTB4/BLT1 axis on GM-CSF–triggered adaptive immunity, the expression levels of inflammatory cytokines, IL-2, IFN-γ, TNF-α (Th1 cytokines), and IL-4 and IL-5 (Th2 cytokines) secreted from splenocytes harvested from KO/WGM mice were compared with those from WT/WGM mice. On the coculture with irradiated WEHI3B cells, the expression levels of IL-2, IFN-γ, TNF-α, IL-4, and IL-5 from KO/WGM mice, on day 10 after FTC, were increased compared with those from WT/WGM mice. Only levels of IL-4 were significantly altered. All other cytokines tested did not show significant differences between the 2 groups but exhibited the same tendency in 3 independent experiments (Figure 4A-E).
Absence of LTB4/BLT1 axis promotes systemic activation of TAA-specific Th1 and Th2 subsets in KO/WGM mice in intermediate phase. In vitro Th1/Th2 cytokine production profiles of splenocytes harvested from WT/W, KO/W, WT/WGM, or KO/WGM mice on day 10 after the FTC. Approximately 1 × 106 splenocytes harvested were cultured with or without 4 × 105 irradiated WEHI3B cells for 20 hours. The concentrations of mouse (A) IL-4, (B) IL-5, (C) IL-2, (D) IFN-γ, and (E) TNF-α in the culture supernatants (n = 3) were measured by cytometric bead array assay. Bar graphs represent mean ± SEM. *Significant differences (P < .05). Representative data from 3 independent experiments with similar results are shown.
Absence of LTB4/BLT1 axis promotes systemic activation of TAA-specific Th1 and Th2 subsets in KO/WGM mice in intermediate phase. In vitro Th1/Th2 cytokine production profiles of splenocytes harvested from WT/W, KO/W, WT/WGM, or KO/WGM mice on day 10 after the FTC. Approximately 1 × 106 splenocytes harvested were cultured with or without 4 × 105 irradiated WEHI3B cells for 20 hours. The concentrations of mouse (A) IL-4, (B) IL-5, (C) IL-2, (D) IFN-γ, and (E) TNF-α in the culture supernatants (n = 3) were measured by cytometric bead array assay. Bar graphs represent mean ± SEM. *Significant differences (P < .05). Representative data from 3 independent experiments with similar results are shown.
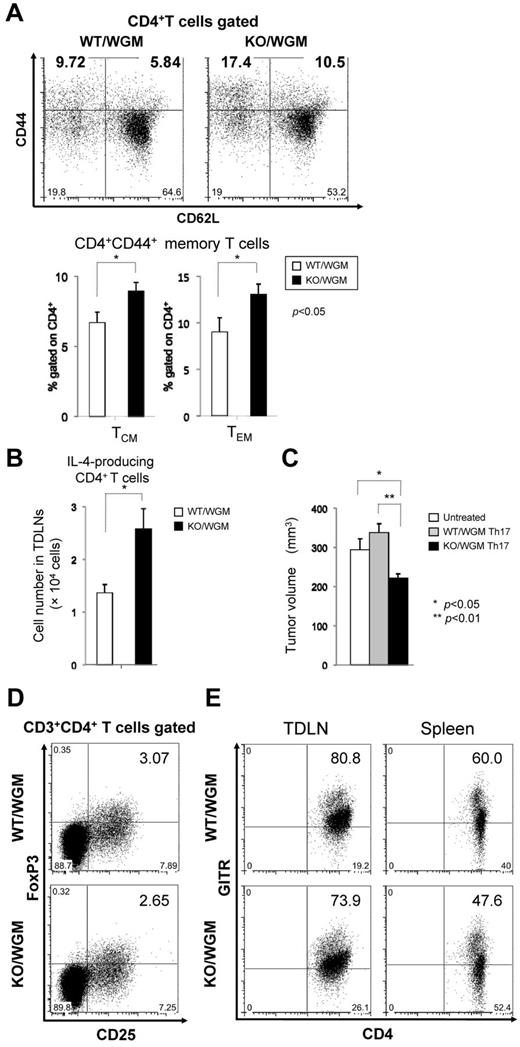
Absence of LTB4/BLT1 axis in GM-CSF-induced antitumor immunity increases diverse memory CD4+ T subsets skewing Th balance toward Th2 and Th17 predominance with antitumor phenotype
We speculated that the defective LTB4/BLT1 axis could have a positive impact on phenotypic profile of different memory CD4+ or CD8+ T subset, which might account for the marked rejection of STC. Thus, we compared the rates of central memory T subset (TCM) and effector memory T subset (TEM) in TDLNs between WT/WGM and KO/WGM mice on day 46 in late phase. Surface marker of CD44 was used as memory phenotype. TDLNs harvested from KO/WGM mice contained significantly higher proportions of the CD4+CD44+CD62L+ (TCM) and CD4+CD44+CD62L− (TEM) subsets relative to the total CD4+ T-cell population compared with those from WT/WGM mice (P < .05; Figure 5A), suggesting that the defective LTB4/BLT1 axis could serve to generate long-surviving TAA-specific memory CD4+ T cells. Subsequently, the proportion of Th1, Th2, and Th17 subsets in TDLNs and spleen in the late phase was evaluated between the 2 groups. The results showed that the frequency of Th2 and Th17 subset in TDLNs from KO/WGM mice increased by 32% and 47%, respectively, compared with that from WT/WGM mice (data not shown), indicating that the defective LTB4/BLT1 axis in GM-CSF–triggered immunity could cause a shift of the Th balance toward Th2 and Th17 predominance. Because IL-4 production fromTh2 cells is reported to be essential for maximal systemic antitumor immunity induced by GM-CSF–based tumor immunization,4,28 we analyzed the total number of IL-4–producing CD4+ T cells from TDLNs between the 2 groups. The total number of IL-4–producing Th2 cells in TDLNs from KO/WGM mice was significantly higher than in WT/WGM mice (P < .05), suggesting that the defective LTB4/BLT1 axis facilitates effective induction of antitumor Th2 responses driven by GM-CSF (Figure 5B). To determine whether the Th17 subset has an in vivo antitumor activity, we treated recipient BALB/c mice with adoptive cell transfer of splenic Th17-skewed cells derived from WT/WGM or KO/WGM mice and subcutaneously challenged them with parental WEHI3B cells. Only the transfer of KO/WGM mice-derived Th17 cells significantly suppressed the WEHI3B tumor development compared with untreated and WT/WGM mice (P < .05; Figure 5C).
Absence of LTB4/BLT1 axis facilitates induction of diverse memory CD4+ T subsets and polarized Th2/Th17 cells with antitumor phenotype in KO/WGM mice in late phase. TDLNs cells harvested from WT/WGM or WT/WGM mice (n = 3-5/group) on day 46 after the FTC were subjected to polychromatic flow cytometric analyses. (A) Dot plot profiles (top panels) and graphs (bottom panels) represent the percentages of CD4+CD44+CD62L+ TCM or CD4+CD44+CD62L− TEM subset relative to the total CD4+ T-cell population. Numbers in boldface in dot plot profiles show the representative percentages of TCM and TEM in WT/WGM and KO/WGM and are reflected to the graphs. (B) Bar graphs represent the total number of IL-4-producing T cells in TDLNs derived from WT/WGM and KO/WGM mice (n = 3 or 4). Combined data from 2 independent experiments are shown. (C) Th17 adoptive T-cell transfer (ACT) assay. Splenic CD4+ T cells from WT/WGM or KO/WGM mice on day 46 were MACS-sorted and stimulated with plate-bound anti-CD3 mAb and soluble anti-CD28 mAb under Th17 condition. After 4 days of incubation, 1 million cells were intravenously transferred into recipient syngeneic BALB/c mice. On the next day, they received subcutaneous challenge with WEHI3B cells in the right flank. Bar graph represents the tumor volume (mm3) of untreated, mice treated with WT/WGM mice- or KO/WGM mice-derived Th17 ACT, assessed on day 10 after the Th17 ACT therapy. (D-E) Phenotypic profile of immune-regulatory T cells in late phase. Shown are representative dot plots depicting the percentages of (D) CD3+CD4+CD25+FoxP3+ cells in TDLNs harvested from WT/WGM or KO/WGM mice, and of (E) CD3+CD4+GITR+ T cells in TDLNs and spleen from WT/WGM or KO/WGM mice. Bar graphs represent the mean ± SEM. Significant differences: *P < .05, **P < .01. Representative data from 3 independent experiments with similar results or combined data (A-C) from 2 independent experiments are shown.
Absence of LTB4/BLT1 axis facilitates induction of diverse memory CD4+ T subsets and polarized Th2/Th17 cells with antitumor phenotype in KO/WGM mice in late phase. TDLNs cells harvested from WT/WGM or WT/WGM mice (n = 3-5/group) on day 46 after the FTC were subjected to polychromatic flow cytometric analyses. (A) Dot plot profiles (top panels) and graphs (bottom panels) represent the percentages of CD4+CD44+CD62L+ TCM or CD4+CD44+CD62L− TEM subset relative to the total CD4+ T-cell population. Numbers in boldface in dot plot profiles show the representative percentages of TCM and TEM in WT/WGM and KO/WGM and are reflected to the graphs. (B) Bar graphs represent the total number of IL-4-producing T cells in TDLNs derived from WT/WGM and KO/WGM mice (n = 3 or 4). Combined data from 2 independent experiments are shown. (C) Th17 adoptive T-cell transfer (ACT) assay. Splenic CD4+ T cells from WT/WGM or KO/WGM mice on day 46 were MACS-sorted and stimulated with plate-bound anti-CD3 mAb and soluble anti-CD28 mAb under Th17 condition. After 4 days of incubation, 1 million cells were intravenously transferred into recipient syngeneic BALB/c mice. On the next day, they received subcutaneous challenge with WEHI3B cells in the right flank. Bar graph represents the tumor volume (mm3) of untreated, mice treated with WT/WGM mice- or KO/WGM mice-derived Th17 ACT, assessed on day 10 after the Th17 ACT therapy. (D-E) Phenotypic profile of immune-regulatory T cells in late phase. Shown are representative dot plots depicting the percentages of (D) CD3+CD4+CD25+FoxP3+ cells in TDLNs harvested from WT/WGM or KO/WGM mice, and of (E) CD3+CD4+GITR+ T cells in TDLNs and spleen from WT/WGM or KO/WGM mice. Bar graphs represent the mean ± SEM. Significant differences: *P < .05, **P < .01. Representative data from 3 independent experiments with similar results or combined data (A-C) from 2 independent experiments are shown.
Furthermore, we evaluated the effect of the defective LTB4/BLT1 axis on regulatory T-cell subpopulations. The each percentage of CD4+CD25+FoxP3+ regulatory T cells or CD3+CD4+GITR+ cells between WT/WGM and KO/WGM mice was comparatively assessed, as GITR expression is constitutively up-regulated in regulatory T cells but not on resting CD3+CD4+ T lymphocytes.29,30 The percentages of CD4+CD25+Foxp3+ regulatory T cells in TDLNs and CD3+CD4+GITR+ T cells in TDLNs and spleen from KO/WGM mice were more decreased than those from WT/WGM mice (Figure 5D-E).
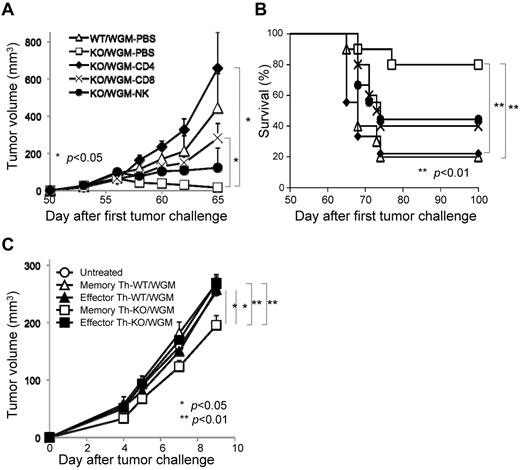
Marked rejection of STC in KO/WGM mice is mainly attributed to CD4+ T cells where memory Th subset possesses an antitumor phenotype
In vivo depletion of CD4+ T, CD8+ T, and NK cells was performed to determine which subsets are essential for the rejection of STC seen in KO/WGM mice. Each depletion of CD4+ T, CD8+ T, or NK cells in KO/WGM mice significantly abrogated the antitumor effects compared with PBS-treated KO/WGM mice (P < .05). Importantly, KO/WGM mice depleted of CD4+ T cells elicited rather accelerated tumor outgrowth (Figure 6A) and a significantly shorter survival (Figure 6B), with a significant decrease in number of mice that rejected the STC (Table 2), compared with WT/WGM mice, demonstrating that the long-lasting antitumor immunity seen in KO/WGM mice was largely dependent on CD4+ T cells.
CD4+ T cells mainly mediate the remarkable rejection of second tumor challenge with WEHI3B cells where memory CD44hiCD4+ T subset has an antitumor phenotype on adoptive cell transfer. All mice that had completely rejected the FTC with WGM cells were then subcutaneously inoculated with the STC on day 50 after the FTC, as described in Figure 1 (n = 4-6). For depletion of CD4+ and CD8+ T cells, mice received repeated intraperitoneal injections of anti–mouse-CD4 mAb or anti–mouse-CD8 mAb on days 3, 4, and 5 before the day of the STC and once every 3 days thereafter up to 13 times. For depletion of NK cells, mice received repeated intraperitoneal injections of rabbit anti-asialo GM1 antiserum 1 day before and 7 and 14 days after the STC. (A) Tumor volume was monitored and (B) a survival curve of the mice groups was examined. (C) For CD4+ T-cell ACT therapy, 5 × 105 CD4+CD44low or CD4+CD44hi T cells harvested from spleen of WT/WGM or KO/WGM mice were flow cytometrically sorted on day 3 after the STC and intravenously injected into recipient syngeneic BLAB/c mice (n = 4-6). On the next day, they received subcutaneous challenge with 2 × 105 parental WEHI3B cells in the right flank. Bar graphs represent mean ± SEM. Significant differences: *P < .05, **P < .01. Representative or combined data from 2 independent experiments with similar results are shown.
CD4+ T cells mainly mediate the remarkable rejection of second tumor challenge with WEHI3B cells where memory CD44hiCD4+ T subset has an antitumor phenotype on adoptive cell transfer. All mice that had completely rejected the FTC with WGM cells were then subcutaneously inoculated with the STC on day 50 after the FTC, as described in Figure 1 (n = 4-6). For depletion of CD4+ and CD8+ T cells, mice received repeated intraperitoneal injections of anti–mouse-CD4 mAb or anti–mouse-CD8 mAb on days 3, 4, and 5 before the day of the STC and once every 3 days thereafter up to 13 times. For depletion of NK cells, mice received repeated intraperitoneal injections of rabbit anti-asialo GM1 antiserum 1 day before and 7 and 14 days after the STC. (A) Tumor volume was monitored and (B) a survival curve of the mice groups was examined. (C) For CD4+ T-cell ACT therapy, 5 × 105 CD4+CD44low or CD4+CD44hi T cells harvested from spleen of WT/WGM or KO/WGM mice were flow cytometrically sorted on day 3 after the STC and intravenously injected into recipient syngeneic BLAB/c mice (n = 4-6). On the next day, they received subcutaneous challenge with 2 × 105 parental WEHI3B cells in the right flank. Bar graphs represent mean ± SEM. Significant differences: *P < .05, **P < .01. Representative or combined data from 2 independent experiments with similar results are shown.
Effect of in vivo depletion of each immune subpopulation on the long-lasting rejection of the second tumor challenge
| Group . | Mice that rejected second tumor challenge, no. (%)* . |
|---|---|
| WT/WGM-PBS | 1/10 (10.0)† |
| KO/WGM-PBS | 9/11 (81.8) |
| KO/WGM-CD4 | 1/10 (10.0)† |
| KO/WGM-CD8 | 3/11 (27.3)† |
| KO/WGM-NK | 2/10 (20.0)† |
| Group . | Mice that rejected second tumor challenge, no. (%)* . |
|---|---|
| WT/WGM-PBS | 1/10 (10.0)† |
| KO/WGM-PBS | 9/11 (81.8) |
| KO/WGM-CD4 | 1/10 (10.0)† |
| KO/WGM-CD8 | 3/11 (27.3)† |
| KO/WGM-NK | 2/10 (20.0)† |
Shown are combined pooled data from 2 independent experiments with similar results.
Assessed at day 15 after the second tumor challenge with WEHI3B cells.
χ2 test (P < .05).
To determine whether the persistent antitumor memory observed in KO/WGM mice was memory CD4+ T subset-dependent, we comparatively investigated therapeutic effects by adoptive T-cell transfer (ACT) between CD4+CD44low T cells (effector Th) and CD4+CD44hi T (memory Th) cells, both of which were sorted from spleens of WT/WGM or KO/WGM mice on day 3 after the STC. We treated mice with intravenous ACT therapy and, on the next day, challenged them with WEHI3B cells in the right flank. Either with or without ACT using KO/WGM mice-derived effector Th subset, WEHI3B tumors continued to grow, whereas ACT using KO/WGM mice-derived splenic memory Th subset significantly inhibited the WEHI3B tumor formation (Figure 6C). On the other hand, all ACT therapies using cells from WT/WGM mice manifested no antitumor activity. These results strongly indicated that memory Th subset has an important role in GM-CSF–induced antitumor memory immunity when the LTB4/BLT1 signaling is not present or dysfunctional.
Discussion
Memory T cells respond promptly to delivered antigens and are critical in the induction of sustained antitumor immunity. They require less costimulation to be activated and release a broader spectrum of cytokines compared with naive T cells.31 One of the major challenges for therapeutic improvement of cancer vaccines is to optimize persistent memory antitumor responses.
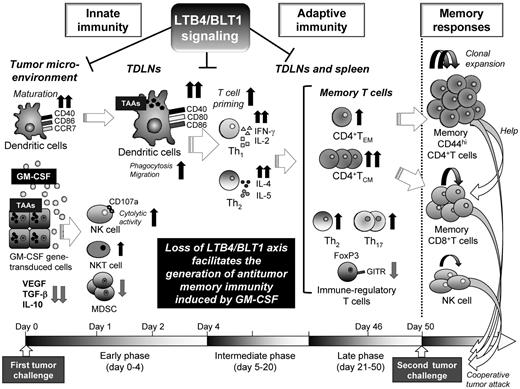
Numerous studies reported that leukotrienes are involved in carcinogenesis and tumor development.24,25,32,33 However, the role of leukotriene-based signaling in tumor immunology, including memory immunity, has never been elucidated. In the current study, we are the first group to find that the defective LTB4/BLT1 signaling induces long-term antitumor memory responses after immunization with WGM cells, demonstrating that the LTB4/BLT1 axis is an adversary for maintaining GM-CSF–triggered antitumor memory responses as summarized in a schematic overview (Figure 7). Namely, blockade of the LTB4/BLT1 signaling elicited numerous positive effects on development of GM-CSF–triggered persistent antitumor memory immunity as follows:
The defective LTB4/BLT1 axis diminished the recruitment of immune-regulatory MDSCs into tumors in early phase (Figure 2B), probably because of abundant BLT1 expression in diverse myeloid cells, including MDSCs.34
The absence of LTB4/BLT1 axis increased recruitment of CD107a, a surrogate for lytic degranulation,35 expressing cytolytic NK cells into tumors (Figure 2E), indicating its negative effects for tumoricidal ability activated by GM-CSF.
The defective LTB4/BLT1 signaling caused a synergistic effect on maturation, homing capacity of TAA-phagocytosed DCs into TDLNs (Figures 2F-H and 3A-D), and further enhanced the capacity of GM-CSF–sensitized DCs to stimulate CD4+ T cells, but not CD8+ T cells (Figure 2J-K), indicating its effective priming of helper T cell-predominant adaptive immunity. These positive effects on DCs were the result of the decreased expression of various immune-regulatory cytokines in tumor microenvironments (Figure 2I). On the contrary, others reported that LTB4 stimulation enhances DC migration and adaptive immune responses.13 This discrepancy may stem from our use of GM-CSF gene-transduced tumor cells, although the detailed molecular crosstalk between LTB4/BLT1 and GM-CSF signaling remains unknown.
The absence of LTB4/BLT1 axis augmented the production of Th1 cytokine and Th2 cytokine from TAA-stimulated splenocytes harvested from GM-CSF–sensitized mice in intermediate phase (Figure 4). Conversely, Toda et al showed that functional BLT1 expression on DCs is important to initiate Th1-type immune response, indicating that GM-CSF–based immunization may bring about the conflicting outcome.36
The absence of LTB4/BLT1 axis increased the proportions of CD4+ TCM and CD4+ TEM subset in TDLNs in late phase before the STC (Figure 5A). As memory T cells possess the capacity to mount immediate recall responses to antigen challenge37 and that TCM subset has superior antitumor abilities compared with TEM subset,38 we hypothesized that the former subset might rapidly migrate to the injection site of STC to confer antitumor effects. Indeed, we succeeded to demonstrate that the KO/WGM mice-derived memory Th subset had significantly stronger antitumor efficacy than the effector Th subset from the results of ACT therapy (Figure 6C).
We showed that the defective LTB4/BLT1 signaling in GM-CSF-triggered antitumor immunity skewed Th2 predominance in late phase (Figure 5B) and that ACT of splenic Th17 cells harvested from KO/WGM mice elicited stronger antitumor effects against WEHI3B challenge than those from WT/WGM mice (Figure 5C). Our results are compatible with the previous reports showing that memory Th2 cells had a potent antitumor immunity through IL-4–activated NK cells39 and that the Th17 subset was decisive for yielding immunologic responses together with effector cytotoxic T lymphocytes.40 These findings indicate that the absence of LTB4/BLT1 signaling facilitates Th2 and Th17 polarization with antitumor phenotype, although the significance of Th2 and Th17 cells in tumor immunology is still controversial.41,42 As frequency of multifunctional CD4+ T cells simultaneously producing IFN-γ, IL-2, and TNF-α is recognized as a sensitive immune correlate for the optimized effector function against antigens,43 we assessed the influence of the defective LTB4/BLT1 signaling on phenotypic profiles of CD4+ T-cell multifunctionality. There was, however, no significant difference in frequency of each single-, or double-, or triple-cytokine producing CD4+ T subset between WT/WGM and KO/WGM mice (supplemental Figure 4).
The absence of LTB4/BLT1 in GM-CSF–induced immunity systemically decreased production levels of the immune-regulatory cytokines in the tumor microenvironment (Figure 2I) and the infiltrated immune-regulatory CD4+ T subsets in lymphoid tissue (Figure 5D-E), probably converting the immunosuppressive milieu to one that helps both generation and maintenance of memory T cells. Although other researchers showed that LTB4 plus IL-2 could generate CD8+ suppressor thymocytes involved in tolerance to self-antigens,44 there have never been relevant data delineating relationship between arachidonic-acid–derived lipid mediator, such as pro-inflammatory leukotrienes and regulatory T cell-mediated immune tolerance. Thereby, our findings could offer a hint to attenuate tumor-driven immune tolerance via the inhibition of the LTB4/ BLT1 signaling.
Finally, our results from in vivo depletion assays unraveled that CD4+ T cells in KO/WGM mice played a predominant role in providing persistent antitumor immunity, in coordination with NK cells and CD8+ T cells (Figure 6A-B), which are compatible with the previous findings that CD4+ T cells are essential in the control of tumor growth through the cooperation with macrophages and cytotoxic T lymphocytes.4,45,46 When we consider to further clarify the molecular crosstalk between LTB4 and GM-CSF signaling pathways, it will be of importance to focus on our findings that the absence of the LTB4/BLT1 axis had positive impact mainly on CD4+ helper T cell-mediated adaptive immunity as evidenced by MLR assay and ACT experiments, as well as innate immunity. This novel insight that potent memory CD4+ T cells in GM-CSF–triggered immunity can be retained in devoid of LTB4/BLT1signaling allows us to expect that blocking LTB4/BLT1signaling using corresponding antagonists or inhibitors may be a promising strategy to improve the therapeutic effects of GM-CSF–based tumor vaccines in clinical settings by shaping a favorable immunologic memory.
Schematic overview of the experimental results. This schematic overview illustrates the effects of the defective LTB4/BLT1 axis on the process of generation of antitumor memory immunity provoked by GM-CSF–transduced tumor cells in mice, and the molecular or cellular components that compose the immune system and are putatively relevant to this phenomenon.
Schematic overview of the experimental results. This schematic overview illustrates the effects of the defective LTB4/BLT1 axis on the process of generation of antitumor memory immunity provoked by GM-CSF–transduced tumor cells in mice, and the molecular or cellular components that compose the immune system and are putatively relevant to this phenomenon.
With regard to another receptor BLT2 for LTB4, it requires very high concentrations of LTB4 for its activation47 and has been well accepted to be a receptor for 12-HHT.48 Accordingly, it is now thought that BLT2 does not mediate any functions of LTB4 in the absence of BLT1 in vivo. We therefore disregarded the impact of BLT2 on the results of our experiments.
Intriguingly, in terms of sexual difference, the mechanism in which a superior antitumor immunity induced by WGM cells was manifested in WT female mice compared with WT male (Table 1) could possibly be explained by the following WT regulatory T cells from female mice expressing significantly lower B7-H1, a coinhibitory signaling molecule that can suppress antitumor immunity49 and possess a more efficient phagocytic ability by resident macrophages,50 compared with those from male mice.
In conclusion, we demonstrated that, even long after the tumor rejection, the defective LTB4/BLT1 axis facilitates effective generation of long-lasting memory CD4+ T cell-dependent antitumor immunity through its positive impacts on innate and adaptive immunologic responses induced by GM-CSF.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kazuko Saeki and Dr Toshiaki Okuno (Kyushu University) for their technical supports.
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Young Scientists 23790446) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant-in-Aid for Scientific Research on Priority Areas “Cancer” 17016053).
Authorship
Contribution: Y.Y., H.I., and K. Tani designed the study and wrote manuscript; Y.Y., H.I., Y.M., C.S., and H.N. executed the experiments; Y.Y. analyzed all data; Y.Y., H.I., T.Y., and K. Tani interpreted the results of experiments; Y.Y., H.N., A.W., and F.S. contributed in the backcross and preparation of mice; and all authors discussed the results and helped conduct experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenzaburo Tani, Department of Molecular Genetics, Medical Institute of Bioregulation, Kyushu University, 3-1-1, Maidashi, Higashi-ku, Fukuoka, Japan 812-8582; e-mail: taniken@bioreg.kyushu-u.ac.jp.
References
Author notes
Y.Y. and H.I. contributed equally to this study.

![Figure 2. Absence of LTB4/BLT1 signaling promotes activation of various innate immune subpopulations in tumor tissues, accompanied with attenuated immune tolerance in KO/WGM mice. (A-E) One day after the FTC with WGM cells, tumors were excised from WT/WGM or KO/WGM mice, minced finely, and treated with collagenase for tissue dissociation. For assessment of innate immunity subpopulations, tumor infiltrating cells were subsequently analyzed by flow cytometry as described in “Analyses for tumor-infiltrating leukocytes.” The numbers of viable cells are shown. (F-H) For detection of mature DCs, cells were stained with anti-CD11c, anti-CD40, anti-CD86, and anti-CCR7 mAb and subjected to flow cytometric analysis. Results shown are representative 2-dimensional dot plots (top panel, F-G) and bar graphs depicting the rates of indicated tumor infiltrating mature DCs (bottom panel, F-G). Numbers in 2-dimensional dot plots reflect the frequencies of (F) CD40+ or CCR7+CD11c+cells, (G) CD40+CD86+, CD40+CCR7+, or CCR7+CD86+CD11c+cells, and (H) CD40+CD86+CCR7+CD11c+ cells relative to the total CD11c+ cells. (I) The concentrations of VEGF (n = 5 or 6), TGF-β (n = 3), and IL-10 (n = 9 or 10) in supernatants derived from smashed tumor tissue of WT/WGM or KO/WGM mice were measured by ELISA assay. (J) MLR assay evaluated by [3H]thymidine incorporation. Various numbers of MACS-sorted splenic (left panel)- or TDLNs (right panel)-derived CD11c+ DCs from WT/WGM or KO/WGM mice in early phase were 30 Gy irradiated as stimulator cells and incubated with 2 × 105 MACS-sorted splenic CD3+ T cells (responder) harvested from splenic C57BL/6 mice at responder to stimulator (R:S) ratios as indicated for 4 days in triplicate. [3H]Thymidine was added 16 hours before the cells were harvested, and thymidine incorporation was assessed using TopCount NXT microplate scintillation counter. Cultures in the absence of DCs (T cells only) were used as a negative control. (K) CFSE-labeled MLR assay. A total of 100 000 MACS-sorted splenic CD11c+ DCs were harvested from WT/WGM or KO/WGM mice, irradiated with 30 Gy, and cocultured with CD3+ T cells at an R:S ratio of 2:1. After 6 days of coculture, the proliferation rate of CD3+CD4+ T cells (left panel) or CD3+CD8+ T cells (right panel) labeled with 2.0μM CFSE in the coculture was assessed by flow cytometric analysis. The histograms are gated on CD3+CD4+ or CD3+CD8+ T cells. Cultures in the absence of DCs (T cells only) were used as a negative control. Bar graphs represent mean ± SEM. *Significant difference (P < .05). **Significant difference (P < .01). Representative data from 3 independent experiments or combined data from 2 independent experiments (D-E) with similar results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/17/10.1182_blood-2011-10-383240/4/m_zh89991297850002.jpeg?Expires=1764957128&Signature=RxQeUiA94yMstFNGHFl128wWKRPpJRvlEVm9jeFlMKkJCYCcq-PNYpjWr8IviUddafZkIno1Kkz1OEn6eYdwW0LUDWwRP0Zn1Of4dLHp~eOV2Cpz167Qzd29XBG0fWt3cT9pFn8tiIfZwtRk6necQC4mKwT~DuwPK50hiePy~dfhVxkRz18Ax2oKwyDwrCd63DetX6ztNRaUCBw4tHNu1ah4U-SoIowff~3AeMSyS0PiczeDD2Qn8Jn8Z4P8Jt0SKOx~FY7g4KWVHCZLHekyH4JLU-uxEwBev4sXHOmrPLqvMuPWZ95vOPFIYXkd1w4zd7EDSbNuNsLAPYFd5kqI2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)